Development and Validation of the Acute PNeumonia Early Assessment Score for Safely Discharging Low-Risk SARS-CoV-2-Infected Patients from the Emergency Department
Abstract
:1. Background
2. Patients and Methods
2.1. Study Design
2.2. Score Calculation
2.3. Six-Minute Walk Test
2.4. Lung Imaging
2.5. Statistical Methods
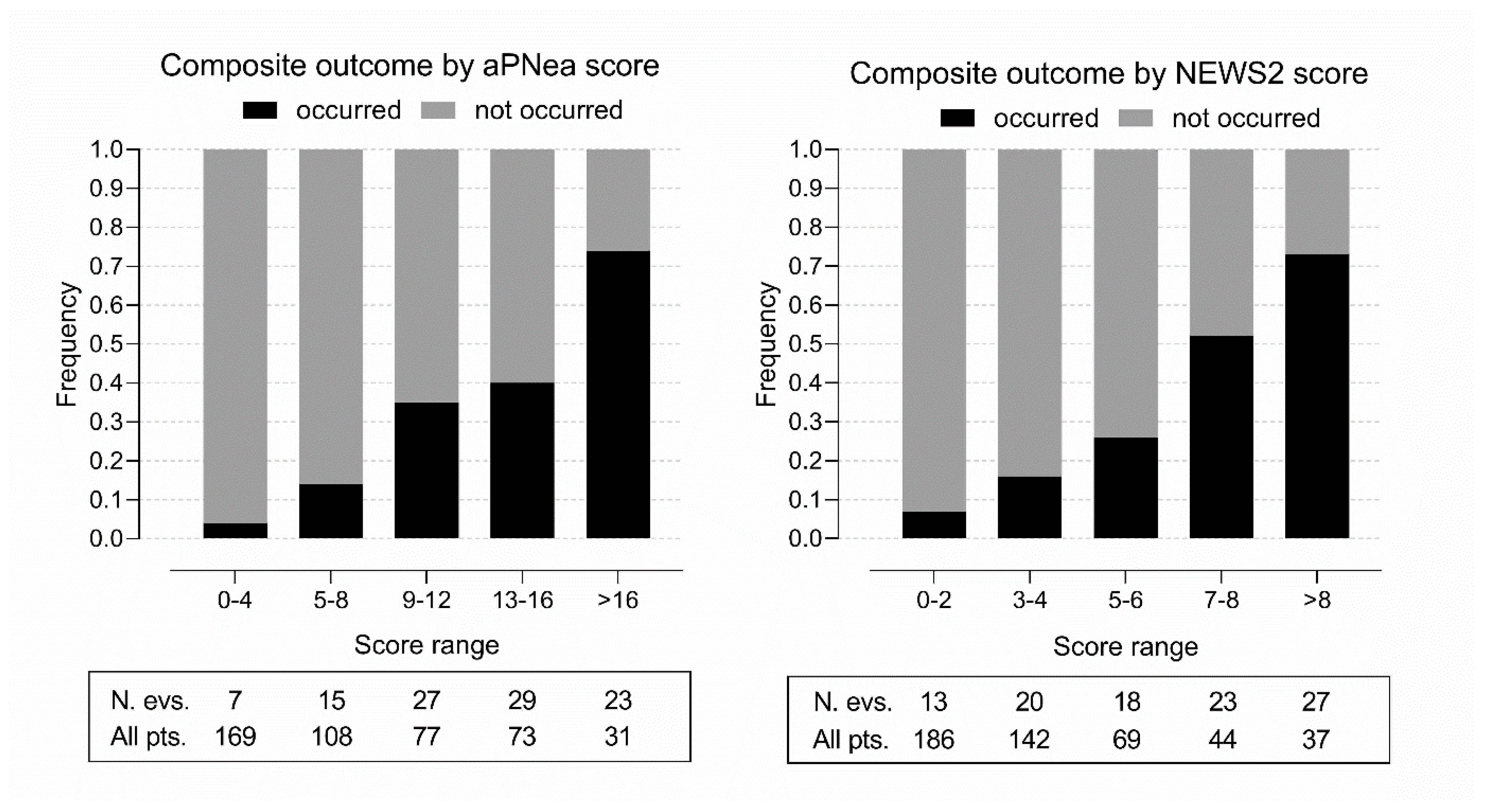
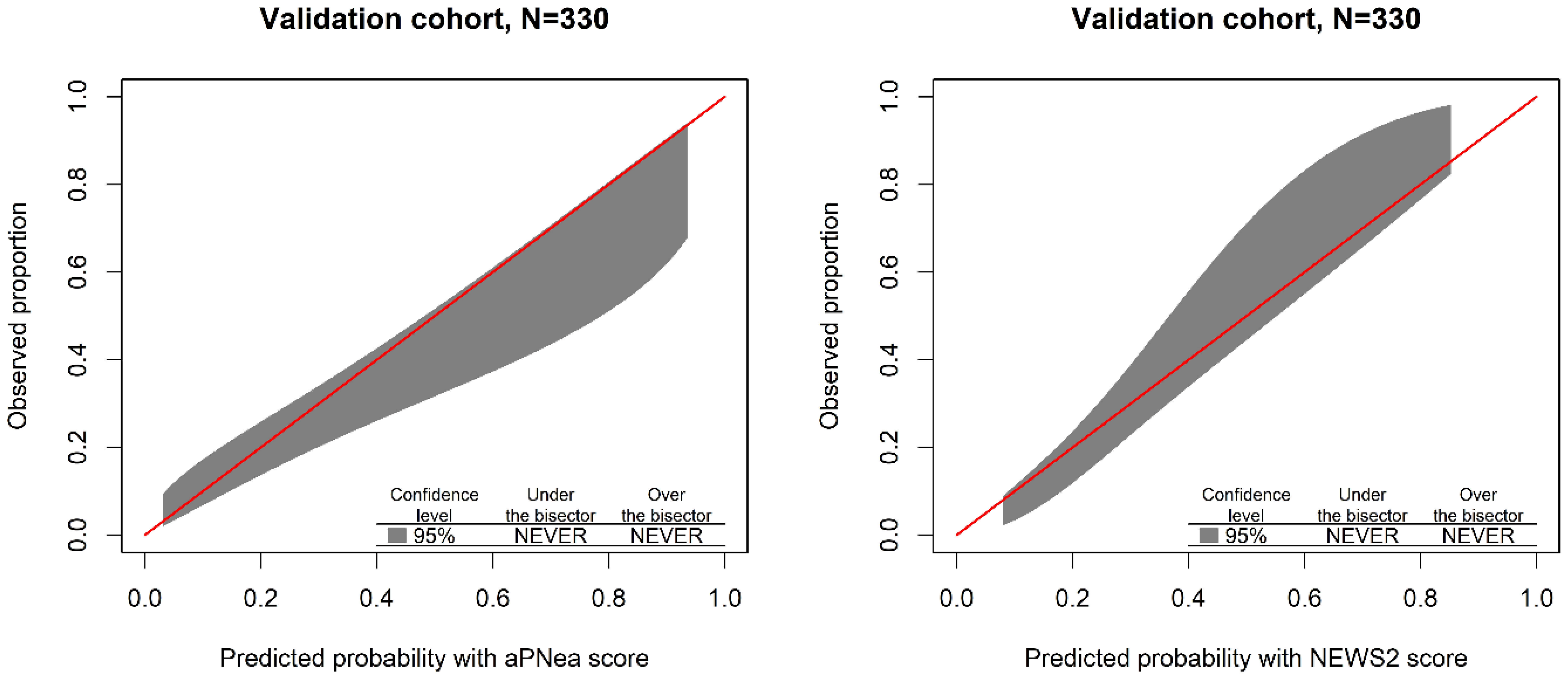
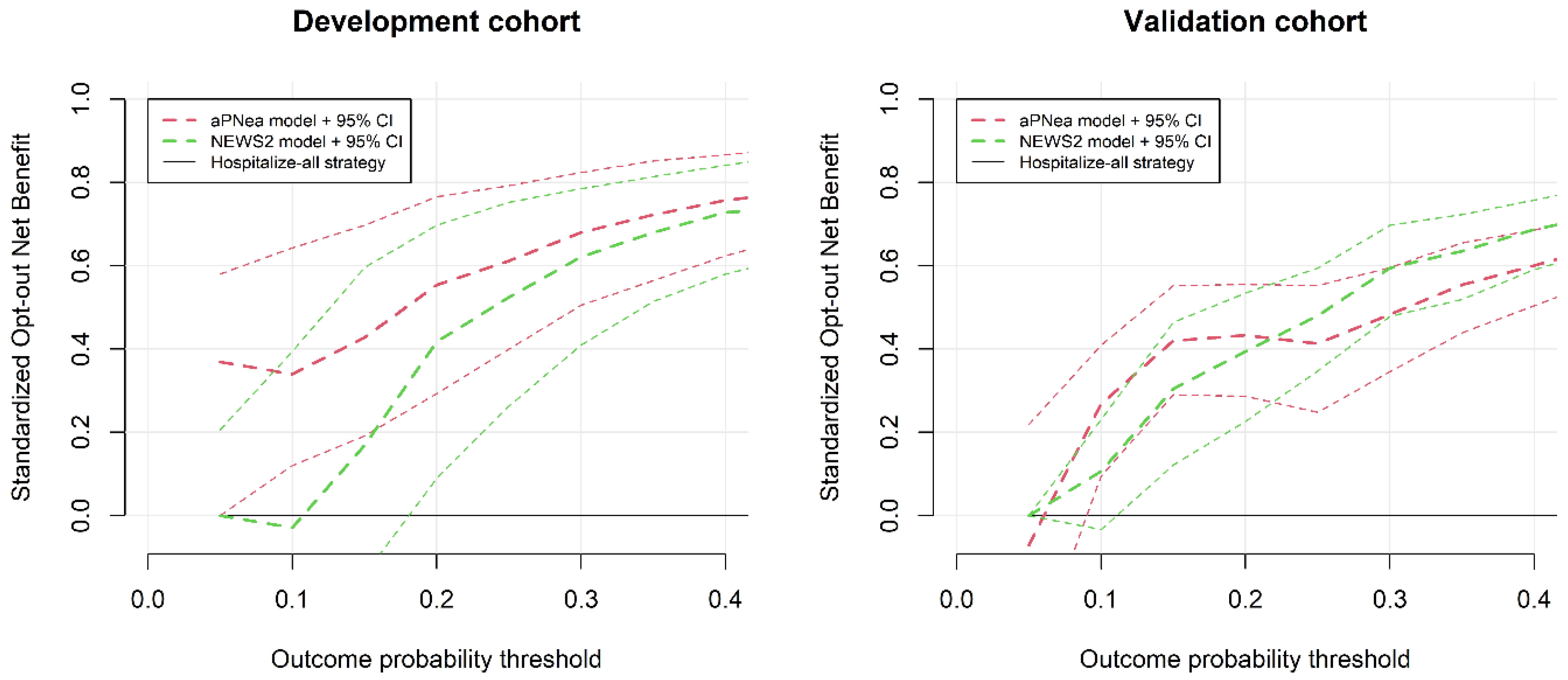
3. Results
Score Performance and Validation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weekly Epidemiological Update—29 December 2020. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update (accessed on 25 July 2021).
