Sexual Differences in Internet Gaming Disorder (IGD): From Psychological Features to Neuroanatomical Networks
Abstract
1. Introduction
1.1. Diagnostic Criteria
1.2. Epidemiology
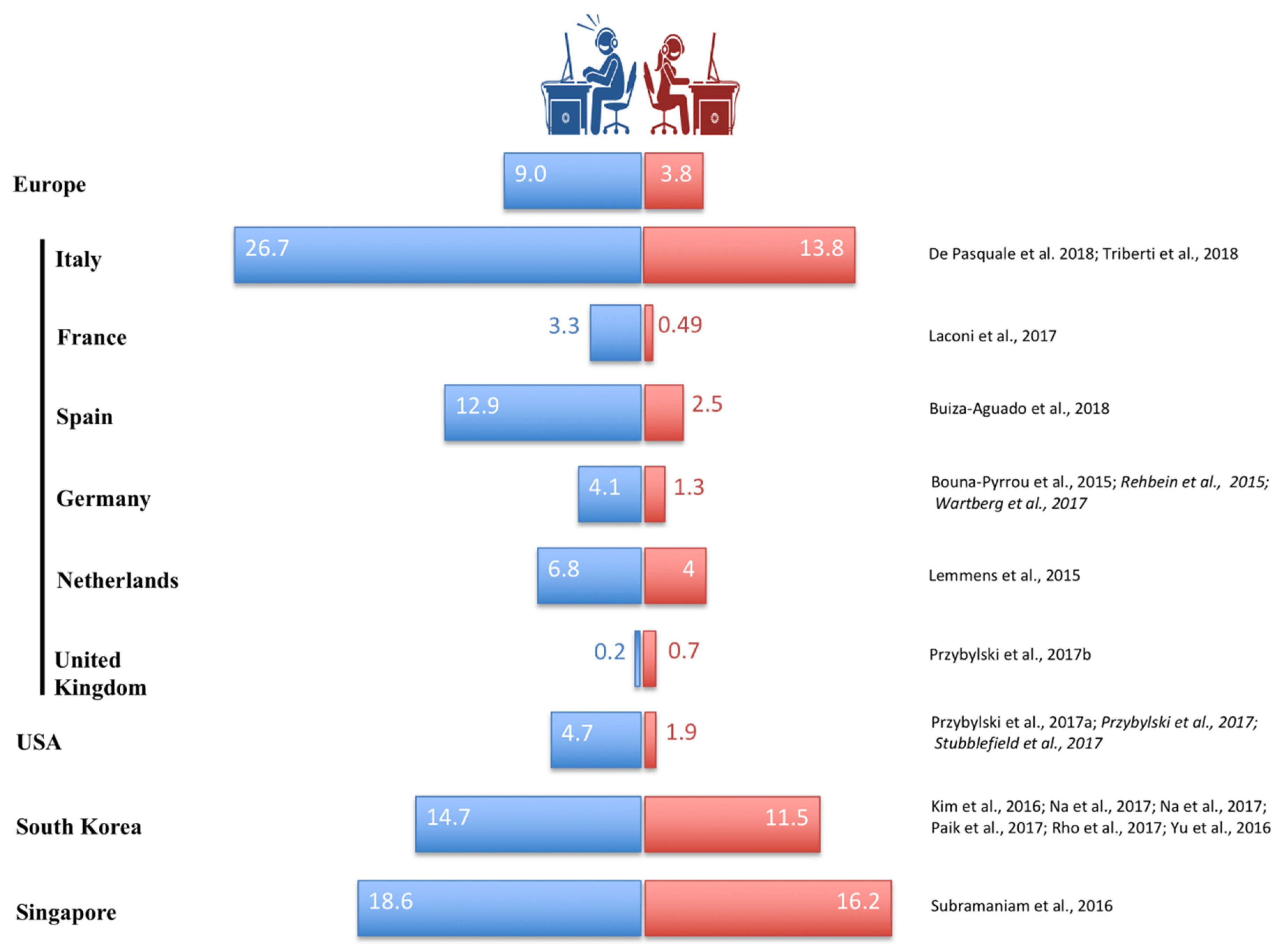
1.3. IGD and Cultural Factors
1.4. Historical Background
2. Objectives
3. Sexual Dimorphism in IGD
4. Psychological Factors and Personality Traits Sexually Dimorphic in IGD
4.1. Impulsivity and Poor Self-Control
4.2. Hostility and Social Phobia
4.3. Depression
4.4. Aggressive Behavior
5. Sexual Dimorphism in the Brain of IGD Gamers
5.1. The Prefrontal Region
5.2. Posterior Cingulate Cortex (PCC)
5.3. Brain Regions Involved in Visual Processing and Cognitive Control
5.4. Mesocorticolimbic Reward System
6. Correlation of Neural Sexual Dimorphisms between IGD and Substance Abuse
7. Limitations in IGD Human Studies
8. Beyond Human Study: The Animal Model for the Study of IGD
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- van den Brink, W. ICD-11 Gaming Disorder: Needed and just in time or dangerous and much too early? J. Behav. Addict. 2017, 6, 290–292. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- WHO. Gaming Disorder. 2018. Available online: http://www.who.int/features/qa/gaming-disorder/en/ (accessed on 14 December 2021).
