Lung Congestion Severity in Kidney Transplant Recipients Is Not Affected by Arteriovenous Fistula Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrasound Assessment
2.2. Study Group
2.3. Statistical Analysis
3. Results
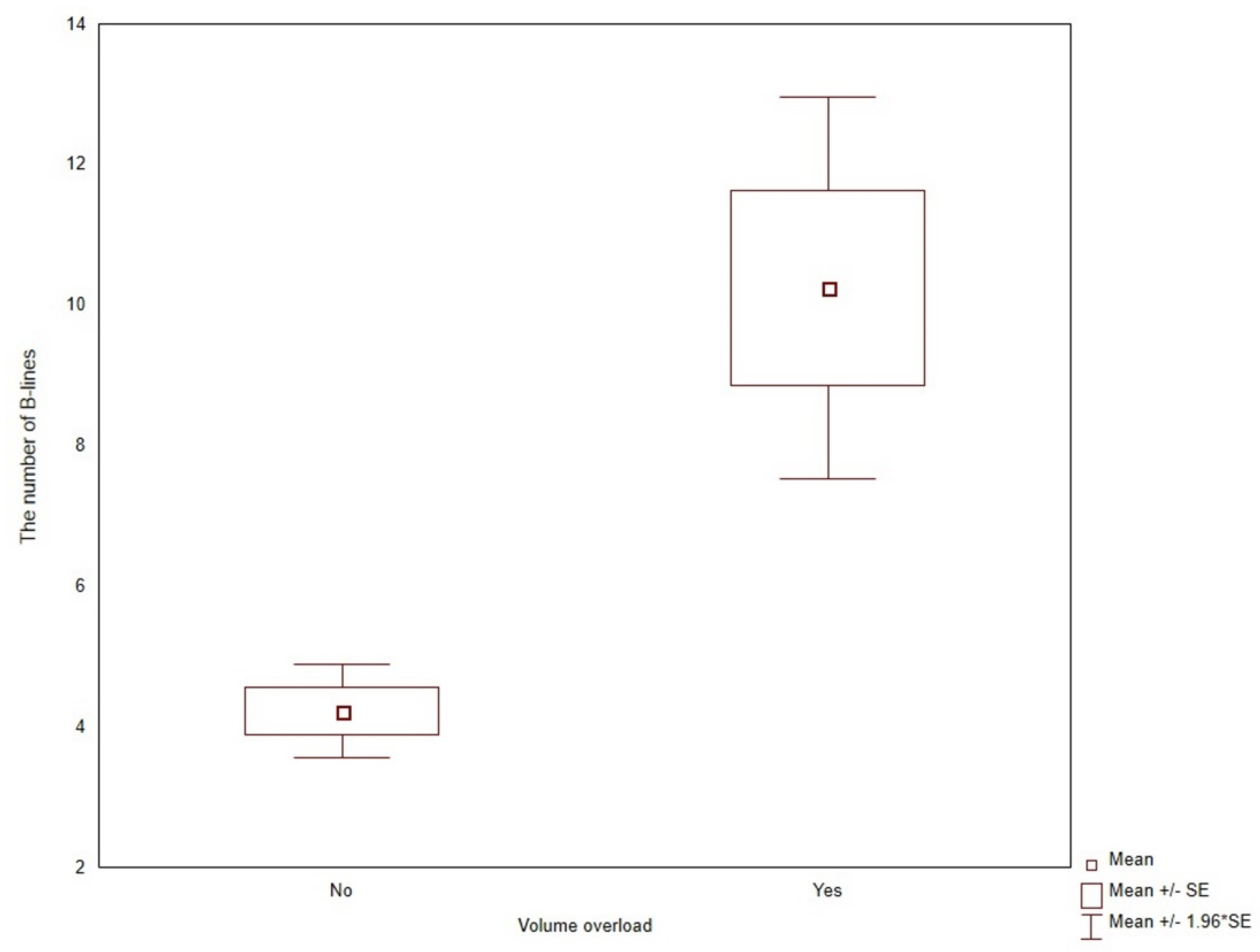
3.1. Lung Ultrasound B-Lines
3.2. Predictors of Lung Congestion
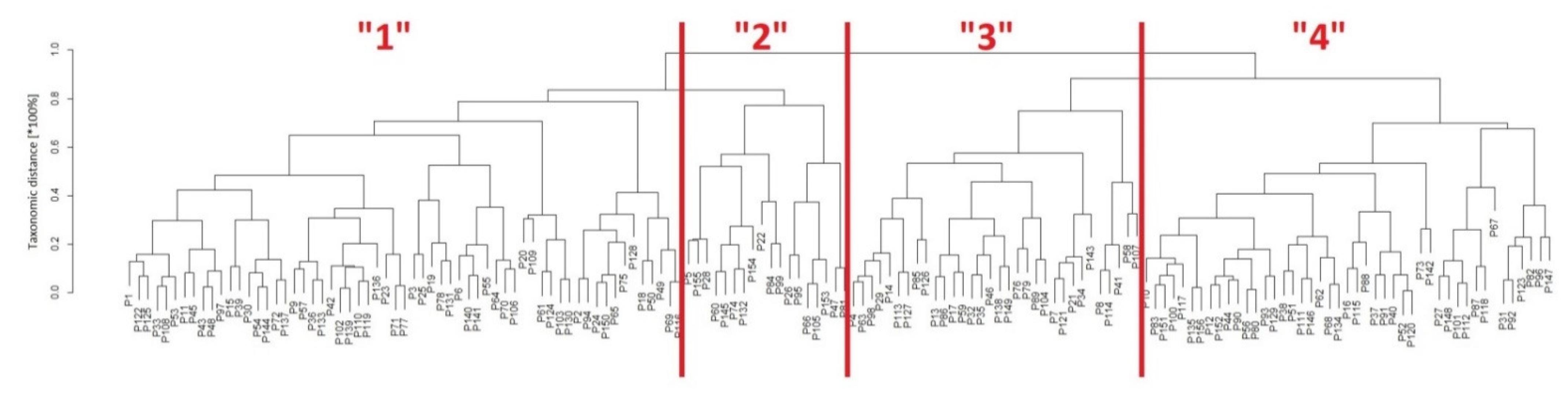
3.3. Classification of Patients with Taxonomy
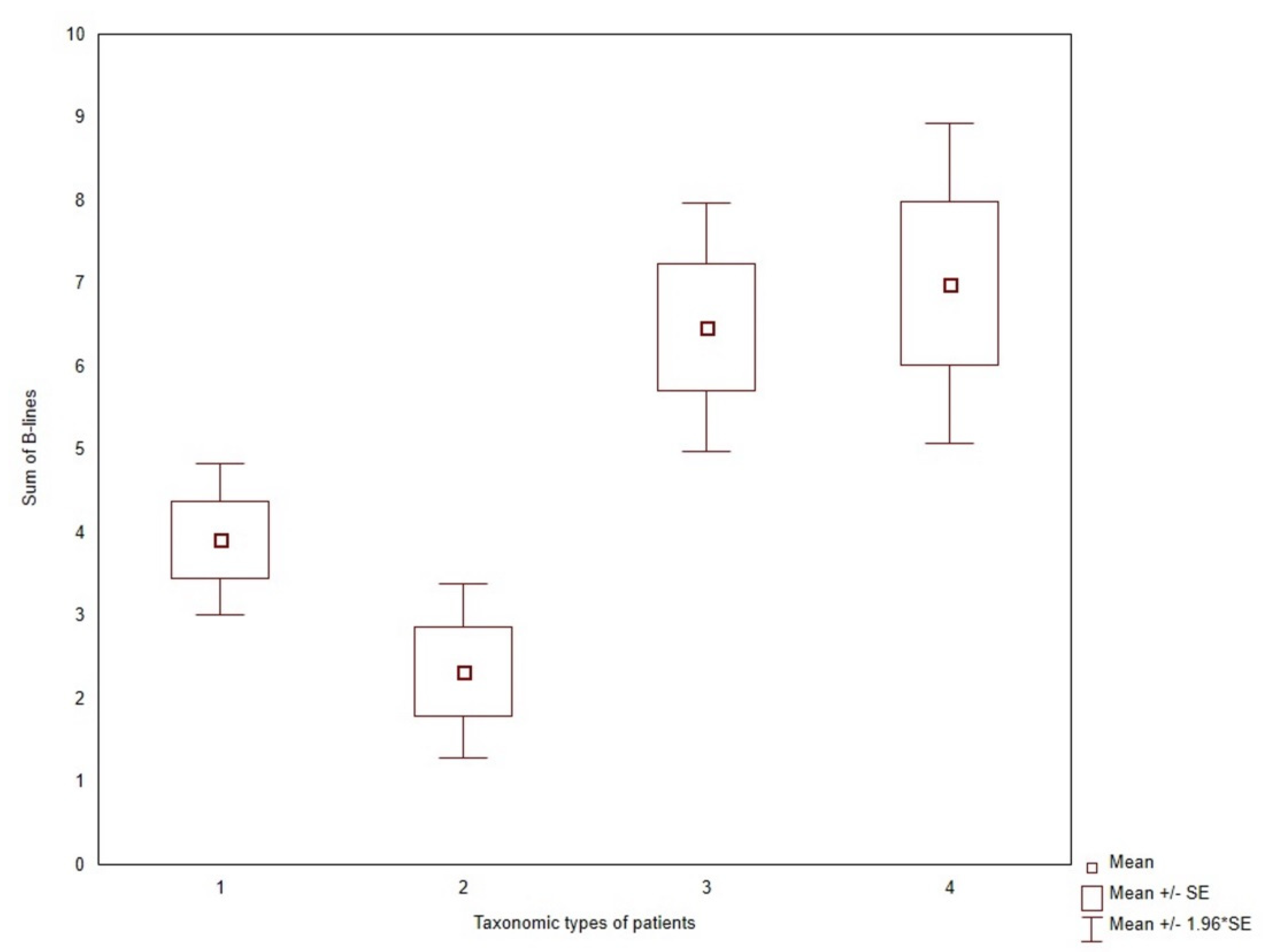
3.4. Lung Ultrasound B-Lines in Clusters of Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenstein, D.; Mézière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.-J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.; Dini, F.L.; Landi, P.; Picano, E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Öhman, J.; Harjola, V.-P.; Karjalainen, P.; Lassus, J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail. 2018, 5, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Torino, C.; Tripepi, R.; Tripepi, G.; D’Arrigo, G.; Postorino, M.; Gargani, L.; Sicari, R.; Picano, E.; Mallamaci, F.; et al. Pulmonary congestion predicts cardiac events and mortality in ESRD. J. Am. Soc. Nephrol. 2013, 24, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Torino, C.; Mallamaci, F.; Sarafidis, P.; Papagianni, A.; Ekart, R.; Hojs, R.; Klinger, M.; Letachowicz, K.; Fliser, D.; et al. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021, 100, 1325–1333. [Google Scholar] [CrossRef]
- Volpicelli, G.; Lamorte, A.; Villén, T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensiv. Care Med. 2020, 46, 1445–1448. [Google Scholar] [CrossRef]
