Diurnal Variability of Platelet Aggregation in Patients with Myocardial Infarction Treated with Prasugrel and Ticagrelor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Endpoints
2.3. Blood Collection
2.4. Assessment of Platelet Function
2.5. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Baseline Characteristics
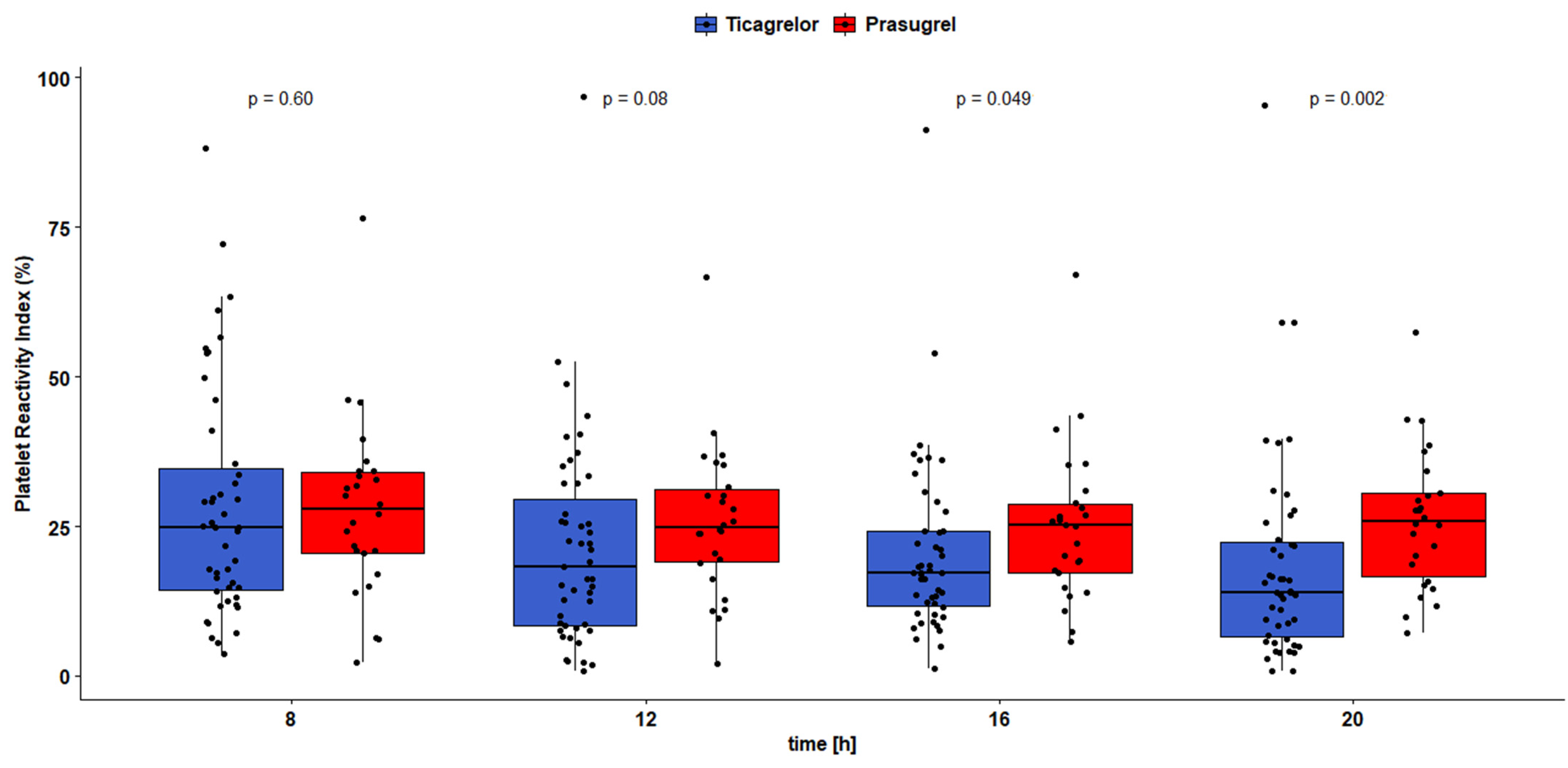
3.2. Pharmacodynamic Outcomes
3.3. Adverse Events
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Medscape. Available online: https://emedicine.medscape.com/article/155919-overview (accessed on 7 January 2022).