- Jo, Y.S.; Bhang, S.Y.; Choi, J.S.; Lee, H.K.; Lee, S.Y.; Kweon, Y.S. Clinical Characteristics of Diagnosis for Internet Gaming Disorder: Comparison of DSM-5 IGD and ICD-11 GD Diagnosis. J. Clin. Med. 2019, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- King, D.L.; Chamberlain, S.R.; Carragher, N.; Billieux, J.; Stein, D.; Mueller, K.; Potenza, M.N.; Rumpf, H.J.; Saunders, J.; Starcevic, V.; et al. Screening and assessment tools for gaming disorder: A comprehensive systematic review. Clin. Psychol. Rev. 2020, 77, 101831. [Google Scholar] [CrossRef]
- Ko, C.H.; Lin, H.C.; Lin, P.C.; Yen, J.Y. Validity, functional impairment and complications related to Internet gaming disorder in the DSM-5 and gaming disorder in the ICD-11. Aust. N. Z. J. Psychiatry 2019, 54, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Sussman, C.J.; Harper, J.M.; Stahl, J.L.; Weigle, P. Internet and Video Game Addictions: Diagnosis, Epidemiology, and Neurobiology. Child Adolesc. Psychiatr. Clin. 2018, 27, 307–326. [Google Scholar] [CrossRef]
- Bouna-Pyrrou, P.; Aufleger, B.; Braun, S.; Gattnar, M.; Kallmayer, S.; Wagner, H.; Kornhuber, J.; Muhle, C.; Lenz, B. Cross-Sectional and Longitudinal Evaluation of the Social Network Use Disorder and Internet Gaming Disorder Criteria. Front. Psychiatry 2018, 9, 692. [Google Scholar] [CrossRef]
- Mihara, S.; Higuchi, S. Cross-sectional and longitudinal epidemiological studies of Internet gaming disorder: A systematic review of the literature. Psychiatry Clin. Neurosci. 2017, 71, 425–444. [Google Scholar] [CrossRef]
- Darvesh, N.; Radhakrishnan, A.; Lachance, C.C.; Nincic, V.; Sharpe, J.P.; Ghassemi, M.; Straus, S.E.; Tricco, A.C. Exploring the prevalence of gaming disorder and Internet gaming disorder: A rapid scoping review. Syst. Rev. 2020, 9, 68. [Google Scholar] [CrossRef]
- Pontes, H.M.; Griffiths, M.D. Measuring DSM-5 internet gaming disorder: Development and validation of a short psychometric scale. Comput. Hum. Behav. 2015, 45, 137–143. [Google Scholar] [CrossRef]
- Gonzalez-Bueso, V.; Santamaria, J.J.; Oliveras, I.; Fernandez, D.; Montero, E.; Bano, M.; Jimenez-Murcia, S.; Del Pino-Gutierrez, A.; Ribas, J. Internet Gaming Disorder Clustering Based on Personality Traits in Adolescents, and Its Relation with Comorbid Psychological Symptoms. Int. J. Environ. Res. Public Health 2020, 17, 1516. [Google Scholar] [CrossRef]
- van den Eijnden, R.; Koning, I.; Doornwaard, S.; van Gurp, F.; Ter Bogt, T. The impact of heavy and disordered use of games and social media on adolescents’ psychological, social, and school functioning. J. Behav. Addict. 2018, 7, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Sussman, S.; Arnett, J.J. Emerging Adulthood: Developmental Period Facilitative of the Addictions. Eval. Health Prof. 2014, 37, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, K.; Lee, H.W.; Hong, J.P.; Cho, M.J.; Fava, M.; Mischoulon, D.; Heo, J.Y.; Jeon, H.J. Internet Game Addiction, Depression, and Escape From Negative Emotions in Adulthood: A Nationwide Community Sample of Korea. J. Nerv. Ment. Dis. 2017, 205, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.L.M.; Stavropoulos, V.; Burleigh, T.L.; Liew, L.W.L.; Beard, C.L.; Griffiths, M.D. Internet Gaming Disorder Behaviors in Emergent Adulthood: A Pilot Study Examining the Interplay Between Anxiety and Family Cohesion. Int. J. Ment. Health Addict. 2019, 17, 828–844. [Google Scholar] [CrossRef]
- Burleigh, T.L.; Stavropoulos, V.; Liew, L.W.L.; Adams, B.L.M.; Griffiths, M.D. Depression, Internet Gaming Disorder, and the Moderating Effect of the Gamer-Avatar Relationship: An Exploratory Longitudinal Study. Int. J. Ment. Health Addict. 2018, 16, 102–124. [Google Scholar] [CrossRef]
- Dong, G.; Wang, L.; Du, X.; Potenza, M.N. Gender-related differences in neural responses to gaming cues before and after gaming: Implications for gender-specific vulnerabilities to Internet gaming disorder. Soc. Cogn. Affect. Neurosci. 2018, 13, 1203–1214. [Google Scholar] [CrossRef]
- Carli, V.; Durkee, T.; Wasserman, D.; Hadlaczky, G.; Despalins, R.; Kramarz, E.; Wasserman, C.; Sarchiapone, M.; Hoven, C.W.; Brunner, R.; et al. The association between pathological internet use and comorbid psychopathology: A systematic review. Psychopathology 2013, 46, 1–13. [Google Scholar] [CrossRef]
- Leonhardt, M.; Overa, S. Are There Differences in Video Gaming and Use of Social Media among Boys and Girls?—A Mixed Methods Approach. Int. J. Environ. Res. Public Health 2021, 18, 6085. [Google Scholar] [CrossRef]
- Snodgrass, J.G.; Zhao, W.; Lacy, M.G.; Zhang, S.; Tate, R. The cross-cultural expression of internet gaming distress in North America, Europe, and China. Addict. Behav. Rep. 2019, 9, 100146. [Google Scholar] [CrossRef]
