Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives
Abstract
:1. Introduction
2. Prosthetic Materials for Clinical Use
3. Surgical Mesh Related Infection
4. Types of Antimicrobial Surgical Meshes
4.1. Surgical Meshes with Antimicrobial Metals
4.2. Surgical Meshes with Antiseptics
4.3. Surgical Meshes with Antibiotics
5. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- See, C.W.; Kim, T.; Zhu, D. Hernia Mesh and Hernia Repair: A Review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- Falco, E.E.; Roth, J.S.; Fisher, J.P. Skeletal muscle tissue engineering approaches to abdominal wall hernia repair. Birth Defects Res. Part C Embryo Today Rev. 2008, 84, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kalaba, S.; Gerhard, E.; Winder, J.S.; Pauli, E.M.; Haluck, R.S.; Yang, J. Design strategies and applications of biomaterials and devices for Hernia repair. Bioact. Mater. 2016, 1, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, S.M.; Savi, F.M.; Ren, J.; Bas, O.; O’Rourke, N.; Maher, C.; Hutmacher, D.W. The Patenting and Technological Trends in Hernia Mesh Implants. Tissue Eng. Part B Rev. 2021, 27, 48–73. [Google Scholar] [CrossRef]
- Weyhe, D. Improving outcomes in hernia repair by the use of light meshes–a comparison of different implant constructions based on a critical appraisal of the literature. World J. Surg. 2007, 31, 234–244. [Google Scholar] [CrossRef]
- Zogbi, L. The Use of Biomaterials to Treat Abdominal Hernias. In Biomaterials Applications for Nanomedicine; Rosario, P., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.; Gómez-Gil, V.; Pérez-Köhler, B.; Pascual, G.; Bellón, J.M. Polymer Hernia Repair Materials: Adapting to Patient Needs and Surgical Techniques. Materials 2021, 14, 2790. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Pavan, P.G.; Natali, A.N. Synthetic surgical meshes used in abdominal wall surgery: Part I—materials and structural conformation. J. Biomed. Mater. Res. Part B 2017, 105B, 689–699. [Google Scholar] [CrossRef]
- Guillaume, O. Emerging trends in abdominal wall reinforcement: Bringing bio-functionality to meshes. Adv. Healthc. Mater. 2015, 4, 1763–1789. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Bayon, Y.; Bellón, J.M. Mesh Infection and Hernia Repair: A Review. Surg. Infect. 2016, 17, 124–137. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Lockhart, K.; Dunn, D.; Teo, S.; Ng, J.Y.; Dhillon, M.; Teo, E.; van Driel, M.L. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst. Rev. 2018, 13, CD011517. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Gil, V.; Pascual, G.; Bellón, J.M. Biomaterial Implants in Abdominal Wall Hernia Repair: A Review on the Importance of the Peritoneal Interface. Processes 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Miserez, M.; Jairam, A.P.; Boersema Geesien, S.A.; Bayon, Y.; Jeekel, J.; Lange, J.F. Resorbable Synthetic Meshes for Abdominal Wall Defects in Preclinical Setting: A Literature Review. J. Surg. Res. 2019, 237, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, A.S.; Pous-Serrano, S. Prosthetic meshes for hernia repair: State of art, classification, biomaterials, antimicrobial approaches, and fabrication methods. J. Biomed. Mater. Res. 2021, 109, 2695–2719. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Adamo, S.; Gossetti, F.; D’Amore, L.; Ceci, F.; Negro, P.; Bruzzone, P. Biological Scaffolds for Abdominal Wall Repair: Future in Clinical Application? Materials 2019, 12, 2375. [Google Scholar] [CrossRef] [Green Version]
- Plymale, M.A.; Davenport, D.L.; Walsh-Blackmore, S.; Hess, J.; Griffiths, W.S.; Plymale, M.C.; Totten, C.F.; Roth, J.S. Costs and Complications Associated with Infected Mesh for Ventral Hernia Repair. Surg. Infect. 2020, 21, 344–349. [Google Scholar] [CrossRef]
- Bilsel, Y.; Abci, I. The search for ideal hernia repair; mesh materials and types. Int. J. Surg. 2012, 10, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Young, R.M.; Gustafson, R.; Dinsmore, R.C. Sepramesh vs. Dualmesh for abdominal wall hernia repairs in a rabbit model. Curr. Surg. 2004, 61, 77–79. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Müller, M.; Schumpelick, V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur. J. Surg. 1999, 165, 665–673. [Google Scholar] [CrossRef]
- Robinson, T.N.; Clarke, J.H.; Schoen, J.; Walsh, M.D. Major mesh-related complications following hernia repair: Events reported to the Food and Drug Administration. Surg. Endosc. 2005, 19, 1556–1560. [Google Scholar] [CrossRef]
- Amid, P.K. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997, 1, 15–21. [Google Scholar] [CrossRef]
- Klinge, U.; Schumpelick, V.; Klosterhalfen, B. Functional assessment and tissue response of short-and long-term absorbable surgical meshes. Biomaterials 2001, 22, 1415–1424. [Google Scholar] [CrossRef]
- Krause, H.G.; Galloway, S.J.; Khoo, S.K.; Lourie, R.; Goh, J.W. Biocompatible properties of surgical mesh using an animal model. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.E., Jr.; Gonzalez, J.J., Jr.; Michaelson, R.P.; Glass, J.L.; Chock, D.A. Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia 2002, 6, 171–174. [Google Scholar] [CrossRef]
- Ruiz-Jasbon, F.; Norrby, J.M.; Ivarsson, L.; Björck, S. Inguinal hernia repair using a synthetic long-term resorbable mesh: Results from a 3-year prospective safety and performance study. Hernia 2014, 18, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Hjort, H.; Mathisen, T.; Alves, A.; Clermont, G.; Boutrand, J.P. Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics. Hernia 2012, 16, 191–197. [Google Scholar] [CrossRef] [Green Version]
- López-Cano, M.; Armengol, M.; Quiles, M.T.; Biel, A.; Velasco, J.; Huguet, P.; Mestre, A.; Delgado, L.M.; Gil, F.X.; Arbós, M.A. Preventive midline laparotomy closure with a new bioabsorbable mesh: An experimental study. J. Surg. Res. 2013, 1, 160–169. [Google Scholar] [CrossRef]
- Burgess, P.L.; Brockmeyer, J.R.; Johnson, E.K. Amyand hernia repaired with Bio-A: A case report and review. J. Surg. Educ. 2011, 68, 62–66. [Google Scholar] [CrossRef]