- Covic, A.; Siriopol, D.; Voroneanu, L. Use of Lung Ultrasound for the Assessment of Volume Status in CKD. Am. J. Kidney Dis. 2018, 71, 412–422. [Google Scholar] [CrossRef]
- Marino, F.; Martorano, C.; Tripepi, R.; Bellantoni, M.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Subclinical pulmonary congestion is prevalent in nephrotic syndrome. Kidney Int. 2016, 89, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ciumanghel, A.; Siriopol, I.; Blaj, M.; Siriopol, D.; Gavrilovici, C.; Covic, A. B-lines score on lung ultrasound as a direct measure of respiratory dysfunction in ICU patients with acute kidney injury. Int. Urol. Nephrol. 2018, 50, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, V.; Tripepi, R.; Parlongo, G.; Mafrica, A.; Caridi, G.; Catalano, F.; Marino, F.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Lung ultrasound to detect and monitor pulmonary congestion in patients with acute kidney injury in nephrology wards: A pilot study. J. Nephrol. 2020, 33, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Girerd, N.; Coiro, S.; Lamiral, Z.; Rossignol, P.; Frimat, L.; Girerd, S. Evaluation of Subclinical Fluid Overload Using Lung Ultrasound and Estimated Plasma Volume in the Postoperative Period Following Kidney Transplantation. Transplant. Proc. 2018, 50, 1336–1341. [Google Scholar] [CrossRef]
- Vanderweckene, P.; Weekers, L.; Lancellotti, P.; Jouret, F. Controversies in the management of the haemodialysis-related arteriovenous fistula following kidney transplantation. Clin. Kidney J. 2018, 11, 406–412. [Google Scholar] [CrossRef]