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, S.; Quattrocchi, A.; Gabel, J.; Heraclides, A.; Kolokotroni, O.; Constantinou, C.; Pagola Ugarte, M.; Nicolaou, N.; Rodriguez-Llanes, J.M.; Bennett, C.M.; et al. Excess All-Cause Mortality and COVID-19-Related Mortality: A Temporal Analysis in 22 Countries, from January until August 2020. Int. J. Epidemiol. 2021, dyab123. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Illness Severity in the NHS. Updated Report of a Working Party; RCP: London, UK, 2017; ISBN 978-1-86016-682-2. [Google Scholar]
- Spagnolli, W.; Rigoni, M.; Torri, E.; Cozzio, S.; Vettorato, E.; Nollo, G. Application of the National Early Warning Score (NEWS) as a Stratification Tool on Admission in an Italian Acute Medical Ward: A Perspective Study. Int. J. Clin. Pract. 2017, 71, e12934. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.; Bendayan, R.; Bean, D.; Stammers, M.; Wang, W.; Zhang, H.; Searle, T.; Kraljevic, Z.; Shek, A.; Phan, H.T.T.; et al. Evaluation and Improvement of the National Early Warning Score (NEWS2) for COVID-19: A Multi-Hospital Study. BMC Med. 2021, 19, 23. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. Interim Guidance. Pediatr. Med. Rodz. 2020, 16, 9–26. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Bella, F.; Pes, C.; Sini, L.; Cossu, C.; Vecchiato, A.; Melis, A.; Pinna Parpaglia, P. Dealing with patients with suspected COVID-19 active infection: A challenge for emergency physicians. Ital. J. Emerg. Med. 2020, 9, 106–111. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Hantzidiamantis, P.J.; Amaro, E. Physiology, Alveolar to Arterial Oxygen Gradient. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An Official European Respiratory Society/American Thoracic Society Technical Standard: Field Walking Tests in Chronic Respiratory Disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Neri, E.; Coppola, F.; Larici, A.R.; Sverzellati, N.; Mazzei, M.A.; Sacco, P.; Dalpiaz, G.; Feragalli, B.; Miele, V.; Grassi, R. Structured Reporting of Chest CT in COVID-19 Pneumonia: A Consensus Proposal. Insights Imaging 2020, 11, 92. [Google Scholar] [CrossRef]
- Ruch, Y.; Kaeuffer, C.; Ohana, M.; Labani, A.; Fabacher, T.; Bilbault, P.; Kepka, S.; Solis, M.; Greigert, V.; Lefebvre, N.; et al. CT Lung Lesions as Predictors of Early Death or ICU Admission in COVID-19 Patients. Clin. Microbiol. Infect. 2020, 26, 1417.e5–1417.e8. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the Performance of Prediction Models: A Framework for Some Traditional and Novel Measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, W.; Macheret, F.; Gabriel, R.A.; Ohno-Machado, L. A Tutorial on Calibration Measurements and Calibration Models for Clinical Prediction Models. J. Am. Med. Inform. Assoc. 2020, 27, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, S.; Poole, D.; Luciani, D.; Cogo, P.E.; Bertolini, G. Calibration Belt for Quality-of-Care Assessment Based on Dichotomous Outcomes. PLoS ONE 2011, 6, e16110. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decisison Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.F.; Brown, M.D.; Marsh, T.L.; Janes, H. Assessing the Clinical Impact of Risk Models for Opting Out of Treatment. Med. Decision Mak. 2019, 39, 86–90. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- World Health Organization. Clinical Care of Severe Acute Respiratory Infections–Tool Kit. Available online: https://www.who.int/publications-detail-redirect/clinical-care-of-severe-acute-respiratory-infections-tool-kit (accessed on 28 September 2021).