- Martin, D.P.; Badhwar, A.; Shah, D.V.; Rizk, S.; Eldridge, S.N.; Gagne, D.H.; Ganatra, A.; Darois, R.E.; Williams, S.F.; Tai, H.C.; et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J. Surg. Res. 2013, 184, 766–773. [Google Scholar] [CrossRef]
- Earle, D.B.; Mark, L.A. Prosthetic material in inguinal hernia repair: How do I choose? Surg. Clin. N. Am. 2008, 88, 179–201. [Google Scholar] [CrossRef]
- Helton, W.S.; Fisichella, P.M.; Berger, R.; Horgan, S.; Espat, N.J.; Abcarian, H. Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch. Surg. 2005, 140, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Paraiso, M.F.; Barber, M.D.; Muir, T.W.; Walters, M.D. Rectocele repair: A randomized trial of three surgical techniques including graft augmentation. Am. J. Obs. Gynecol. 2006, 195, 1762–1771. [Google Scholar] [CrossRef]
- Liang, H.C.; Chang, Y.; Hsu, C.K.; Lee, M.H.; Sung, H.W. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 2004, 25, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.M.; Armstrong, P.J.; Frizzi, J.D.; North, J.H., Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr. Surg. 2006, 63, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Harth, K.C.; Rosen, M.J. Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg. Innov. 2009, 16, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Mulier, K.E.; Nguyen, A.H.; Delaney, J.P.; Marquez, S. Comparison of Permacol™ and Strattice™ for the repair of abdominal wall defects. Hernia 2011, 15, 315–319. [Google Scholar] [CrossRef]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.F.; Richards, R.G.; Eglin, D.; Puchner, A.P. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Kasiakou, S.K. Mesh-related infections after hernia repair surgery. Clin. Microbiol. Infect. 2005, 11, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Aihemaiti, M.; Zhang, H.; Jiang, L.; Zhang, G.; Qin, M.; Pan, Y.; Wen, X.; Chan, F.S.Y.; Fan, J.K.M. Mesh-preservation approach to treatment of mesh infection after large incisional ventral hernia repair-how I do it. Ann. Transl. Med. 2019, 7, 698. [Google Scholar] [CrossRef]
- Demirer, S.; Geçim, I.E.; Aydinuraz, K.; Ataoğlu, H.; Yerdel, M.A.; Kuterdem, E. Affinity of Staphylococcus epidermidis to various prosthetic graft materials. J. Surg. Res. 2001, 99, 70–74. [Google Scholar] [CrossRef]
- Sanders, D.; Lambie, J.; Bond, P.; Moate, R.; Steer, J.A. An in vitro study assessing the effect of mesh morphology and suture fixation on bacterial adherence. Hernia 2013, 17, 779–789. [Google Scholar] [CrossRef]
- Von Eiff, C.; Jansen, B.; Kohnen, W.; Becker, K. Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs 2005, 65, 179–214. [Google Scholar] [CrossRef]