- Eurostat. Available online: https://ec.europa.eu/eurostat/ (accessed on 7 January 2022).
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Schüpke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef]
- Dawwas, G.K.; Dietrich, E.; Winchester, D.E.; Winterstein, A.G.; Segal, R.; Park, H. Comparative Effectiveness and Safety of Ticagrelor versus Prasugrel in Patients with Acute Coronary Syndrome: A Retrospective Cohort Analysis. Pharmacotherapy 2019, 39, 912–920. [Google Scholar] [CrossRef]
- Welsh, R.C.; Sidhu, R.S.; Cairns, J.A.; Lavi, S.; Kedev, S.; Moreno, R.; Cantor, W.J.; Stankovic, G.; Meeks, B.; Yuan, F.; et al. Outcomes Among Clopidogrel, Prasugrel, and Ticagrelor in ST-Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention From the Total Trial. Can. J. Cardiol. 2019, 35, 1377–1385. [Google Scholar] [CrossRef]
- Khan, M.S.; Memon, M.M.; Usman, M.S.; Alnaimat, S.; Khan, S.U.; Khan, A.R.; Yamani, N.; Fugar, S.; Mookadam, F.; Krasuski, R.A.; et al. Prasugrel vs. Ticagrelor for Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Am. J. Cardiovasc. Drugs 2019, 19, 465–476. [Google Scholar] [CrossRef]
- Kubica, J.; Jaguszewski, M. ISAR-REACT 5—What have we learned? Cardiol. J. 2019, 26, 427–428. [Google Scholar] [CrossRef] [Green Version]
- Venetsanos, D.; Träff, E.; Erlinge, D.; Hagström, E.; Nilsson, J.; Desta, L.; Lindahl, B.; Mellbin, L.; Omerovic, E.; Szummer, K.E.; et al. Prasugrel versus ticagrelor in patients with myocardial infarction undergoing percutaneous coronary intervention. Heart 2021, 107, 1145–1151. [Google Scholar] [CrossRef]
- Parodi, G.; Valenti, R.; Bellandi, B.; Migliorini, A.; Marcucci, R.; Comito, V.; Carrabba, N.; Santini, A.; Gensini, G.F.; Abbate, R.; et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J. Am. Coll. Cardiol. 2013, 61, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Michelson, A.D.; Frelinger, A.L., 3rd; Braunwald, E.; Downey, W.E.; Angiolillo, D.J.; Xenopoulos, N.P.; Jakubowski, J.A.; Li, Y.; Murphy, S.A.; Qin, J.; et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur. Heart J. 2009, 30, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Angiolillo, D.J.; Patil, S.B.; Desai, B.; Ecob, R.; Husted, S.; Emanuelsson, H.; Cannon, C.P.; Becker, R.C.; Wallentin, L. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: The PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J. Am. Coll. Cardiol. 2010, 56, 1456–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamski, P.; Koziński, M.; Ostrowska, M.; Fabiszak, T.; Navarese, E.P.; Paciorek, P.; Grześk, G.; Kubica, J. Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb. Haemost. 2014, 112, 224–242. [Google Scholar] [CrossRef] [Green Version]
- Niitsu, Y.; Jakubowski, J.A.; Sugidachi, A.; Asai, F. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin. Thromb. Hemost. 2005, 31, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Algaier, I.; Jakubowski, J.A.; Asai, F.; von Kügelgen, I. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J. Thromb. Haemost. 2008, 6, 1908–1914. [Google Scholar] [CrossRef]
