Through the Looking Glass: Unraveling the Stage-Shift of Acute Rejection in Renal Allografts
Abstract
:1. Introduction
2. Methods
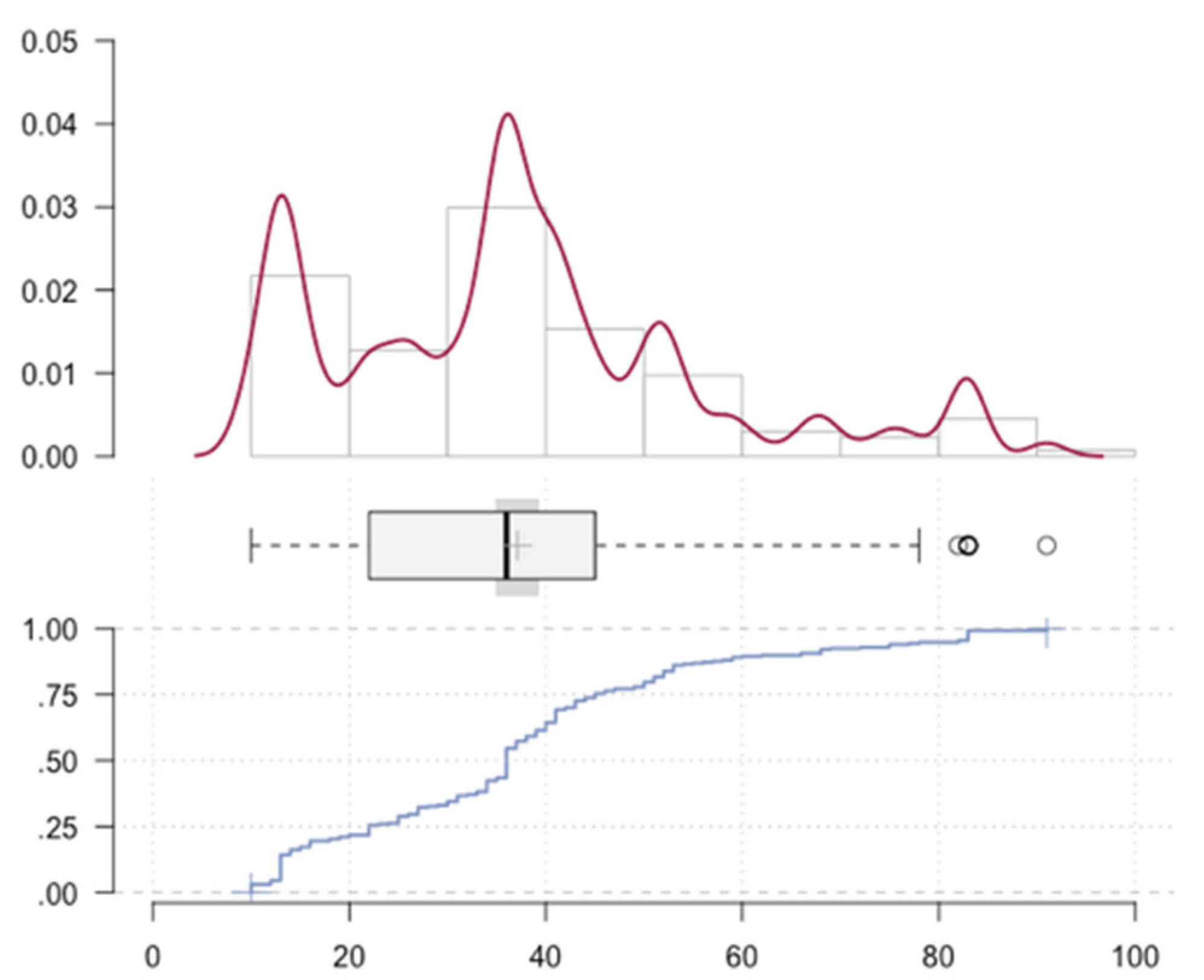
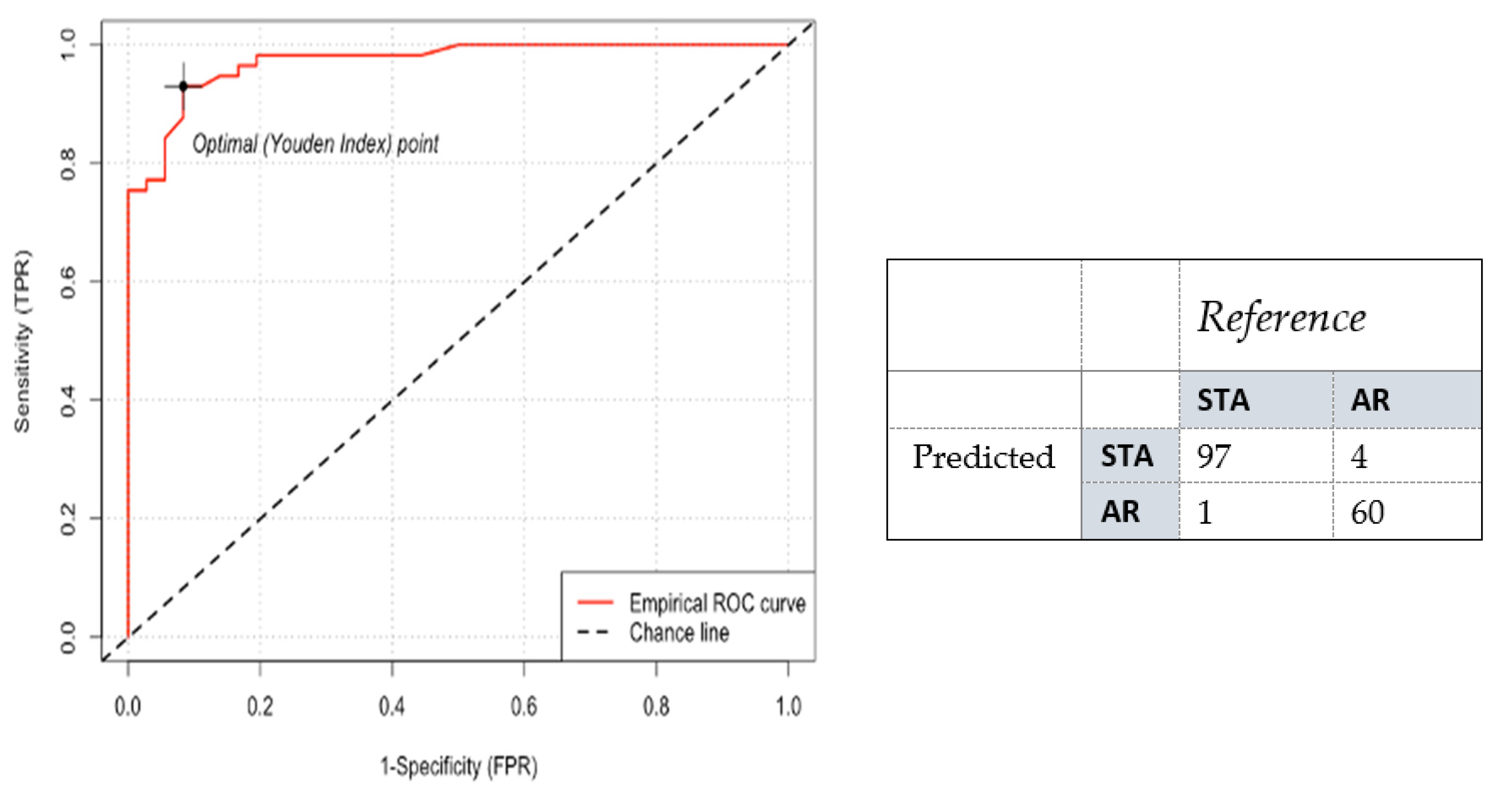
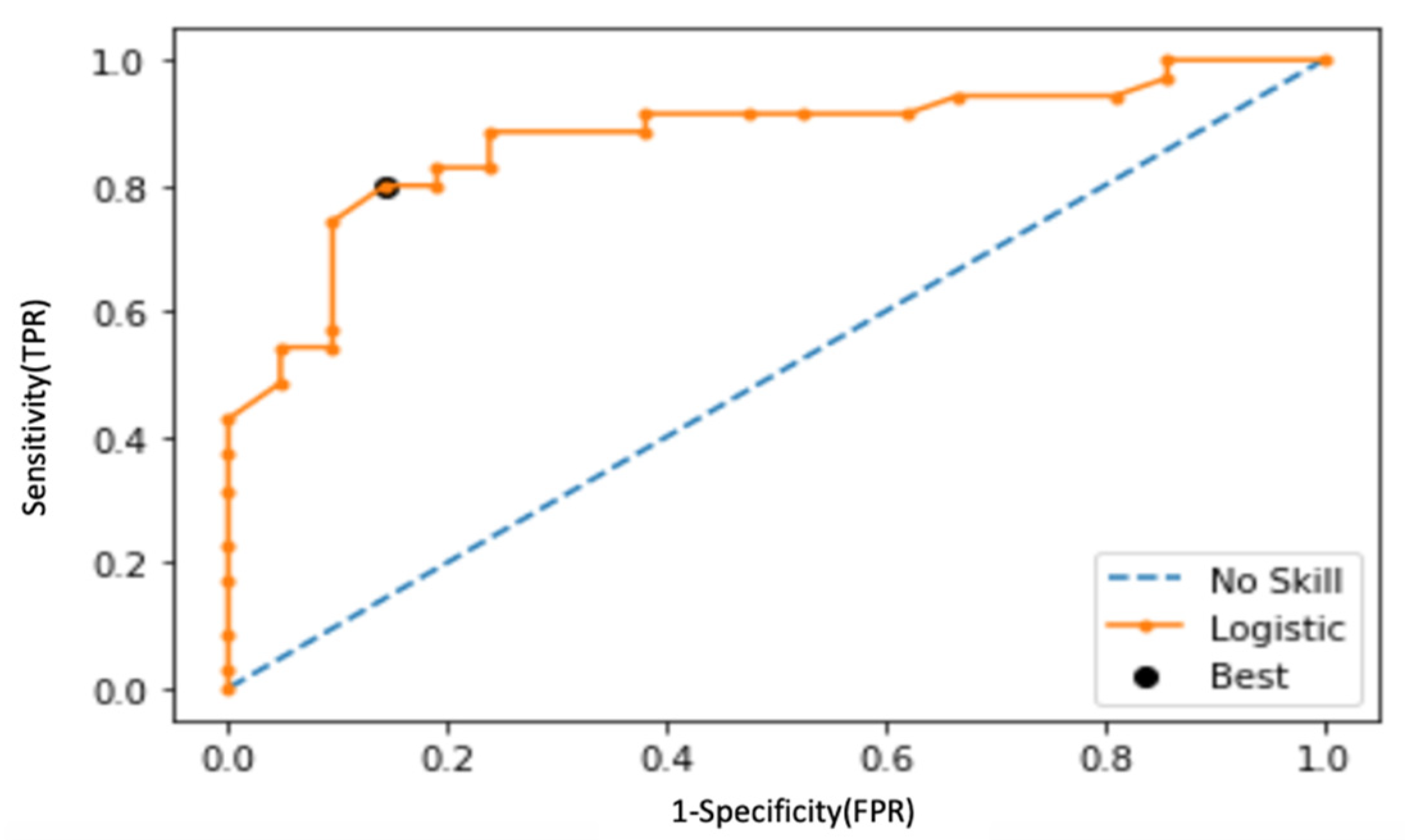
3. Results