- Harrell, A.G.; Novitsky, Y.W.; Kercher, K.W.; Foster, M.; Burns, J.M.; Kuwada, T.S.; Heniford, B.T. In vitro infectability of prosthetic mesh by methicillin-resistant Staphylococcus aureus. Hernia 2006, 10, 120–124. [Google Scholar] [CrossRef]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirel, S.; Voica, C.; Colobățiu, L.; Mirel, V.; Matinca, D.; Flonta, M.; Lupșe, M. In vitro Comparison of the Antimicrobial Efficiency of Commercially Available Silver-Wound Dressings Correlated with the Evaluation of Silver Release by Inductively Coupled Plasma–Mass Spectrometry. Anal. Lett. 2019, 52, 163–176. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhang, J.; Yan, R.L.; Wang, Q.; Fan, L.Y.; Zhang, Q.; Wang, W.J.; Hu, Z.Q. Improving the antibacterial property of porcine small intestinal submucosa by nano-silver supplementation: A promising biological material to address the need for contaminated defect repair. Ann. Surg. 2011, 253, 1033–1041. [Google Scholar] [CrossRef]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. (Larchmt) 2007, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nergiz Adıgüzel, E.; Esen, E.; Aylaz, G.; Keskinkılıç Yağız, B.; Kıyan, M.; Doğan, A.; Ünal, A.E. Do Nano-crystalline Silver-Coated Hernia Grafts Reduce Infection? World J. Surg. 2018, 42, 3537–3542. [Google Scholar] [CrossRef]
- Badiou, W.; Lavigne, J.P.; Bousquet, P.J.; O’Callaghan, D.; Marès, P.; de Tayrac, R. In vitro and in vivo assessment of silver-coated polypropylene mesh to prevent infection in a rat model. Int. Urogynecology J. 2011, 22, 265–272. [Google Scholar] [CrossRef]
- Muzio, G.; Perero, S.; Miola, M.; Oraldi, M.; Ferraris, S.; Vernè, E.; Festa, F.; Canuto, R.A.; Festa, V.; Ferraris, M. Biocompatibility versus peritoneal mesothelial cells of polypropylene prostheses for hernia repair, coated with a thin silica/silver layer. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kaige, X.; Liangpeng, G.; Mohammad, D.A.; Xie, F.; Derakhshanfar, S.; Liu, Y.; Xing, M.M.Q.; Hong, H. A novel nano-silver coated and hydrogel-impregnated polyurethane nanofibrous mesh for ventral hernia repair. RSC Adv. 2016, 6, 90571–90578. [Google Scholar] [CrossRef]
- Saygun, O.; Agalar, C.; Aydinuraz, K.; Agalar, F.; Daphan, C.; Saygun, M.; Ceken, S.; Akkus, A.; Denkbas, E.B. Gold and gold-palladium coated polypropylene grafts in a S. epidermidis wound infection model. J. Surg. Res. 2006, 131, 73–79. [Google Scholar] [CrossRef]
- Cazalini, E.M.; Miyakawa, W.; Teodoro, G.R.; Sobrinho, A.S.S.; Matieli, J.E.; Massi, M.; Koga-Ito, C.Y. Antimicrobial and anti-biofilm properties of polypropylene meshes coated with metal-containing DLC thin films. J. Mater. Sci. Mater. Med. 2017, 28, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelsman, A.F.; van der Mei, H.C.; Busscher, H.J.; Ploeg, R.J. Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br. J. Surg. 2008, 95, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Yurtkap, Y.; Jairam, A.P.; Kaufmann, R.; Kroese, L.F.; Clahsen-van Groningen, M.C.; Mouton, J.W.; Menon, A.G.; Kleinrensink, G.J.; Jeekel, J.; Lange, J.F.; et al. Zinc-Impregnated Mesh for Abdominal Wall Repair Reduces Infection in a Rat Model of Peritonitis. J. Surg. Res. 2020, 246, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Abdulagatov, I.M.; Ragimov, R.M.; Khamidov, М.А.; Maksumova, A.M.; Abdullaeva, N.M. ALD coated polypropylene hernia meshes for prevention of mesh-related post-surgery complications: An experimental study in animals. Biomed. Mater. 2021, 17, 015006. [Google Scholar] [CrossRef]