- Coons, J.C.; Schwier, N.; Harris, J.; Seybert, A.L. Pharmacokinetic evaluation of prasugrel for the treatment of myocardial infarction. Expert Opin. Drug Metab. Toxicol. 2014, 10, 609–620. [Google Scholar] [CrossRef]
- Teng, R. Ticagrelor: Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Profile: An Update. Clin. Pharmacokinet. 2015, 54, 1125–1138. [Google Scholar] [CrossRef] [Green Version]
- Husted, S.; Emanuelsson, H.; Heptinstall, S.; Sandset, P.M.; Wickens, M.; Peters, G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: A double-blind comparison to clopidogrel with aspirin. Eur. Heart J. 2006, 27, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- VAN Giezen, J.J.; Nilsson, L.; Berntsson, P.; Wissing, B.M.; Giordanetto, F.; Tomlinson, W.; Greasley, P.J. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J. Thromb. Haemost. 2009, 7, 1556–1565. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. Recent advances in circadian rhythms in cardiovascular system. Front. Pharmacol. 2015, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozinski, M.; Bielis, L.; Wisniewska-Szmyt, J.; Boinska, J.; Stolarek, W.; Marciniak, A.; Kubica, A.; Grabczewska, Z.; Navarese, E.P.; Andreotti, F.; et al. Diurnal variation in platelet inhibition by clopidogrel. Platelets 2011, 22, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.; Guenat, F.; Fournier, A.; Alberio, L.; Bonny, O.; Bertaggia Calderara, D.; Bardy, D.; Lauriers, N.; Harbaoui, B.; Monney, P.; et al. Circadian variation of ticagrelor-induced platelet inhibition in healthy adulty. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Mogabgab, O.; Wiviott, S.D.; Cannon, C.P.; Sloan, S.; Sabatine, M.S.; Antman, E.M.; Braunwald, E.; Giugliano, R.P. Circadian variation of stent thrombosis and the effect of more robust platelet inhibition: A post hoc analysis of the TRITON-TIMI 38 trial. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 555–559. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Adamski, P.; Buszko, K.; Sikora, J.; Niezgoda, P.; Fabiszak, T.; Ostrowska, M.; Barańska, M.; Karczmarska-Wódzka, A.; Navarese, E.P.; Kubica, J. Determinants of high platelet reactivity in patients with acute coronary syndromes treated with ticagrelor. Sci. Rep. 2019, 9, 3924. [Google Scholar] [CrossRef]
- Adamski, P.; Sikora, J.; Laskowska, E.; Buszko, K.; Ostrowska, M.; Umińska, J.M.; Sikora, A.; Skibińska, N.; Sobczak, P.; Adamska, U.; et al. Comparison of bioavailability and antiplatelet action of ticagrelor in patients with ST-elevation myocardial infarction and non-ST-elevation myocardial infarction: A prospective, observational, single-centre study. PLoS ONE 2017, 12, e0186013. [Google Scholar] [CrossRef] [Green Version]
- Kubica, J.; Adamski, P.; Buszko, K.; Barańska, M.; Sikora, J.; Marszałł, M.P.; Sobczak, P.; Sikora, A.; Kuliczkowski, W.; Fabiszak, T.; et al. Platelet inhibition with standard vs. lower maintenance dose of ticagrelor early after myocardial infarction (ELECTRA): A randomized, open-label, active-controlled pharmacodynamic and pharmacokinetic study. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 139–148. [Google Scholar] [CrossRef]