- Wilsnack, R.W.; Vogeltanz, N.D.; Wilsnack, S.C.; Harris, T.R.; Ahlstrom, S.; Bondy, S.; Csemy, L.; Ferrence, R.; Ferris, J.; Fleming, J.; et al. Gender differences in alcohol consumption and adverse drinking consequences: Cross-cultural patterns. Addiction 2000, 95, 251–265. [Google Scholar] [CrossRef]
- Stavropoulos, V.; Anderson, E.E.; Beard, C.; Latifi, M.Q.; Kuss, D.; Griffiths, M. A preliminary cross-cultural study of Hikikomori and Internet Gaming Disorder: The moderating effects of game-playing time and living with parents. Addict. Behav. Rep. 2019, 9, 100137. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.P.; Couture, M.E.; Cooper, M.L.; Kuntsche, E.; O’Connor, R.M.; Stewart, S.H.; Team, D. Cross-cultural comparisons of drinking motives in 10 countries: Data from the DRINC project. Drug Alcohol. Rev. 2017, 36, 721–730. [Google Scholar] [CrossRef]
- Griffiths, M.D.; Hunt, N. Dependence on computer games by adolescents. Psychol. Rep. 1998, 82, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, V.; Baynes, K.L.; O’Farrel, D.L.; Gomez, R.; Mueller, A.; Yucel, M.; Griffiths, M. Inattention and Disordered Gaming: Does Culture Matter? Psychiatr. Q. 2020, 91, 333–348. [Google Scholar] [CrossRef] [PubMed]
- OReilly, M. Internet addiction: A new disorder enters the medical lexicon. CMAJ 1996, 154, 1882–1883. [Google Scholar]
- Bonnaire, C.; Baptista, D. Internet gaming disorder in male and female young adults: The role of alexithymia, depression, anxiety and gaming type. Psychiatry Res. 2019, 272, 521–530. [Google Scholar] [CrossRef]
- Hartmann, T.; Klimmt, C. Gender and computer games: Exploring females’ dislikes. J. Comput.-Med. Comm. 2006, 11, 910–931. [Google Scholar] [CrossRef]
- Kuss, D.J.; Griffiths, M.D. Internet Gaming Addiction: A Systematic Review of Empirical Research. Int. J. Ment. Health Addict. 2012, 10, 278–296. [Google Scholar] [CrossRef]
- Kuss, D.J.; Griffiths, M.D. Social Networking Sites and Addiction: Ten Lessons Learned. Int. J. Environ. Res. Public Health 2017, 14, 311. [Google Scholar] [CrossRef]
- Dufour, M.; Brunelle, N.; Tremblay, J.; Leclerc, D.; Cousineau, M.M.; Khazaal, Y.; Legare, A.A.; Rousseau, M.; Berbiche, D. Gender Difference in Internet Use and Internet Problems among Quebec High School Students. Can. J. Psychiatry 2016, 61, 663–668. [Google Scholar] [CrossRef]
- Fortson, B.L.; Scotti, J.R.; Chen, Y.C.; Malone, J.; Del Ben, K.S. Internet use, abuse, and dependence among students at a southeastern regional university. J. Am. Coll. Health 2007, 56, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ciarrochi, J.; Parker, P.; Sahdra, B.; Marshall, S.; Jackson, C.; Gloster, A.T.; Heaven, P. The development of compulsive internet use and mental health: A four-year study of adolescence. Dev. Psychol. 2016, 52, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.A.; Krishnan-Sarin, S.; Cavallo, D.; Potenza, M.N. Video-gaming among high school students: Health correlates, gender differences, and problematic gaming. Pediatrics 2010, 126, e1414–e1424. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fernandez, O.; Williams, A.J.; Kuss, D.J. Measuring Female Gaming: Gamer Profile, Predictors, Prevalence, and Characteristics from Psychological and Gender Perspectives. Front. Psychol. 2019, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Royse, P.; Lee, J.; Undrahbuyan, B.; Hopson, M.; Consalvo, M. Women and games: Technologies of the gendered self. New Media Soc. 2007, 9, 555–576. [Google Scholar] [CrossRef]
- Shen, C.H.; Ratan, R.; Cai, Y.D.; Leavitt, A. Do Men Advance Faster Than Women? Debunking the Gender Performance Gap in Two Massively Multiplayer Online Games. J. Comput.-Med. Comm. 2016, 21, 312–329. [Google Scholar] [CrossRef]
- Rho, M.J.; Lee, H.; Lee, T.H.; Cho, H.; Jung, D.J.; Kim, D.J.; Choi, I.Y. Risk Factors for Internet Gaming Disorder: Psychological Factors and Internet Gaming Characteristics. Int. J. Environ. Res. Public Health 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Koh, Y.Y. Online social networking addiction among college students in Singapore: Comorbidity with behavioral addiction and affective disorder. Asian J. Psychiatry 2017, 25, 175–178. [Google Scholar] [CrossRef]
- Yen, J.Y.; Ko, C.H.; Yen, C.F.; Chen, S.H.; Chung, W.L.; Chen, C.C. Psychiatric symptoms in adolescents with Internet addiction: Comparison with substance use. Psychiatry Clin. Neurosci. 2008, 62, 9–16. [Google Scholar] [CrossRef]
- Ding, W.N.; Sun, J.H.; Sun, Y.W.; Chen, X.; Zhou, Y.; Zhuang, Z.G.; Li, L.; Zhang, Y.; Xu, J.R.; Du, Y.S. Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with internet gaming addiction revealed by a Go/No-Go fMRI study. Behav. Brain Funct. 2014, 10, 20. [Google Scholar] [CrossRef]