- Kiladze, M.; Gogoladze, M.; Chkhikvadze, T. Hernioplasty with new antiseptic polymeric biocomposite meshes. TCMGMJ 2018, 3, 17–19. [Google Scholar]
- Pérez-Köhler, B.; Benito-Martínez, S.; Rodríguez, M.; García-Moreno, F.; Pascual, G.; Bellón, J.M. Experimental study on the use of a chlorhexidine-loaded carboxymethylcellulose gel as antibacterial coating for hernia repair meshes. Hernia 2019, 23, 789–800. [Google Scholar] [CrossRef]
- Carbonell, A.M.; Matthews, B.D.; Dréau, D.; Foster, M.; Austin, C.E.; Kercher, K.W.; Sing, R.F.; Heniford, B.T. The susceptibility of prosthetic biomaterials to infection. Surg. Endosc. 2005, 19, 430–435. [Google Scholar] [CrossRef]
- Cobb, W.S.; Paton, B.L.; Novitsky, Y.W.; Rosen, M.J.; Kercher, K.W.; Kuwada, T.S.; Heniford, B.T. Intra-abdominal placement of antimicrobial-impregnated mesh is associated with noninfectious fever. Am. Surg. 2006, 72, 1205–1209. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Fernández-Gutiérrez, M.; Pascual, G.; García-Moreno, F.; San Román, J.; Bellón, J.M. In vitro assessment of an antibacterial quaternary ammonium-based polymer loaded with chlorhexidine for the coating of polypropylene prosthetic meshes. Hernia 2016, 20, 869–878. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; García-Moreno, F.; Bayon, Y.; Pascual, G.; Bellón, J.M. Inhibition of Staphylococcus aureus Adhesion to the Surface of a Reticular Heavyweight Polypropylene Mesh Soaked in a Combination of Chlorhexidine and Allicin: An In vitro Study. PLoS ONE 2015, 10, e0126711. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Köhler, B.; García-Moreno, F.; Brune, T.; Pascual, G.; Bellón, J.M. Preclinical Bioassay of a Polypropylene Mesh for Hernia Repair Pretreated with Antibacterial Solutions of Chlorhexidine and Allicin: An In Vivo Study. PLoS ONE 2015, 10, e0142768. [Google Scholar] [CrossRef] [Green Version]
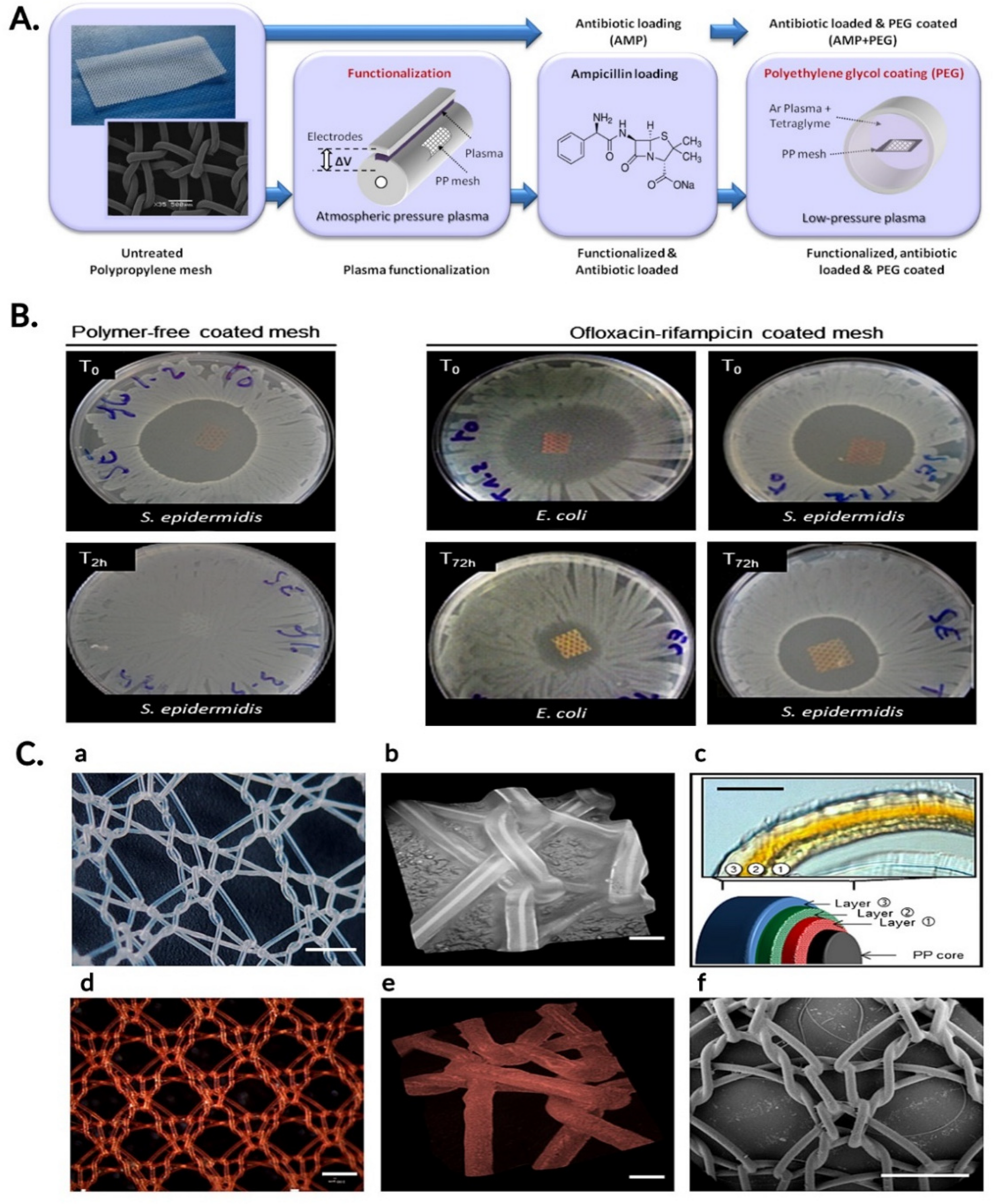
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Sinha, B.; Spor, L.; Klinge, U.; Steger, U.; Germer, C.T.; Dietz, U.A. Gentamicin for prevention of intraoperative mesh contamination: Demonstration of high bactericide effect (in vitro) and low systemic bioavailability (in vivo). Hernia 2014, 18, 691–700. [Google Scholar] [CrossRef]
- Junge, K.; Rosch, R.; Klinge, U.; Krones, C.; Klosterhalfen, B.; Mertens, P.R.; Lynen, P.; Kunz, D.; Preiss, A.; Peltroche-Llacsahuanga, H.; et al. Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 2005, 26, 787–793. [Google Scholar] [CrossRef]
- Kilic, D.; Agalar, C.; Ozturk, E.; Denkbas, E.B.; Cime, A.; Agalar, F. Antimicrobial activity of cefazolin-impregnated mesh grafts. ANZ J. Surg. 2007, 77, 256–260. [Google Scholar] [CrossRef]