- Thomas, B.; Goodacre, S.; Lee, E.; Sutton, L.; Bursnall, M.; Loban, A.; Waterhouse, S.; Simmonds, R.; Biggs, K.; Marincowitz, C.; et al. Prognostic Accuracy of Emergency Department Triage Tools for Adults with Suspected COVID-19: The PRIEST Observational Cohort Study. Emerg. Med. J. 2021, 38, 587–593. [Google Scholar] [CrossRef]
- Fan, G.; Tu, C.; Zhou, F.; Liu, Z.; Wang, Y.; Song, B.; Gu, X.; Wang, Y.; Wei, Y.; Li, H.; et al. Comparison of Severity Scores for COVID-19 Patients with Pneumonia: A Retrospective Study. Eur. Respir. J. 2020, 56, 2002113. [Google Scholar] [CrossRef]
- Ma, X.; Ng, M.; Xu, S.; Xu, Z.; Qiu, H.; Liu, Y.; Lyu, J.; You, J.; Zhao, P.; Wang, S.; et al. Development and Validation of Prognosis Model of Mortality Risk in Patients with COVID-19. Epidemiol. Infect. 2020, e168, 1–7. [Google Scholar] [CrossRef]
- Bradley, P.; Frost, F.; Tharmaratnam, K.; Wootton, D.G. NW Collaborative Organisation for Respiratory Research Utility of Established Prognostic Scores in COVID-19 Hospital Admissions: Multicentre Prospective Evaluation of CURB-65, NEWS2 and QSOFA. BMJ Open Respir. Res. 2020, 7, e000729. [Google Scholar] [CrossRef]
- Gold, M.S.; Sehayek, D.; Gabrielli, S.; Zhang, X.; McCusker, C.; Ben-Shoshan, M. COVID-19 and Comorbidities: A Systematic Review and Meta-Analysis. Postgrad. Med. 2020, 132, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk Factors for Mortality in Patients with Coronavirus Disease 2019 (COVID-19) Infection: A Systematic Review and Meta-Analysis of Observational Studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Leoni, M.L.G.; Lombardelli, L.; Colombi, D.; Bignami, E.G.; Pergolotti, B.; Repetti, F.; Villani, M.; Bellini, V.; Rossi, T.; Halasz, G.; et al. Prediction of 28-Day Mortality in Critically Ill Patients with COVID-19: Development and Internal Validation of a Clinical Prediction Model. PLoS ONE 2021, 16, e0254550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial Dysfunction Contributes to COVID-19-Associated Vascular Inflammation and Coagulopathy. Rev. Cardiovasc Med. 2020, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Haberecker, M.; Schwarz, E.I.; Steiger, P.; Frontzek, K.; Scholkmann, F.; Zeng, X.; Höller, S.; Moch, H.; Varga, Z. Autopsy-Based Pulmonary and Vascular Pathology: Pulmonary Endotheliitis and Multi-Organ Involvement in COVID-19 Associated Deaths. Respiration 2021, 101, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-Dimer Levels on Admission to Predict in-Hospital Mortality in Patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Gungor, B.; Atici, A.; Baycan, O.F.; Alici, G.; Ozturk, F.; Tugrul, S.; Asoglu, R.; Cevik, E.; Sahin, I.; Barman, H.A. Elevated D-Dimer Levels on Admission Are Associated with Severity and Increased Risk of Mortality in COVID-19: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2021, 39, 173–179. [Google Scholar] [CrossRef]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.A.; Debray, T.P.A.; et al. Prediction Models for Diagnosis and Prognosis of COVID-19: Systematic Review and Critical Appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk Stratification of Patients Admitted to Hospital with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol: Development and Validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef] [PubMed]