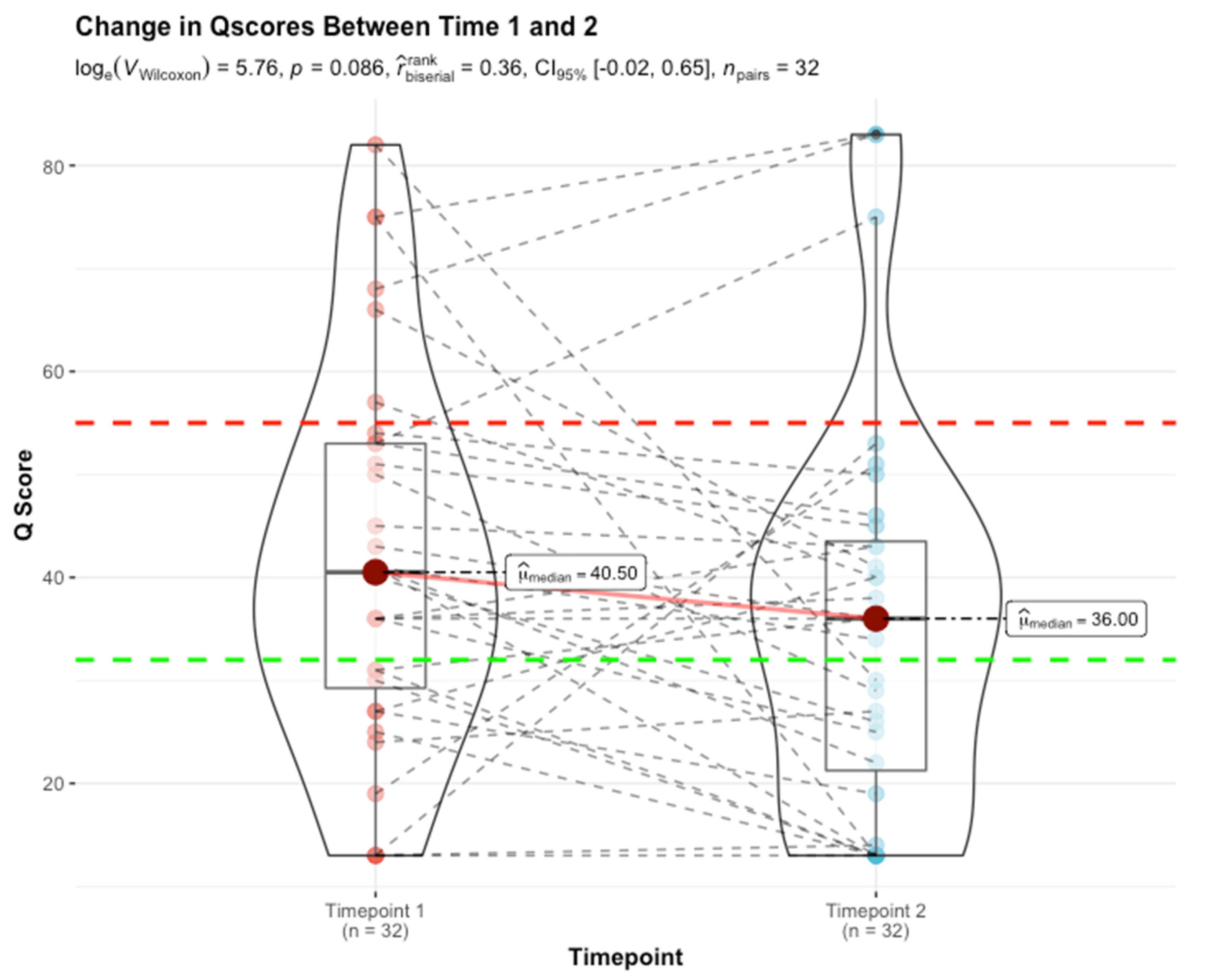
- (A).
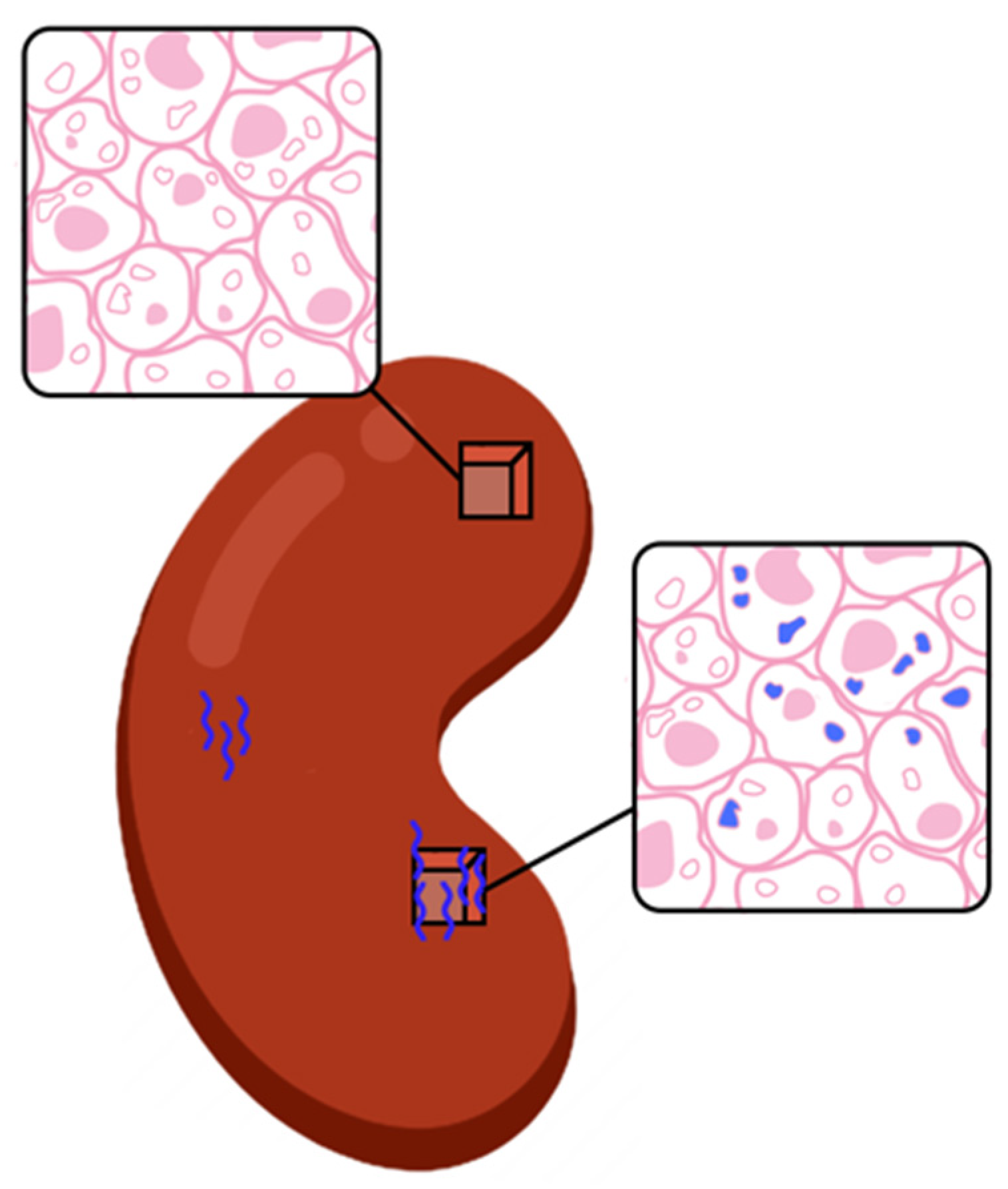
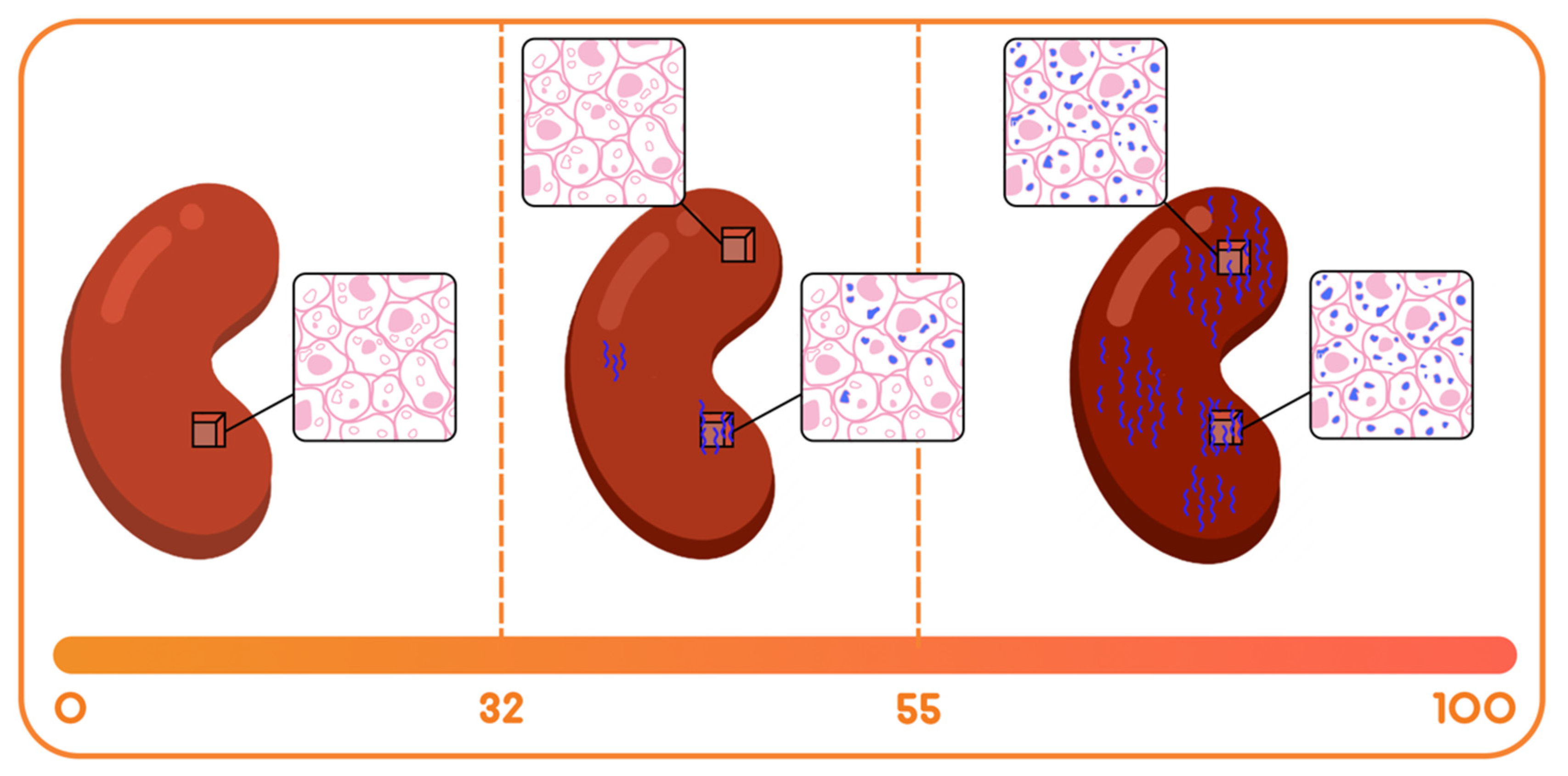
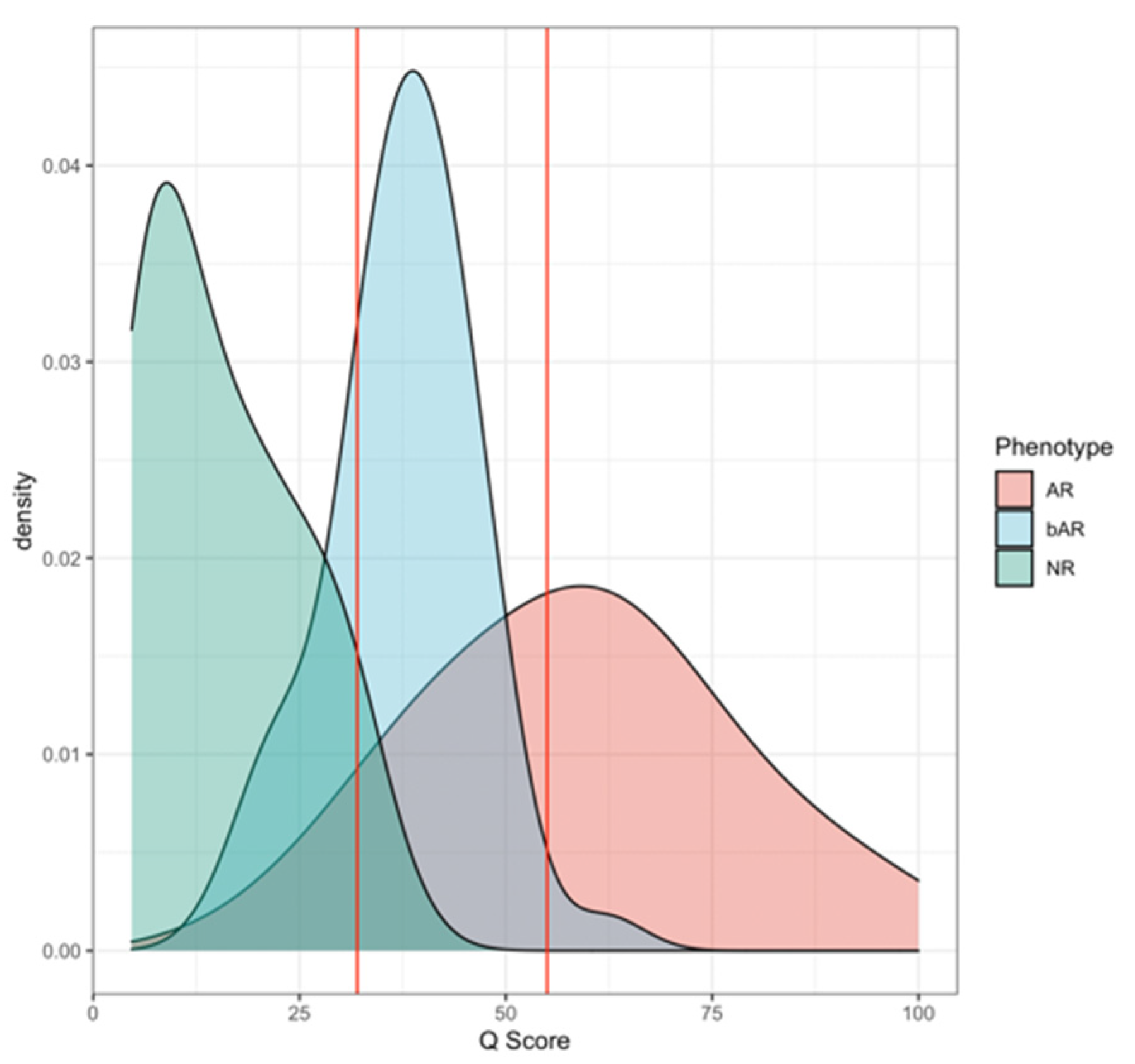
- The Immune quiescence domain of Q-Score < 32: Allograft health is demarcated by this domain; this has been validated in published data [10] and classifies stable (STA) allograft recipients. Patients in this range are on immunosuppression that is adequate to suppress alloimmunity and persisting low Q-Score < 32 may possibly allow for immunosuppression to be safely maintained or diminished.
- (B).