- Suárez-Grau, J.M.; Morales-Conde, S.; González Galán, V.; Martín Cartes, J.A.; Docobo Durantez, F.; Padillo Ruiz, F.J. Antibiotic embedded absorbable prosthesis for prevention of surgical mesh infection: Experimental study in rats. Hernia 2015, 19, 187–194. [Google Scholar] [CrossRef]
- Harth, K.C.; Rosen, M.J.; Thatiparti, T.R.; Jacobs, M.R.; Halaweish, I.; Bajaksouzian, S.; Furlan, J.; von Recum, H.A. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: An in vivo study. J. Surg. Res. 2010, 163, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Grafmiller, K.T.; Zuckerman, S.T.; Petro, C.; Liu, L.; von Recum, H.A.; Rosen, M.J.; Korley, J.N. Antibiotic-releasing microspheres prevent mesh infection in vivo. J. Surg. Res. 2016, 206, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blatnik, J.A.; Thatiparti, T.R.; Krpata, D.M.; Zuckerman, S.T.; Rosen, M.J.; von Recum, H.A. Infection prevention using affinity polymer-coated, synthetic meshes in a pig hernia model. J. Surg. Res. 2017, 219, 5–10. [Google Scholar] [CrossRef]
- Sanbhal, N.; Li, Y.; Khatri, A.; Peerzada, M.; Wang, L. Chitosan Cross-Linked Bio-based Antimicrobial Polypropylene Meshes for Hernia Repair Loaded HCl via Cold Oxygen Plasma. Coatings 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Hall Barrientos, I.J.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.L.; Ma, Y.J.; Xia, G.G.; Wang, Y.; Kapadia, W.; Sun, Z.Y.; Wu, W.; Gu, H.C.; Cui, W.G.; Huang, X.Y. In vitro and in vivo combined antibacterial effect of levofloxacin /silver coloaded electrospun fibrous membranes. J. Mater. Chem. B 2017, 5, 7632–7643. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Nisticò, R.; Faga, M.G.; D’Angelo, D.; Boot, E.A.; Lamberti, R.; Martorana, S.; Calza, P.; Fabbri, D.; Magnacca, G. Hernia-repair prosthetic devices functionalised with chitosan and ciprofloxacin coating: Controlled release and antibacterial activity. J. Mater. Chem. B 2014, 2, 5287–5294. [Google Scholar] [CrossRef] [Green Version]
- Laurent, T.; Kacem, I.; Blanchemain, N.; Cazaux, F.; Neut, C.; Hildebrand, H.F.; Martel, B. Cyclodextrin and maltodextrin finishing of a polypropylene abdominal wall implant for the prolonged delivery of ciprofloxacin. Acta Biomater. 2011, 7, 3141–3149. [Google Scholar] [CrossRef]
- Qamar, N.; Abbas, N.; Irfan, M.; Hussain, A.; Arshad, M.S.; Latif, S.; Mehmood, F.; Ghori, M.U. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. J. Drug Deliv. Sci. Technol. 2019, 53, 101164. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Bahrami, S.H.; Nazarpak, M.H.; Solouk, A. Biomimetic double-sided polypropylene mesh modified by DOPA and ofloxacin loaded carboxyethyl chitosan/polyvinyl alcohol-polycaprolactone nanofibers for potential hernia repair applications. Int. J. Biol. Macromol. 2020, 165, 902–917. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Rodríguez, M.; Pascual, G.; Bellón, J.M. Preclinical bioassay of a novel antibacterial mesh for the repair of abdominal hernia defects. Surgery 2020, 167, 598–608. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Urich, L.; Uhde, A.K.; Müller, I.; Weindl, T.; Vogel, U.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Biodegradable rifampicin-releasing coating of surgical meshes for the prevention of bacterial infections. Drug Des. Dev. 2017, 18, 2753–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.E.; Imahiyerobo, T.A.; Scott, J.R.; Spector, J.A. Comparison of Antibiotic-Coated versus Uncoated Porcine Dermal Matrix. Plast. Reconstr. Surg. 2016, 138, 844e–855e. [Google Scholar] [CrossRef]
- Baker, E.H.; Lepere, D.; Lundgren, M.P.; Greaney, P.J.; Ehrlich, D.A.; Copit, S.E.; Murphree, A.L.; Canfield, A.J.; Parker, G.; Iannitti, D.A. Early Clinical Outcomes of a Novel Antibiotic-Coated, Non-Crosslinked Porcine Acellular Dermal Graft after Complex Abdominal Wall Reconstruction. J. Am. Coll. Surg. 2016, 223, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Garric, X.; Lavigne, J.P.; Van Den Berghe, H.; Coudane, J. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: Preparation and antimicrobial efficacy in vitro. J. Control. Release 2012, 162, 492–501. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Linardi, F.; Pascual, G.; Bellón, J.M.; Eglin, D.; Guillaume, O. Efficacy of antimicrobial agents delivered to hernia meshes using an adaptable thermo-responsive hyaluronic acid-based coating. Hernia 2020, 24, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-Generation Surgical Meshes for Drug Delivery and Tissue Engineering Applications: Materials, Design and Emerging Manufacturing Technologies. Bio-Des. Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; Gómez-Gil, V.; Rodríguez, M.; Pascual, G.; Bellón, J.M. New Insights into the Application of 3D-Printing Technology in Hernia Repair. Materials 2021, 14, 7092. [Google Scholar] [CrossRef] [PubMed]
- Pantermehl, S.; Emmert, S.; Foth, A.; Grabow, N.; Alkildani, S.; Bader, R.; Barbeck, M.; Jung, O. 3D Printing for Soft Tissue Regeneration and Applications in Medicine. Biomedicines 2021, 9, 336. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, N.; Tang, R. Functionalized Strategies and Mechanisms of the Emerging Mesh for Abdominal Wall Repair and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 2064–2082. [Google Scholar] [CrossRef]

| Antimicrobial Agent | Mesh | Test Method | Antibacterial Activity Tested | Ref. |
|---|---|---|---|---|
| NcAg | PP | In vitro | S. aureus | [49] |
| NcAg | PP | In vivo | MRSA | [50] |
| AgNP | PP | In vitro In vivo | E. coli | [51] |
| AgNP | PSIS | In vitro In vivo | S. aureus, S. epidermidis, E. coli, P. aeruginosa | [48] |
| Ag/SiO2 | PP | In vitro | S. aureus | [52] |
| Nano-Ag | PEG/Gel-Hy/PU | In vitro In vivo | S. aureus, E. coli | [53] |
| Au, Au-Pd | PP | In vitro In vivo | S. epidermidis | [54] |
| Me/Me-DLC | PP | In vitro | C. albicans, E. coli, S. aureus, P. aeruginosa | [55] |
| Ti | PP | In vitro | S. aureus, P. aeruginosa, E. coli | [56] |
| Ti | PP | In vitro | S. epidermidis, S. aureus | [42] |
| Zn | PP | In vivo | Enterococcus, Staphylococcus | [57] |