- Rikkers, W.; Lawrence, D.; Hafekost, J.; Zubrick, S.R. Internet use and electronic gaming by children and adolescents with emotional and behavioural problems in Australia—Results from the second Child and Adolescent Survey of Mental Health and Wellbeing. BMC Public Health 2016, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.Y.; Liu, T.L.; Wang, P.W.; Chen, C.S.; Yen, C.F.; Ko, C.H. Association between Internet gaming disorder and adult attention deficit and hyperactivity disorder and their correlates: Impulsivity and hostility. Addict. Behav. 2017, 64, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Loton, D.; Borkoles, E.; Lubman, D.; Polman, R. Video Game Addiction, Engagement and Symptoms of Stress, Depression and Anxiety: The Mediating Role of Coping. Int. J. Ment. Health Addict. 2016, 14, 565–578. [Google Scholar] [CrossRef]
- Sroufe, L.A. Psychopathology as an outcome of development. Dev. Psychopathol. 1997, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S. Emotion Regulation and Psychopathology: The Role of Gender. Annu. Rev. Clin. Psychol. 2012, 8, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J.; Coulson, M.; Barnett, J. A meta-analysis of pathological gaming prevalence and comorbidity with mental health, academic and social problems. J. Psychiatr. Res. 2011, 45, 1573–1578. [Google Scholar] [CrossRef]
- Lemmens, J.S.; Valkenburg, P.M.; Peter, J. The effects of pathological gaming on aggressive behavior. J. Youth Adolesc. 2011, 40, 38–47. [Google Scholar] [CrossRef]
- Vadlin, S.; Aslund, C.; Hellstrom, C.; Nilsson, K.W. Associations between problematic gaming and psychiatric symptoms among adolescents in two samples. Addict. Behav. 2016, 61, 8–15. [Google Scholar] [CrossRef]
- Stavropoulos, V.; Adams, B.L.M.; Beard, C.L.; Dumble, E.; Trawley, S.; Gomez, R.; Pontes, H.M. Associations between attention deficit hyperactivity and internet gaming disorder symptoms: Is there consistency across types of symptoms, gender and countries? Addict. Behav. Rep. 2019, 9, 100158. [Google Scholar] [CrossRef]
- Hyun, G.J.; Han, D.H.; Lee, Y.S.; Kang, K.D.; Yoo, S.K.; Chung, U.-S.; Renshaw, P.F. Risk factors associated with online game addiction: A hierarchical model. Comput. Hum. Behav. 2015, 48, 706–713. [Google Scholar] [CrossRef]
- Weinstein, A.; Weizman, A. Emerging association between addictive gaming and attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 2012, 14, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.Y.; Yen, C.F.; Chen, C.S.; Tang, T.C.; Ko, C.H. The association between adult ADHD symptoms and internet addiction among college students: The gender difference. Cyberpsychol. Behav. 2009, 12, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, V.; Kuss, D.J.; Griffiths, M.D.; Wilson, P.; Motti-Stefanidi, F. MMORPG gaming and hostility predict Internet Addiction symptoms in adolescents: An empirical multilevel longitudinal study. Addict. Behav. 2017, 64, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sioni, S.R.; Burleson, M.H.; Bekerian, D.A. Internet gaming disorder: Social phobia and identifying with your virtual self. Comput. Hum. Behav. 2017, 71, 11–15. [Google Scholar] [CrossRef]
- Kuss, D.J.; Lopez-Fernandez, O. Internet addiction and problematic Internet use: A systematic review of clinical research. World J. Psychiatry 2016, 6, 143–176. [Google Scholar] [CrossRef]
- Ko, C.H.; Yen, J.Y.; Yen, C.F.; Lin, H.C.; Yang, M.J. Factors predictive for incidence and remission of internet addiction in young adolescents: A prospective study. Cyberpsychol. Behav. 2007, 10, 545–551. [Google Scholar] [CrossRef]
- van den Eijnden, R.J.; Meerkerk, G.J.; Vermulst, A.A.; Spijkerman, R.; Engels, R.C. Online communication, compulsive Internet use, and psychosocial well-being among adolescents: A longitudinal study. Dev. Psychol. 2008, 44, 655–665. [Google Scholar] [CrossRef]
- Laconi, S.; Pires, S.; Chabrol, H. Internet gaming disorder, motives, game genres and psychopathology. Comput. Hum. Behav. 2017, 75, 652–659. [Google Scholar] [CrossRef]
- Wang, H.R.; Cho, H.; Kim, D.J. Prevalence and correlates of comorbid depression in a nonclinical online sample with DSM-5 internet gaming disorder. J. Affect. Disord. 2018, 226, 1–5. [Google Scholar] [CrossRef]
- Norton, A.R.; Abbott, M.J. Bridging the Gap between Aetiological and Maintaining Factors in Social Anxiety Disorder: The Impact of Socially Traumatic Experiences on Beliefs, Imagery and Symptomatology. Clin. Psychol. Psychother. 2017, 24, 747–765. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kircaburun, K.; Griffiths, M.D.; Billieux, J. Psychosocial factors mediating the relationship between childhood emotional trauma and internet gaming disorder: A pilot study. Eur. J. Psychotraumatol. 2019, 10, 1565031. [Google Scholar] [CrossRef] [PubMed]
- Finseras, T.R.; Pallesen, S.; Mentzoni, R.A.; Krossbakken, E.; King, D.L.; Molde, H. Evaluating an Internet Gaming Disorder Scale Using Mokken Scaling Analysis. Front. Psychol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, J.S.; Valkenburg, P.M.; Peter, J. Development and Validation of a Game Addiction Scale for Adolescents. Media Psychol. 2009, 12, 77–95. [Google Scholar] [CrossRef]