- Fox, K.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A., Jr.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziński, M.; Obońska, K.; Stankowska, K.; Navarese, E.P.; Fabiszak, T.; Stolarek, W.; Kasprzak, M.; Siller-Matula, J.M.; Rość, D.; Kubica, J.; et al. Prasugrel overcomes high on-clopidogrel platelet reactivity in the acute phase of acute coronary syndrome and maintains its antiplatelet potency at 30-day follow-up. Cardiol. J. 2014, 21, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Kubica, A.; Kasprzak, M.; Siller-Matula, J.; Koziński, M.; Navarese, E.P.; Obońska, K.; Andruszkiewicz, A.; Sztuba, B.; Fabiszak, T.; Swiątkiewicz, I.; et al. Time-related changes in determinants of antiplatelet effect of clopidogrel in patients after myocardial infarction. Eur. J. Pharmacol. 2014, 742, 47–54. [Google Scholar] [CrossRef]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [Green Version]
- Rehmel, J.L.; Eckstein, J.A.; Farid, N.A.; Heim, J.B.; Kasper, S.C.; Kurihara, A.; Wrighton, S.A.; Ring, B.J. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab. Dispos. 2006, 34, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Huang, M.S.; Chao, T.H.; Lin, S.H.; Li, Y.H. Reduced-Dose Prasugrel versus Clopidogrel for Patients Undergoing Percutaneous Coronary Intervention. Int. Heart J. 2021, 62, 246–255. [Google Scholar] [CrossRef]
- Teng, R.; Oliver, S.; Hayes, M.A.; Butler, K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab. Dispos. 2010, 38, 1514–1521. [Google Scholar] [CrossRef]
- Adamski, P.; Ostrowska, M.; Navarese, E.P.; Kubica, J. Pharmacodynamic and clinical efficacy of reduced ticagrelor maintenance doses in patients with coronary artery disease. Curr. Med. Res. Opin. 2021, 37, 195–206. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Gorog, D.A.; Kubica, A.; Jilma, B.; Budaj, A.; Siller-Matula, J.M.; Gurbel, P.A.; Alexopoulos, D.; Badarienė, J.; et al. Low-dose ticagrelor with or without acetylsalicylic acid in patients with acute coronary syndrome: Rationale and design of the ELECTRA-SIRIO 2 trial. Cardiol. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bednar, F.; Kroupa, J.; Ondrakova, M.; Osmancik, P.; Kopa, M.; Motovska, Z. Antiplatelet efficacy of P2Y12 inhibitors (prasugrel, ticagrelor, clopidogrel) in patients treated with mild therapeutic hypothermia after cardiac arrest due to acute myocardial infarction. J. Thromb. Thrombolysis 2016, 41, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kerneis, M.; Silvain, J.; Abtan, J.; Hauguel, M.; Barthélémy, O.; Payot, L.; Brugier, D.; Galier, S.; Collet, J.P.; Montalescot, G. Platelet effect of prasugrel and ticagrelor in patients with ST-segment elevation myocardial infarction. Arch. Cardiovasc. Dis. 2015, 108, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freynhofer, M.K.; Hein-Rothweiler, R.; Haller, P.M.; Aradi, D.; Dézsi, D.A.; Gross, L.; Orban, M.; Trenk, D.; Geisler, T.; Huczek, Z.; et al. Diurnal Variability of On-Treatment Platelet Reactivity in Clopidogrel versus Prasugrel Treated Acute Coronary Syndrome Patients: A Pre-Specified TROPICAL-ACS Sub-Study. Thromb. Haemost. 2019, 119, 660–667. [Google Scholar] [CrossRef]