| V/TiO2 | PP | In vivo | S. aureus, E. coli | [58] |
| Antimicrobial Agent | Mesh | Test Method | Antibacterial Activity Tested | Ref. |
|---|---|---|---|---|
| CHX | PP | In vitro In vivo | S. aureus | [59] |
| CHX | CMC | In vitro In vivo | S. aureus | [60] |
| Ag-CHX | ePTFE | In vivo | S. aureus | [61] |
| Ag-CHX | ePTFE | In vitro | MRSA | [44] |
| Ag-CHX | ePTFE | In vivo | S. aureus | [62] |
| CHX-QAC | PP | In vitro | S. aureus S. epidermidis E. coli | [63] |
| CHX-All | PP | In vitro | S. aureus | [64] |
| In vivo | [65] |
| Antimicrobial Agent | Meshes | Test Methods | Antibacterial Activity Tested | Ref. |
|---|---|---|---|---|
| Ampicillin | PP | In vitro | S. aureus, E. coli | [66] |
| Gentamicin | PE PP/PGC | In vitro In vivo | S. aureus | [67] |
| Gentamicin | PVDF | In vivo | S. aureus, E. coli, S. epidermidis | [68] |
| Cefazolin | PE | In vitro In vivo | MRSA | [69] |
| Cefazolin | PGA–TMC | In vivo | S. aureus | [70] |
| Vancomycin * | PE | In vitro In vivo | S. aureus | [71] |
| Vancomycin * | PE | In vitro | S. aureus | [72] |
| Vancomycin * | PE | In vivo | MRSA | [73] |
| Levofloxacin Levofloxacin | PP | In vitro | S. aureus, E. coli S. aureus, E. coli | [74] |
| PCL | In vitro | [75] | ||
| Levofloxacin + silver | PLLA | In vitro In vivo | MRSA | [76] |
| Ciprofloxacin | PP | In vitro | S. aureus, E. coli | [77] |
| Ciprofloxacin | PP | In vitro | S. aureus, E. coli, S. epidermidis | [78] |
| Ciprofloxacin | PP, PVC (3D printing) | In vitro In vivo | [79] | |
| Ofloxacin | PCL/L-DOPA and PCL or CECS/PVA | In vitro | S. aureus, E. coli | [80] |
| Rifampicin | PP | In vitro In vivo | S. aureus S. epidermidis | [81] |
| Rifampicin | PP/PLGA | In vitro In vivo | S. aureus | [82] |
| Rifampicin + Minocycline | PADG | In vitro In vivo | S. aureus E. coli | [83] |
| Rifampicin + Minocycline | PADH | In vitro In vivo | MRSA | [84] |
| Rifampicin + Ofloxacin | PP+ PCL + PLA | In vitro | E. coli, S. aureus, S. epidermidis, P. aeruginosa, K. pneumoniae, MRSA | [85] |
| Rifampicin + Gentamicin + Vancomycin + CHX | PP, ePTFE; PGA, PLA, PCL, PSIS, PADG | In vitro | S. aureus, E. coli, S. epidermidis | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirel, S.; Pusta, A.; Moldovan, M.; Moldovan, S. Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives. J. Clin. Med. 2022, 11, 883. https://doi.org/10.3390/jcm11030883
Mirel S, Pusta A, Moldovan M, Moldovan S. Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives. Journal of Clinical Medicine. 2022; 11(3):883. https://doi.org/10.3390/jcm11030883
Chicago/Turabian StyleMirel, Simona, Alexandra Pusta, Mihaela Moldovan, and Septimiu Moldovan. 2022. "Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives" Journal of Clinical Medicine 11, no. 3: 883. https://doi.org/10.3390/jcm11030883
APA StyleMirel, S., Pusta, A., Moldovan, M., & Moldovan, S. (2022). Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives. Journal of Clinical Medicine, 11(3), 883. https://doi.org/10.3390/jcm11030883