- Malik, J.; Valerianova, A.; Tuka, V.; Trachta, P.; Bednarova, V.; Hruskova, Z.; Slavikova, M.; Rosner, M.H.; Tesar, V. The effect of high-flow arteriovenous fistulas on systemic haemodynamics and brain oxygenation. ESC Heart Fail. 2021, 8, 2165–2171. [Google Scholar] [CrossRef]
- Valerianova, A.; Malik, J.; Janeckova, J.; Kovarova, L.; Tuka, V.; Trachta, P.; Lachmanova, J.; Hladinova, Z.; Hruskova, Z.; Tesar, V. Reduction of arteriovenous access blood flow leads to biventricular unloading in haemodialysis patients. Int. J. Cardiol. 2021, 334, 148–153. [Google Scholar] [CrossRef]
- Mudoni, A.; Caccetta, F.; Caroppo, M.; Musio, F.; Accogli, A.; Zacheo, M.D.; Burzo, M.D.; Gallieni, M.; Nuzzo, V. Echo color Doppler ultrasound: A valuable diagnostic tool in the assessment of arteriovenous fistula in hemodialysis patients. J. Vasc. Access 2016, 17, 446–452. [Google Scholar] [CrossRef]
- Jambrik, Z.; Monti, S.; Coppola, V.; Agricola, E.; Mottola, G.; Miniati, M.; Picano, E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am. J. Cardiol. 2004, 93, 1265–1270. [Google Scholar] [CrossRef]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef]
- Gargani, L.; Sicari, R.; Raciti, M.; Serasini, L.; Passera, M.; Torino, C.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; et al. Efficacy of a remote web-based lung ultrasound training for nephrologists and cardiologists: A LUST trial sub-project. Nephrol. Dial. Transplant. 2016, 31, 1982–1988. [Google Scholar] [CrossRef]