- Hauge, M.R.; Gentile, D.A. Video game addiction among adolescents: Associations with academic performance and aggression. In Proceedings of the 2003 Society for Research in Child Development Bien-Nial Conference, Tampa, FL, USA, 24–27 April 2003. [Google Scholar]
- Anderson, C.A.; Bushman, B.J. Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: A meta-analytic review of the scientific literature. Psychol. Sci. 2001, 12, 353–359. [Google Scholar] [CrossRef]
- Smith, S.L.; Lachlan, K.; Tamborini, R. Popular Video Games: Quantifying the Presentation of Violence and Its Context. J. Broadcasting Electron. Media 2003, 47, 58–76. [Google Scholar] [CrossRef]
- Anderson, C.A.; Dill, K.E. Video games and aggressive thoughts, feelings, and behavior in the laboratory and in life. J. Pers. Soc. Psychol. 2000, 78, 772–790. [Google Scholar] [CrossRef]
- Koepp, M.J.; Gunn, R.N.; Lawrence, A.D.; Cunningham, V.J.; Dagher, A.; Jones, T.; Brooks, D.J.; Bench, C.J.; Grasby, P.M. Evidence for striatal dopamine release during a video game. Nature 1998, 393, 266–268. [Google Scholar] [CrossRef]
- Li, W.W.; Li, Y.D.; Yang, W.J.; Zhang, Q.L.; Wei, D.T.; Li, W.F.; Hitchman, G.; Qiu, J. Brain structures and functional connectivity associated with individual differences in Internet tendency in healthy young adults. Neuropsychologia 2015, 70, 134–144. [Google Scholar] [CrossRef]
- Choi, J.; Cho, H.; Kim, J.Y.; Jung, D.J.; Ahn, K.J.; Kang, H.B.; Choi, J.S.; Chun, J.W.; Kim, D.J. Structural alterations in the prefrontal cortex mediate the relationship between Internet gaming disorder and depressed mood. Sci. Rep. 2017, 7, 1245. [Google Scholar] [CrossRef]
- Hoeft, F.; Watson, C.L.; Kesler, S.R.; Bettinger, K.E.; Reiss, A.L. Gender differences in the mesocorticolimbic system during computer game-play. J. Psychiatr. Res. 2008, 42, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, H.; Du, X.; Dong, G. Mapping Internet gaming disorder using effective connectivity: A spectral dynamic causal modeling study. Addict. Behav. 2019, 90, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wang, Z.; Wang, Y.; Du, X.; Potenza, M.N. Gender-related functional connectivity and craving during gaming and immediate abstinence during a mandatory break: Implications for development and progression of internet gaming disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 1–10. [Google Scholar] [CrossRef]
- Weinstein, A.; Livny, A.; Weizman, A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017, 75, 314–330. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, M.F.; Yen, J.Y.; Chen, C.S.; Liu, G.C.; Yen, C.F.; Ko, C.H. Brain correlates of response inhibition in Internet gaming disorder. Psychiatry Clin. Neurosci. 2015, 69, 201–209. [Google Scholar] [CrossRef]
- Wang, L.; Shen, H.; Lei, Y.; Zeng, L.L.; Cao, F.; Su, L.; Yang, Z.; Yao, S.; Hu, D. Altered default mode, fronto-parietal and salience networks in adolescents with Internet addiction. Addict. Behav. 2017, 70, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.P.; Osunde, I.; Li, X.; Zhou, S.K.; Zheng, H.R.; Li, L.J. Increased regional homogeneity in internet addiction disorder: A resting state functional magnetic resonance imaging study. Chin. Med. J. 2010, 123, 1904–1908. [Google Scholar]
- Weng, C.B.; Qian, R.B.; Fu, X.M.; Lin, B.; Han, X.P.; Niu, C.S.; Wang, Y.H. Gray matter and white matter abnormalities in online game addiction. Eur. J. Radiol. 2013, 82, 1308–1312. [Google Scholar] [CrossRef]
- Ding, W.N.; Sun, J.H.; Sun, Y.W.; Zhou, Y.; Li, L.; Xu, J.R.; Du, Y.S. Altered Default Network Resting-State Functional Connectivity in Adolescents with Internet Gaming Addiction. PLoS ONE 2013, 8, e59902. [Google Scholar] [CrossRef]
- Sun, Y.W.; Sun, J.H.; Zhou, Y.; Ding, W.N.; Chen, X.; Zhuang, Z.G.; Xu, J.R.; Du, Y.S. Assessment of in vivo microstructure alterations in gray matter using DKI in internet gaming addiction. Behav. Brain Funct. 2014, 10, 37. [Google Scholar] [CrossRef]
- Wang, H.M.; Jin, C.W.; Yuan, K.; Shakir, T.M.; Mao, C.P.; Niu, X.; Niu, C.; Guo, L.P.; Zhang, M. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Yuan, K.; Yin, J.S.; Feng, D.; Bi, Y.Z.; Li, Y.D.; Yu, D.H.; Jin, C.W.; Qin, W.; Tian, J. Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging Behav. 2016, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Qin, W.; Yu, D.H.; Bi, Y.Z.; Xing, L.H.; Jin, C.W.; Tian, J. Core brain networks interactions and cognitive control in internet gaming disorder individuals in late adolescence/early adulthood. Brain Struct. Funct. 2016, 221, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.H.; Zhou, S.K.; Zhang, L.; Wang, Z.Y.; Zhang, Y.; Jiang, Y.B.; Li, L.J. Functional characteristics of the brain in college students with internet gaming disorder. Brain Imaging Behav. 2016, 10, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Y.B.; Wang, Z.L.; Du, X.X.; Dong, G.H. Sex difference in the effect of Internet gaming disorder on the brain functions: Evidence from resting-state fMRI. Neurosci. Lett. 2019, 698, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, Y.; Zheng, H.; Yuan, K.; Du, X.; Dong, G. Females are more vulnerable to Internet gaming disorder than males: Evidence from cortical thickness abnormalities. Psychiatry Res. Neuroimaging 2019, 283, 145–153. [Google Scholar] [CrossRef]