- Alloimmune injury domain of Q-Scores in the range of 32–55: expose a state of alloimmunity flux as the graft switches from a state of allograft health to early alloimmune injury. This domain enriches for molecular allograft injury, where early rejection may not have histologically developed fully yet and could be missed by a biopsy [15].
- (C).
- High-grade acute rejection domain of Q-Scores in the range of 55–100: provide a zone of advanced alloimmunity, associating with simultaneous histological and molecular rejection injury.
- (i)
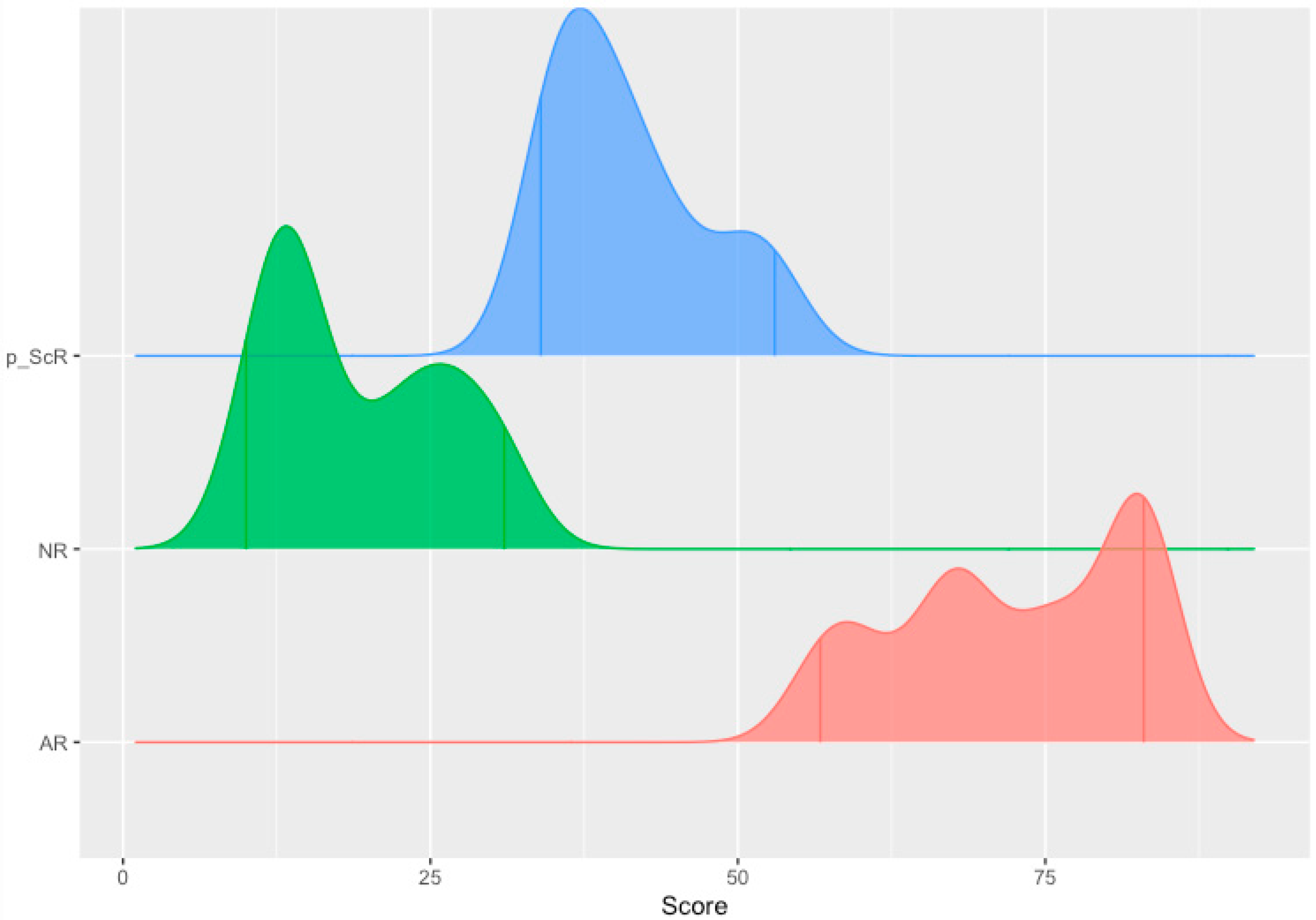
- A planar gradient in Q-Score across time, showing persistence of alloimmunity (28% [9/32]);
- (ii)
- An increasing gradient, delineating patients moving into the mature AR domain (3% [1/32]);
- (iii)
- A decreasing gradient, delineating patients moving into the immunoquiescent domain, putatively in response to immunomodulation following assessment of chronic/active/mixed rejection (22% [7/32]). Albeit a small sample size (16% [5/32]), the evolving and resolving nature of higher-grade acute rejection (Q-Score > 55) in response to immunomodulation is exemplified here.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nickerson, P.; Jeffery, J.; Gough, J.; Grimm, P.; McKenna, R.; Birk, P.; Rush, D. Effect of increasing baseline immunosuppression on the prevalence of clinical and subclinical rejection: A pilot study. J. Am. Soc. Nephrol. 1999, 10, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Evaluation and Treatment of Acute Rejection in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2020, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Schinstock, C.A.; Sapir-Pichhadze, R.; Naesens, M.; Batal, I.; Bagnasco, S.; Bow, L.; Campbell, P.; Cooper, M.; Cozzi, E.; Kraus, E.; et al. Banff Survey on Antibody-Mediated Rejection Clinical Practices in Kidney Transplantation: Diagnostic Misinterpretation Has Potential Therapeutic Implications. Am. J. Transplant. 2018, 19, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Mengel, M.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; et al. The Banff 2015 Kidney Meeting Report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Sis, B.; Jhangri, G.S.; Bunnag, S.; Allanach, K.; Kaplan, B.; Halloran, P.F. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am. J. Transplant. 2009, 9, 2312–2323. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.; Coresh, J.; Balk, E.; Kausz, A.; Levin, A.; Steffes, M.; Eknoyan, G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137. [Google Scholar] [CrossRef] [PubMed]