- Verdoia, M.; Rolla, R.; Pergolini, P.; Gioscia, R.; Nardin, M.; Negro, F.; Viglione, F.; Suryapranata, H.; Kedhi, E.; De Luca, G. Low hemoglobin predicts high-platelet reactivity and major cardiovascular ischemic events at long-term follow-up among ACS patients receiving dual antiplatelet therapy with ticagrelor. Catheter Cardiovasc. Interv. 2021, 98, 1309–1316. [Google Scholar] [CrossRef]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Barbieri, L.; Schaffer, A.; Bellomo, G.; Marino, P.; Suryapranata, H.; De Luca, G. Mean platelet volume and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Expert Opin. Pharmacother. 2015, 16, 1739–1747. [Google Scholar] [CrossRef]
- Chang, H.Y.; Hsu, L.W.; Lee, C.H.; Lin, C.C.; Huang, C.W.; Chen, P.W.; Yang, P.K.; Hsueh, Y.C.; Liu, P.Y. Impact of Platelet Volume on the Clinical Outcomes of Patients with Acute Coronary Syndrome. Acta Cardiol. Sin. 2019, 35, 563–570. [Google Scholar] [CrossRef]


| Variable | Ticagrelor (n = 47) | Prasugrel (n = 26) | p Value |
|---|---|---|---|
| Age, years | 59 [51–63] | 58 [48–63] | 0.95 |
| Female | 16 (34%) | 4 (15%) | 0.15 |
| Body mass index, kg/m2 | 27.9 [25.5–30.1] | 27.8 [24.9–29.4] | 0.91 |
| STEMI | 37 (79%) | 21 (81%) | 0.92 |
| Hypertension | 30 (64%) | 16 (62%) | 0.95 |
| Diabetes mellitus | 9 (19%) | 3 (12%) | 0.31 |
| Hyperlipidemia | 44 (94%) | 20 (77%) | 0.06 |
| Current smoker | 29 (62%) | 20 (77%) | 0.28 |
| Prior CAD | 6 (13%) | 7 (27%) | 0.23 |
| Prior AMI | 5 (11%) | 4 (15%) | 0.72 |
| Prior PCI | 5 (11%) | 4 (15%) | 0.72 |
| Prior CABG | 1 (2%) | 0 | n/a |
| Peripheral arterial disease | 3 (6%) | 1 (4%) | 0.55 |
| Prior heart failure | 1 (2%) | 2 (8%) | 0.29 |
| COPD | 3 (6%) | 0 | n/a |
| Chronic renal disease | 1 (2%) | 2 (8%) | 0.29 |
| Gout | 0 | 2 (8%) | 0.12 |
| LVEF at discharge, % | 45 [40–50] | 47 [38–50] | 0.69 |
| Creatinine, mg/dL | 0.83 [0.74–1.02] | 0.84 [0.77–1.02] | 0.73 |
| GFR, mL/min | 86 [72–97] | 74 [60–95] | 0.73 |
| CRP, mg/L | 8.6 [3.8–24.0] | 10.2 [4.0–20.3] | 0.59 |
| BNP, pg/mL | 120 [72–185] | 107 [79–234] | 0.93 |
| Uric acid, mg/dL | 5.9 [4.8–6.4] | 5.7 [5.1–6.4] | 0.67 |
| Hemoglobin, g/dL | 14.9 [13.8–15.5] | 14.9 [14.4–15.9] | 0.63 |
| RBC, 1012/L | 4.8 [4.8–5.1] | 4.8 [4.5–5.3] | 0.52 |
| HCT, % | 44 [41–46] | 44 [41–47] | 0.66 |
| WBC, 109/L | 10.5 [8.6–13.4] | 10.6 [7.7–13.3] | 0.60 |
| PLT, 109/L | 239 [203–279] | 256 [214–301] | 0.32 |
| MPV, fL | 10.9 [10.2–11.4] | 10.7 [9.7–11.3] | 0.29 |
| Sampling Time Point (Hour) | VASP | Multiplate | ||||
|---|---|---|---|---|---|---|
| Ticagrelor (n = 47) | Prasugrel (n = 26) | p Value | Ticagrelor (n = 47) | Prasugrel (n = 26) | p Value | |
| 08:00 | 8 (17.0%) | 1 (3.9%) | 0.20 | 5 (10.6%) | 2 (7.7%) | 0.52 |
| 12:00 | 2 (4.3%) | 1 (3.9%) | 0.71 | 4 (8.5%) | 3 (11.5%) | 0.69 |
| 16:00 | 2 (4.3%) | 1 (3.9%) | 0.71 | 3 (6.4%) | 2 (7.7%) | 0.59 |
| 20:00 | 3 (6.4%) | 1 (3.9%) | 0.55 | 10 (21.3%) | 4 (15.4%) | 0.39 |
| Clinical Variable | VASP | Multiplate | ||||||
|---|---|---|---|---|---|---|---|---|
| Value | SE | p Value | R2 | Value | SE | p Value | R2 | |
| Ticagrelor vs. prasugrel | 28.23 | 5.06 | <0.00001 | 0.31 | 0.07 | 0.05 | 0.12 | 0.04 |