- Ko, C.H.; Hsieh, T.J.; Chen, C.Y.; Yen, C.F.; Chen, C.S.; Yen, J.Y.; Wang, P.W.; Liu, G.C. Altered brain activation during response inhibition and error processing in subjects with Internet gaming disorder: A functional magnetic imaging study. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 661–672. [Google Scholar] [CrossRef]
- Dieter, J.; Hoffmann, S.; Mier, D.; Reinhar, I.; Beutel, M.; Vollstadt-Klein, S.; Kiefer, F.; Mann, K.; Lemenager, T. The role of emotional inhibitory control in specific internet addiction—An fMRI study. Behav. Brain. Res. 2017, 324, 1–14. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Jiang, W.Q.; Bao, X.C.; Sun, Y.W.; Ding, W.N.; Cao, M.Q.; Wu, X.W.; Du, Y.S.; Zhou, Y. Resting-State Activity of Prefrontal-Striatal Circuits in Internet Gaming Disorder: Changes with Cognitive Behavior Therapy and Predictors of Treatment Response. Front. Psychiatry 2018, 9, 341. [Google Scholar] [CrossRef]
- Yuan, K.; Qin, W.; Wang, G.H.; Zeng, F.; Zhao, L.Y.; Yang, X.J.; Liu, P.; Liu, J.X.; Sun, J.B.; von Deneen, K.M.; et al. Microstructure Abnormalities in Adolescents with Internet Addiction Disorder. PLoS ONE 2011, 6, e20708. [Google Scholar] [CrossRef]
- Dong, G.H.; Wu, L.D.; Wang, Z.L.; Wang, Y.F.; Du, X.X.; Potenza, M.N. Diffusion-weighted MRI measures suggest increased white-matter integrity in Internet gaming disorder: Evidence from the comparison with recreational Internet game users. Addict. Behav. 2018, 81, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.K.; Gwak, A.R.; Lim, J.A.; Lee, J.Y.; Jung, H.Y.; Sohn, B.K.; Choi, S.W.; Kim, D.J.; Choi, J.S. Resting-state regional homogeneity as a biological marker for patients with Internet gaming disorder: A comparison with patients with alcohol use disorder and healthy controls. Prog. Neuro-Psychopharmacol. 2015, 60, 104–111. [Google Scholar] [CrossRef]
- Sharaev, M.G.; Zavyalova, V.V.; Ushakov, V.L.; Kartashov, S.I.; Velichkovsky, B.M. Effective Connectivity within the Default Mode Network: Dynamic Causal Modeling of Resting-State fMRI Data. Front. Hum. Neurosci. 2016, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Hong, J.S.; Kim, S.M.; Han, D.H. Bupropion Shows Different Effects on Brain Functional Connectivity in Patients With Internet-Based Gambling Disorder and Internet Gaming Disorder. Front. Psychiatry 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Yao, Y.W.; Potenza, M.N.; Xia, C.C.; Lan, J.; Liu, L.; Wang, L.J.; Liu, B.; Ma, S.S.; Fang, X.Y. Altered resting-state neural activity and changes following a craving behavioral intervention for Internet gaming disorder. Sci. Rep. 2016, 6, 28109. [Google Scholar] [CrossRef]
- Uher, R.; Yoganathan, D.; Mogg, A.; Eranti, S.V.; Treasure, J.; Campbell, I.C.; McLoughlin, D.M.; Schmidt, U. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol. Psychiatry 2005, 58, 840–842. [Google Scholar] [CrossRef]
- Han, D.H.; Lyoo, I.K.; Renshaw, P.F. Differential regional gray matter volumes in patients with on-line game addiction and professional gamers. J. Psychiatr. Res. 2012, 46, 507–515. [Google Scholar] [CrossRef]
- Renier, L.A.; Anurova, I.; De Volder, A.G.; Carlson, S.; VanMeter, J.; Rauschecker, J.P. Preserved Functional Specialization for Spatial Processing in the Middle Occipital Gyrus of the Early Blind. Neuron 2010, 68, 138–148. [Google Scholar] [CrossRef]
- Kojima, H.; Suzuki, T. Hemodynamic change in occipital lobe during visual search: Visual attention allocation measured with NIRS. Neuropsychologia 2010, 48, 349–352. [Google Scholar] [CrossRef]
- Song, G.; Qiu, J.; Li, C.; Li, J.; Gui, S.; Zhu, H.; Zhang, Y. Alterations of regional homogeneity and functional connectivity in pituitary adenoma patients with visual impairment. Sci. Rep. 2017, 7, 13074. [Google Scholar] [CrossRef]
- Tam, E.W.; Widjaja, E.; Blaser, S.I.; Macgregor, D.L.; Satodia, P.; Moore, A.M. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics 2008, 122, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Bruck, C.; Kreifelts, B.; Ethofer, T.; Wildgruber, D. Reduced functional connectivity to the frontal cortex during processing of social cues in autism spectrum disorder. J. Neural Transm. 2016, 123, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.M.; Hasan, B.A.S.; Giordano, B.L.; Belin, P. Gender differences in the temporal voice areas. Front. Neurosci.-Switz. 2014, 8, 228. [Google Scholar] [CrossRef]