- Keane, W.F.; Eknoyan, G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am. J. Kidney Dis. 1999, 33, 1004–1010. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Mengel, M.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Haas, M. Donor Kidney Biopsies: Pathology Matters, and so Does the Pathologist. Kidney Int. 2014, 85, 1016–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Y.; Sarwal, R.D.; Sigdel, T.K.; Damm, I.; Rosenbaum, B.; Liberto, J.M.; Sarwal, M.M.; Chan-on, C.; Arreola-guerra, J.M.; Alberu, J.; et al. A Urine Score for Noninvasive Accurate Diagnosis and Prediction of Kidney Transplant Rejection. Sci. Transl. Med. 2020, 12, 535. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Becker, J.U.; Loupy, A.; Mengel, M.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Stegall, M.D.; Gaston, R.S.; Cosio, F.G.; Matas, A. Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J. Am. Soc. Nephrol. 2015, 26, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Sarwal, M.M.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J. Clin. Med. 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Brennan, D.C.; Matas, A.J.; Mehta, S.; Mannon, R.B.; et al. Cell-free DNA and active rejection in kidney allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Rychkov, D.; Sur, S.; Sirota, M.; Sarwal, M.M. Molecular Diversity of Clinically Stable Human Kidney Allografts. JAMA Netw. Open 2021, 4, e2035048. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwal, R.D.; Yazar, W.; Titzler, N.; Wong, J.; Lai, C.-h.; Chin, C.; Krieger, D.; Stoll, J.; Dias Lourenco, F.; Sarwal, M.M.; et al. Through the Looking Glass: Unraveling the Stage-Shift of Acute Rejection in Renal Allografts. J. Clin. Med. 2022, 11, 910. https://doi.org/10.3390/jcm11040910
Sarwal RD, Yazar W, Titzler N, Wong J, Lai C-h, Chin C, Krieger D, Stoll J, Dias Lourenco F, Sarwal MM, et al. Through the Looking Glass: Unraveling the Stage-Shift of Acute Rejection in Renal Allografts. Journal of Clinical Medicine. 2022; 11(4):910. https://doi.org/10.3390/jcm11040910
Chicago/Turabian StyleSarwal, Reuben D., Wanzin Yazar, Nicholas Titzler, Jeremy Wong, Chih-hung Lai, Christopher Chin, Danielle Krieger, Jeff Stoll, Francisco Dias Lourenco, Minnie M. Sarwal, and et al. 2022. "Through the Looking Glass: Unraveling the Stage-Shift of Acute Rejection in Renal Allografts" Journal of Clinical Medicine 11, no. 4: 910. https://doi.org/10.3390/jcm11040910
APA StyleSarwal, R. D., Yazar, W., Titzler, N., Wong, J., Lai, C.-h., Chin, C., Krieger, D., Stoll, J., Dias Lourenco, F., Sarwal, M. M., & Ghosh, S. (2022). Through the Looking Glass: Unraveling the Stage-Shift of Acute Rejection in Renal Allografts. Journal of Clinical Medicine, 11(4), 910. https://doi.org/10.3390/jcm11040910