- Bertazzoni, G.; Minisola, S.; Franceschi, F.; Mirante, E.; Ruggieri, M.P.; Pugliese, F.R.; Ricciuto, G.M.; Susi, B.; Magnanti, M.; De Vito, C.; et al. Rome Metropolitan Area Multicenter Retrospective Study of Emergency Department Presentation during COVID-19 Pandemic. Ital. J. Emerg. Med. 2020, 9, 118–125. [Google Scholar] [CrossRef]
- Dell’Aquila, P.; Raimondo, P.; De Matteis, S.; De Luca, P.; Grasso, S.; Procacci, V. Lung Ultrasound Score and Management Strategies in the Critically COVID-19 Patients. Ital. J. Emerg. Med. 2020, 9, 126–130. [Google Scholar] [CrossRef]
- Tobin, M.J. Basing Respiratory Management of COVID-19 on Physiological Principles. Am. J. Respir. Crit. Care Med. 2020, 201, 1319–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, R.; Vaity, C.; Mulakavalupil, B.; Matthew, A.; Sabnis, K.; Joshi, S. Unmasking Hypoxia in COVID 19-Six Minute Walk Test. J. Assoc. Physicians India 2020, 68, 50–51. [Google Scholar] [PubMed]
- Colussi, G.; Perrotta, G.; Pillinini, P.; Dibenedetto, A.G.; Da Porto, A.; Catena, C.; Sechi, L.A. Prognostic Scores and Early Management of Septic Patients in the Emergency Department of a Secondary Hospital: Results of a Retrospective Study. BMC Emerg. Med. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Myrstad, M.; Ihle-Hansen, H.; Tveita, A.A.; Andersen, E.L.; Nygård, S.; Tveit, A.; Berge, T. National Early Warning Score 2 (NEWS2) on Admission Predicts Severe Disease and in-Hospital Mortality from COVID-19—A Prospective Cohort Study. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 66. [Google Scholar] [CrossRef] [PubMed]
| Points | |
|---|---|
| Need for hospitalization independent of COVID-19 severity | 5 |
| Respiratory rate >30 acts per minute | 5 |
| Arterial partial pressure of oxygen <60 mm Hg | 5 |
| Horowitz index <300 | 5 |
Horowitz index <330 and
| 4 2 |
| Positive 6-min walk test | 2 |
| Lung parenchymal involvement ≥50% on the best available lung imaging | 2 |
| Lung parenchymal involvement <50% on the best available lung imaging | 1 |
| Fever >38 °C in the previous 7 days | 1 |
| Plasma D-dimer levels >1000 ng/mL FEU | 1 |
| Variable | All Patients | Deceased or a Need for Oral Intubation | Alive without a Need for Oral Intubation | p |
|---|---|---|---|---|
| Sample number | 458 | 101 | 357 | - |
| General clinical characteristics | ||||
| Age (years) | 67 ± 17 | 78 ± 13 | 63 ± 17 | <0.001 |
| Female sex (n (%)) | 189 (41) | 36 (36) | 153 (43) | 0.209 |
| Obesity (n (%)) | 99 (22) | 30 (30) | 69 (19) | 0.029 |
| Diabetes (n (%)) | 61 (13) | 20 (20) | 41 (11) | 0.045 |
| Hypertension (n (%)) | 239 (52) | 76 (75) | 163 (46) | <0.001 |
| Past or active smoking (n (%)) | 64 (14) | 15 (15) | 49 (14) | 0.747 |
| Congestive heart failure (n (%)) | 32 (7.0) | 16 (16) | 16 (4.5) | <0.001 |
| Coronary artery disease (n (%)) | 50 (11) | 18 (18) | 32 (9.0) | 0.018 |
| Stroke or TIA (n (%)) | 39 (8.5) | 18 (18) | 21 (5.9) | <0.001 |
| 30-day death (n (%)) | 87 (19) | 87 (19) | - | - |
| Oral intubation (n (%)) | 28 (6.1) | 28 (28) | - | - |
| Vital signs | ||||
| Glasgow Coma Scale | 14.8 ± 1.0 | 14.4 ± 2.0 | 15.0 ± 0.3 | <0.001 |
| Body temperature (°C) | 37.0 ± 1.0 | 37.2 ± 1.0 | 36.9 ± 0.9 | 0.022 |