- Xu, C.S.; Li, C.F.; Wu, H.L.; Wu, Y.Y.; Hu, S.; Zhu, Y.F.; Zhang, W.; Wang, L.Y.; Zhu, S.H.; Liu, J.P.; et al. Gender Differences in Cerebral Regional Homogeneity of Adult Healthy Volunteers: A Resting-State fMRI Study. Biomed. Res. Int. 2015, 2015, 183074. [Google Scholar] [CrossRef]
- Sanchez Panchuelo, R.M.; Besle, J.; Schluppeck, D.; Humberstone, M.; Francis, S. Somatotopy in the Human Somatosensory System. Front. Hum. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Vergara, V.M.; Liu, J.Y.; Claus, E.D.; Hutchison, K.; Calhou, V. Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage 2017, 151, 45–54. [Google Scholar] [CrossRef]
- Wang, L.; Wu, L.; Wang, Y.; Li, H.; Liu, X.; Du, X.; Dong, G. Altered Brain Activities Associated with Craving and Cue Reactivity in People with Internet Gaming Disorder: Evidence from the Comparison with Recreational Internet Game Users. Front. Psychol. 2017, 8, 1150. [Google Scholar] [CrossRef]
- Matsuda, G.; Hiraki, K. Sustained decrease in oxygenated hemoglobin during video games in the dorsal prefrontal cortex: A NIRS study of children. Neuroimage 2006, 29, 706–711. [Google Scholar] [CrossRef]
- Cho, S.S.; Strafella, A.P. rTMS of the Left Dorsolateral Prefrontal Cortex Modulates Dopamine Release in the Ipsilateral Anterior Cingulate Cortex and Orbitofrontal Cortex. PLoS ONE 2009, 4, e6725. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F.; Baler, R. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 2010, 32, 748–755. [Google Scholar] [CrossRef]
- Spanagel, R.; Weiss, F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999, 22, 521–527. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Baler, R.; Telang, F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 2009, 56, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Granero, R.; Penelo, E.; Martinez-Gimenez, R.; Alvarez-Moya, E.; Gomez-Pena, M.; Aymami, M.N.; Bueno, B.; Fernandez-Aranda, F.; Jimenez-Murcia, S. Sex differences among treatment-seeking adult pathologic gamblers. Compr. Psychiatry 2009, 50, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Canterberry, M.; Peltier, M.R.; Brady, K.T.; Hanlon, C.A. Attenuated neural response to emotional cues in cocaine-dependence: A preliminary analysis of gender differences. Am. J. Drug Alcohol. Abus. 2016, 42, 577–586. [Google Scholar] [CrossRef]
- Hasler, B.P.; Casement, M.D.; Sitnick, S.L.; Shaw, D.S.; Forbes, E.E. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence 2 years later. Behav. Brain Res. 2017, 327, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kilts, C.D.; Gross, R.E.; Ely, T.D.; Drexler, K.P. The neural correlates of cue-induced craving in cocaine-dependent women. Am. J. Psychiatry 2004, 161, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, T.; Cai, C.; Bi, Y.; Li, Y.; Yu, D.; Zhang, M.; Yuan, K. Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging Behav. 2016, 10, 719–729. [Google Scholar] [CrossRef]
- Zanchi, D.; Brody, A.; Borgwardt, S.; Haller, S. Sex Effects on Smoking Cue Perception in Non-Smokers, Smokers, and Ex-Smokers: A Pilot Study. Front. Psychiatry 2016, 7, 187. [Google Scholar] [CrossRef][Green Version]
- Kogachi, S.; Chang, L.; Alicata, D.; Cunningham, E.; Ernst, T. Sex differences in impulsivity and brain morphometry in methamphetamine users. Brain Struct. Funct. 2017, 222, 215–227. [Google Scholar] [CrossRef]
- Tanabe, J.; York, P.; Krmpotich, T.; Miller, D.; Dalwani, M.; Sakai, J.T.; Mikulich-Gilbertson, S.K.; Thompson, L.; Claus, E.; Banich, M.; et al. Insula and orbitofrontal cortical morphology in substance dependence is modulated by sex. AJNR Am. J. Neuroradiol. 2013, 34, 1150–1156. [Google Scholar] [CrossRef]
- Petry, N.M.; Rehbein, F.; Gentile, D.A.; Lemmens, J.S.; Rumpf, H.J.; Mossle, T.; Bischof, G.; Tao, R.; Fung, D.S.; Borges, G.; et al. An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction 2014, 109, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Hunt, M.; Harper, D. An animal model of slot machine gambling: The effect of structural characteristics on response latency and persistence. J. Gambl. Stud. 2010, 26, 521–531. [Google Scholar] [CrossRef]
- Rokosik, S.L.; Napier, T.C. Intracranial self-stimulation as a positive reinforcer to study impulsivity in a probability discounting paradigm. J. Neurosci. Methods 2011, 198, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Tedford, S.E.; Holtz, N.A.; Persons, A.L.; Napier, T.C. A new approach to assess gambling-like behavior in laboratory rats: Using intracranial self-stimulation as a positive reinforcer. Front. Behav. Neurosci. 2014, 8, 215. [Google Scholar] [CrossRef]