| Age, years | 0.20 | 0.30 | 0.50 | <0.01 | 0.08 | 0.18 | 0.66 | <0.01 |
| Female | 9.31 | 6.42 | 0.15 | 0.03 | −4.37 | 3.96 | 0.27 | 0.02 |
| Body mass index, kg/m2 | −0.37 | 0.67 | 0.58 | <0.01 | −0.36 | 0.41 | 0.38 | 0.01 |
| Obesity | 1.05 | 6.74 | 0.88 | <0.01 | −6.57 | 4.05 | 0.11 | 0.04 |
| STEMI vs. NSTEMI | −5.94 | 7.16 | 0.41 | <0.01 | 2.97 | 4.39 | 0.50 | <0.01 |
| Hypertension | 3.93 | 6.00 | 0.51 | <0.01 | −0.94 | 3.68 | 0.80 | <0.01 |
| Diabetes mellitus | 10.62 | 7.74 | 0.17 | 0.03 | 1.23 | 4.80 | 0.80 | <0.01 |
| Hyperlipidemia | 15.02 | 8.66 | 0.09 | 0.04 | 1.68 | 5.41 | 0.76 | <0.01 |
| Current smoker | −5.95 | 6.15 | 0.34 | 0.01 | −0.38 | 3.79 | 0.92 | <0.01 |
| Prior CAD | −1.90 | 7.59 | 0.80 | <0.01 | −0.66 | 4.65 | 0.89 | <0.01 |
| Prior PCI | 2.57 | 8.83 | 0.77 | <0.01 | 6.17 | 5.36 | 0.25 | 0.02 |
| LVEF at discharge, % | 0.14 | 0.42 | 0.74 | <0.01 | 0.16 | 0.26 | 0.55 | <0.01 |
| Creatinine, mg/dL | −0.05 | 13.25 | 0.99 | <0.01 | 2.15 | 8.11 | 0.79 | <0.01 |
| GFR, mL/min | −0.02 | 0.15 | 0.87 | <0.01 | 0.06 | 0.09 | 0.48 | <0.01 |
| BNP, pg/mL | −0.01 | 0.01 | 0.66 | <0.01 | <0.01 | 0.01 | 0.77 | <0.01 |
| Uric acid, mg/dL | 0.54 | 2.11 | 0.80 | <0.01 | −0.93 | 1.28 | 0.47 | <0.01 |
| Hemoglobin, g/dL | −1.16 | 2.41 | 0.63 | <0.01 | −3.19 | 1.43 | 0.028 | 0.06 |
| RBC, 1012/L | −2.06 | 6.39 | 0.75 | <0.01 | −5.09 | 3.87 | 0.19 | 0.02 |
| HCT, % | −0.67 | 0.88 | 0.45 | <0.01 | −1.06 | 0.53 | 0.047 | 0.06 |
| WBC, 109/L | 0.32 | 0.87 | 0.71 | <0.01 | −0.01 | 0.53 | 0.99 | <0.01 |
| PLT, 109/L | −0.06 | 0.05 | 0.27 | 0.02 | <0.01 | 0.03 | 0.96 | <0.01 |
| MPV, fL | 0.28 | 0.40 | 0.48 | <0.01 | −0.52 | 0.24 | 0.03 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamski, P.; Barańska, M.; Ostrowska, M.; Kuliczkowski, W.; Buszko, K.; Kościelska-Kasprzak, K.; Karolko, B.; Mysiak, A.; Kubica, J. Diurnal Variability of Platelet Aggregation in Patients with Myocardial Infarction Treated with Prasugrel and Ticagrelor. J. Clin. Med. 2022, 11, 1124. https://doi.org/10.3390/jcm11041124
Adamski P, Barańska M, Ostrowska M, Kuliczkowski W, Buszko K, Kościelska-Kasprzak K, Karolko B, Mysiak A, Kubica J. Diurnal Variability of Platelet Aggregation in Patients with Myocardial Infarction Treated with Prasugrel and Ticagrelor. Journal of Clinical Medicine. 2022; 11(4):1124. https://doi.org/10.3390/jcm11041124
Chicago/Turabian StyleAdamski, Piotr, Malwina Barańska, Małgorzata Ostrowska, Wiktor Kuliczkowski, Katarzyna Buszko, Katarzyna Kościelska-Kasprzak, Bożena Karolko, Andrzej Mysiak, and Jacek Kubica. 2022. "Diurnal Variability of Platelet Aggregation in Patients with Myocardial Infarction Treated with Prasugrel and Ticagrelor" Journal of Clinical Medicine 11, no. 4: 1124. https://doi.org/10.3390/jcm11041124
APA StyleAdamski, P., Barańska, M., Ostrowska, M., Kuliczkowski, W., Buszko, K., Kościelska-Kasprzak, K., Karolko, B., Mysiak, A., & Kubica, J. (2022). Diurnal Variability of Platelet Aggregation in Patients with Myocardial Infarction Treated with Prasugrel and Ticagrelor. Journal of Clinical Medicine, 11(4), 1124. https://doi.org/10.3390/jcm11041124