| SBP (mm Hg) | 136 ± 22 | 135 ± 28 | 136 ± 20 | 0.770 |
| Mean arterial pressure (mm Hg) | 98 ± 14 | 95 ± 19 | 98 ± 12 | 0.130 |
| Heart rate (beats per min) | 84 ± 16 | 91 ± 21 | 82 ± 14 | <0.001 |
| Respiratory rate (acts per min) | 20 ± 6 | 25 ± 8 | 19 ± 4 | <0.001 |
| Arterial oxygen saturation (%) | 94 ± 5 | 90 ± 7 | 95 ± 4 | <0.001 |
| Biochemical variables | ||||
| Plasma creatinine (mg/dl) | 0.92 (0.75–1.16) | 1.19 (0.86–1.60) | 0.88 (0.74–1.08) | <0.001 |
| BUN (mg/dl) | 17 (13–25) | 28 (19–47) | 15 (12–21) | <0.001 |
| eGFR (ml/min/1.73 m2) | 75 (57–91) | 53 (41–78) | 78 (64–93) | <0.001 |
| C-reactive protein (mg/dl) | 4.6 (1.4–10.7) | 12 (5.9–17.8) | 3.2 (0.9–7.5) | <0.001 |
| Procalcitonin (ng/mL) | 0.03 (0.01–0.11) | 0.19 (0.06–0.50) | 0.02 (0.01–0.07) | <0.001 |
| D-dimer (ng/mL FEU) | 705 (439–1306) | 1038 (654–1606) | 619 (399–1158) | <0.001 |
| Prognostic scores | ||||
| aPNea score | 6 (1–11) | 12 (8–14) | 5 (1–8) | <0.001 |
| NEWS2 score | 2 (1–4) | 5 (3–8) | 2 (1–3) | <0.001 |
| Variable | Development | Validation | External |
|---|---|---|---|
| Sample number | 128 | 330 | 97 |
| Outcome prevalence (n (%)) | 25 (20) | 76 (23) | 28 (29) |
| General clinical characteristics and laboratory variables | |||
| Age (years) | 65 ± 19 | 67 ± 16 | 71 ± 18 * |
| Female sex (n (%)) | 55 (43) | 134 (41) | 30 (31) |
| Obesity (n (%)) | 19 (15) | 80 (24) | 25 (26) |
| Diabetes (n (%)) | 15 (12) | 46 (14) | 21 (22) |
| Hypertension (n (%)) | 56 (44) | 183 (55) | 65 (67) ** |
| Past or active smoking (n (%)) | 12 (9.4) | 52 (16) | 15 (15) |
| Congestive heart failure (n (%)) | 12 (9.4) | 20 (6.1) | 9 (9.3) |
| Coronary artery disease (n (%)) | 15 (12) | 35 (11) | 9 (9.3) |
| Stroke or TIA (n (%)) | 10 (7.8) | 29 (8.8) | 13 (13) |
| 30-day death (n (%)) | 23 (18) | 64 (19) | 27 (28) |
| Oral intubation (n (%)]) | 5 (4.0) | 23 (7.0) | 3 (3.1) |
| Plasma creatinine (mg/dl) | 0.9 (0.7–1.1) | 0.9 (0.8–1.2) | 1.0 (0.8–1.3) |
| Blood urea nitrogen (mg/dl) | 16 (11–23) | 18 (13–26) | 21 (16–28) *** |
| eGFR (ml/min/1.73 m2) | 78 ± 28 | 73 ± 29 | 69 ± 27 |
| C-reactive protein (mg/dl) | 3.1 (0.8–7.0) | 5.1 (1.7–12.1) *** | 5.1 (1.6–11.1)* |
| Procalcitonin (ng/mL) | 0.02 (0.01–0.08) | 0.04 (0.01–0.13) | 0.05 (0.01–0.14) |
| Vital signs | |||
| Glasgow Coma Scale | 14.9 ± 0.6 | 14.8 ± 1.1 | 14.7 ± 1.1 |
| Body temperature (°C) | 36.8 ± 0.8 | 37.1 ± 1.0 ** | 37.3 ± 0.9 *** |
| Systolic blood pressure (mm Hg) | 132 ± 19 | 137 ± 22 | 135 ± 19 |
| Mean arterial pressure (mm Hg) | 96 ± 13 | 98 ± 14 | 96 ± 11 |
| Heart rate (beats per minute) | 82 ± 15 | 85 ± 17 | 83 ± 14 |
| Respiratory rate (acts per minute) | 20 ± 6 | 20 ± 6 | 20 ± 6 |
| Arterial oxygen saturation (%) | 96 (94–98) | 95 (91–97) *** | 95 (93–97) * |
| Variables used for calculating the aPNea score | |||
| Hospitalization COVID-independent (n (%)) | 23 (18) | 39 (12) | 21 (22) |
| Arterial O2 partial pressure (mm Hg) | 75 (64–84) | 67 (59–78) *** | 68 (61–80) * |
| Horowitz index | 332 ± 86 | 290 ± 96 *** | 279 ± 97 *** |
| Positive walk test (n (%)) | 16 (13) | 31 (9.4) | 1 (1.0) ** |
| Calculated A-a O2 gradient (mm Hg) | 40 (28–55) | 47 (33–93) ** | 53 (39–150) *** |
| Expected A-a O2 gradient (mm Hg) | 21 (17–24) | 22 (18–24) | 24 (19–25) * |
| Lung involvement ≥ 50% on X-ray (%) | 32 (25) | 113 (35) | 39 (42) |