- Horner, A.E.; Heath, C.J.; Hvoslef-Eide, M.; Kent, B.A.; Kim, C.H.; Nilsson, S.R.; Alsio, J.; Oomen, C.A.; Holmes, A.; Saksida, L.M.; et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat. Protoc. 2013, 8, 1961–1984. [Google Scholar] [CrossRef] [PubMed]
| DMS-5 | ICD-11 |
|---|---|
| 1. Preoccupation | 1. Impaired control Impairment—Personal Impairment—Social Impairment—Education Impairment—Work Impairment—Financial |
| 2. Withdrawal | |
| 3. Tolerance | |
| 4. Unsuccessful attempts | |
| 5. Loss of interests | |
| 6. Continued use | |
| 7. Deception | |
| 8. Escape | 2. Increasing priority |
| 9 Jeopardized life | 3. Continuation |
| Neuro Anatomical Region | Sample (IGD/ Ctrl) | M/F | Sex-by-Group-Analysis | Race/ Geographical Regions | Age | Methods | Observed Effects | References |
|---|---|---|---|---|---|---|---|---|
| Cerebellum, brainstem, rCG, bpHIPP, rFL, lSFG, left pre-cuneus, fPCG, rMOG, rITG, lSTG, MTG | 19/19 | 11/8 IGD 11/8 Ctrl | No | Chinese | n.c | fMRI to measure ReHo | ↑ ReHo | [80] |
| rOFCR, bilateral insula, rSMA, right genu of corpus callosum, bFL, rEC | 17/17 | 4/13 IGD 2/15 Ctrl | No | Chinese | 16.25 ± 3.0 15.54 ± 3.2 | MRI (VBMA and TBSS) to measure GM density and WM density changes | GM atrophy in the rOFC, bilateral insula, and rSMA ↓ FA in WM of right genu of corpus callosum, bFL rEC | [81] |
| Cerebellum, TG, iPL, iPG | 17 /24 | 13/4 IGD 16/8 Ctrl | No | n.c. | 16.94 ± 2.7 15.87 ± 2.7 | fMRI to detect FC | ↑ FC in the bilateral cerebellum posterior lobe and mTG ↓ FC in the biPL and riTG | [82] |
| Right anterolateral cerebellum, risTG, rSMA, MOG, right pre-cuneus, PCG, riFG, lLLG, lPCL, laCC, mCC, bFFG, insula, PCC, thalamus | 18/21 | 15/3 IGD 18/3 Ctrl | No | n.c. | 20.5 ± 3.5 21.95 ± 2.4 | MRI followed by DKI in the detection of GM diffusion | ↓ GM diffusion in all analyzed regions | [83] |
| Cerebellum, ACC, SMA, sPL, precuneus, insula, DLPFC, FG | 28/28 | 18/10 IGD 20/8 Ctrl | No | n.c. | 18.8 ± 1.3 19.3 ± 2.6 | fMRI to measure GM volume, FC and VMHC method | ↓ GM volume of the bilateral ACC, pre-cuneus, SMA, SPL, left DLPFC, left insula, and bilateral cerebellum ↓ VMHC between the left and right sFG (orbital part), iFG (orbital part), mFG and sFG | [84] |
| Striatal nuclei (caudate, putamen, and nucleus accumbens) | 27/30 | 23/4 IGD 22/8 Ctrl | No | n.c. | 17.9 ± 0.9 18.3 ± 1.6 | fMRI to detect FC and to measure volumes | ↑ volumes caudate and nucleus accumbens | [85] |
| ACC, DLPFC | 28/25 | 17/11 IGD 16/9 Ctrl | No | n.c. | 19.3 ± 2.1 19.7 ± 3.8 | fMRI to detect FC | ↓ FA in the salience network, right central executive network tracts, and between-network (the ACC-right DLPFC tracts) | [86] |
| sPL, precuneus, CG, sTG, brainstem | 19/19 | 11/8 IGD 11/8 Ctrl | No | n.c. | 21.4 ± 1.0 20.8 ± 1.1 | Task-state in fMRI | ↑ activation in the right SPL, right insular lobe, right precuneus, rCG, right STG, and left brainstem. | [87] |
| PCC, mPFC, iPL | 64/63 | 36/28 IGD 41/22 Ctrl | No | n.c. | 22.577 ± 2.2 23.085 ± 2.5 | Resting-state fMRI to detect connectivity | ↓ interactions between the left IPL-mPFC-PCC (↑inhibition) | [75] |
| rPCC, lMOG, rMTG, and rPCG | 46/58 | 23/23 IGD 29/29 Ctrl | Yes | n.c | 23.0 ± 1.1 22.7 ± 1.4 | fMRI to measure ReHo | ♂: ↓ ReHo in rPCC, ↑ ReHo in lMOG and MTG ♀: ↓ ReHo in lMOG and MTG | [88] |
| DLPFC, striatum, thalamus and insula | 54/65 | 29/25 IGD 34/31 Ctrl | Yes | n.c | 21.14 ± 2.4 21.17 ± 2.1 | fMRI to evaluate FC | ♂: ↓ FC between DLPFC and sFG ↑FC between striatum and thalamus/insula ♀: ↑FC between striatum and thalamus/insula | [76] |
| bRMFG, sFG, lSMG, rPCC, rsPL | 62/71 | 29/33 IGD 37/34 Ctrl | Yes | n.c. | 21.1 ± 1.4 20.7 ± 1.8 | Structural MRI to evaluate cortical thickness | ♂: ↑ bRMFG, sFG, lSMG ↓ rPCC ♀: ↓ bRMFG, sFG, lSMG ↑ rPCC | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marraudino, M.; Bonaldo, B.; Vitiello, B.; Bergui, G.C.; Panzica, G. Sexual Differences in Internet Gaming Disorder (IGD): From Psychological Features to Neuroanatomical Networks. J. Clin. Med. 2022, 11, 1018. https://doi.org/10.3390/jcm11041018
Marraudino M, Bonaldo B, Vitiello B, Bergui GC, Panzica G. Sexual Differences in Internet Gaming Disorder (IGD): From Psychological Features to Neuroanatomical Networks. Journal of Clinical Medicine. 2022; 11(4):1018. https://doi.org/10.3390/jcm11041018
Chicago/Turabian StyleMarraudino, Marilena, Brigitta Bonaldo, Benedetto Vitiello, Giovanna C. Bergui, and GianCarlo Panzica. 2022. "Sexual Differences in Internet Gaming Disorder (IGD): From Psychological Features to Neuroanatomical Networks" Journal of Clinical Medicine 11, no. 4: 1018. https://doi.org/10.3390/jcm11041018
APA StyleMarraudino, M., Bonaldo, B., Vitiello, B., Bergui, G. C., & Panzica, G. (2022). Sexual Differences in Internet Gaming Disorder (IGD): From Psychological Features to Neuroanatomical Networks. Journal of Clinical Medicine, 11(4), 1018. https://doi.org/10.3390/jcm11041018