| Lung involvement ≥ 50% on US (%) | 32 (25) | 113 (35) | 39 (42) |
| Lung involvement ≥ 50% on CT (%) | 32 (25) | 113 (35) | 39 (42) |
| D-dimer (ng/mL FEU) | 656 (423–1155) | 709 (453–1356) | 814 (559–1569) |
| Prognostic scores | |||
| aPNea score | 4 (1–8) | 7 (2–12)* | 6 (2–11) |
| NEWS2 score | 1 (1–3) | 3 (1–4)* | 2 (1–4) |
| Development Cohort n = 128 | Validation Cohort n = 330 | |
|---|---|---|
| AUROC (95% CI) | ||
| aPNea score model | 0.86 (0.78–0.93) | 0.79 (0.73–0.84) |
| NEWS2 score model | 0.72 (0.59–0.85) | 0.81 (0.75–0.87) |
| De Long’s test p-value | 0.025 | 0.443 |
| Brier score (95% CI) | ||
| aPNea score model | 0.108 (0.071–0.145) | 0.148 (0.124–0.172) |
| NEWS2 score model | 0.132 (0.092–0.171) | 0.131 (0.109–0.152) |
| Wald’s test p-value | 0.088 | 0.073 |
| Spiegelhalter test z-score (p-value) | ||
| aPNea score model | −0.010 (0.992) | 1.267 (0.205) |
| NEWS2 score model | −0.045 (0.964) | −1.559 (0.119) |
| Cohort | Score | AUROC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| Validation | aPNea | 0.690 (0.631–0.748) | 0.934 (0.878–0.990) | 0.445 (0.384–0.506) | 0.335 (0.271–0.398) | 0.958 (0.921–0.994) |
| NEWS2 | 0.685 (0.624–0.746) | 0.921 (0.860–0.982) | 0.449 (0.388–0.510) | 0.333 (0.270–0.950) | 0.950 (0.911–0.989) | |
| p-value | 0.837 | 0.200 | 0.906 | 0.917 | 0.675 | |
| External | aPNea | 0.703 (0.597–0.810) | 0.929 (0.833–1.024) | 0.478 (0.360–0.596) | 0.419 (0.297–0.542) | 0.943 (0.866–1.020) |
| NEWS2 | 0.539 (0.392–0.686) | 0.643 (0.465–0.820) | 0.435 (0.318–0.552) | 0.316 (0.195–0.436) | 0.750 (0.616–0.884) | |
| p-value | 0.001 | 0.005 | 0.406 | 0.005 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venturini, S.; Pontoni, E.; Carnelos, R.; Arcidiacono, D.; Da Ros, S.; De Santi, L.; Orso, D.; Cugini, F.; Fossati, S.; Callegari, A.; et al. Development and Validation of the Acute PNeumonia Early Assessment Score for Safely Discharging Low-Risk SARS-CoV-2-Infected Patients from the Emergency Department. J. Clin. Med. 2022, 11, 881. https://doi.org/10.3390/jcm11030881
Venturini S, Pontoni E, Carnelos R, Arcidiacono D, Da Ros S, De Santi L, Orso D, Cugini F, Fossati S, Callegari A, et al. Development and Validation of the Acute PNeumonia Early Assessment Score for Safely Discharging Low-Risk SARS-CoV-2-Infected Patients from the Emergency Department. Journal of Clinical Medicine. 2022; 11(3):881. https://doi.org/10.3390/jcm11030881
Chicago/Turabian StyleVenturini, Sergio, Elisa Pontoni, Rossella Carnelos, Domenico Arcidiacono, Silvia Da Ros, Laura De Santi, Daniele Orso, Francesco Cugini, Sara Fossati, Astrid Callegari, and et al. 2022. "Development and Validation of the Acute PNeumonia Early Assessment Score for Safely Discharging Low-Risk SARS-CoV-2-Infected Patients from the Emergency Department" Journal of Clinical Medicine 11, no. 3: 881. https://doi.org/10.3390/jcm11030881
APA StyleVenturini, S., Pontoni, E., Carnelos, R., Arcidiacono, D., Da Ros, S., De Santi, L., Orso, D., Cugini, F., Fossati, S., Callegari, A., Mancini, W., Tonizzo, M., Grembiale, A., Crapis, M., & Colussi, G. (2022). Development and Validation of the Acute PNeumonia Early Assessment Score for Safely Discharging Low-Risk SARS-CoV-2-Infected Patients from the Emergency Department. Journal of Clinical Medicine, 11(3), 881. https://doi.org/10.3390/jcm11030881