- Abreo, K.; Sachdeva, B.; Abreo, A.P. To ligate or not to ligate hemodialysis arteriovenous fistulas in kidney transplant patients. J. Vasc. Access 2021, 22, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Letachowicz, K.; Banasik, M.; Królicka, A.; Mazanowska, O.; Gołębiowski, T.; Augustyniak-Bartosik, H.; Zmonarski, S.; Kamińska, D.; Kuriata-Kordek, M.; Krajewska, M. Vascular Access Perspectives in Patients After Kidney Transplantation. Front. Surg. 2021, 8, 640986. [Google Scholar] [CrossRef] [PubMed]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. Inflammation is an amplifier of lung congestion by high lv filling pressure in hemodialysis patients: A longitudinal study. J. Nephrol. 2020, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.2. Available online: https://cran.rproject.org/web/packages/cluster/cluster.pdf (accessed on 5 December 2021).
- R Core Team. A Language and Environment for Statistical Computing, version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org (accessed on 5 December 2021).
- Marczewski, E.; Steinhaus, H. On a certain distance of sets and the corresponding distance of functions. Colloq. Math. 1958, 6, 319–327. [Google Scholar] [CrossRef]
- Tukiendorf, A.; Kaźmierski, R.; Michalak, S. The taxonomy statistic uncovers novel clinical patterns in a population of ischemic stroke patients. PLoS ONE 2013, 8, e69816. [Google Scholar] [CrossRef][Green Version]
- Miglioranza, M.H.; Gargani, L.; Sant’Anna, R.T.; Rover, M.M.; Martins, V.M.; Mantovani, A.; Weber, C.; Moraes, M.A.; Feldman, C.J.; Kalil, R.A.K.; et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1141–1151. [Google Scholar] [CrossRef]
- Picano, E.; Scali, M.C. The lung water cascade in heart failure. Echocardiography 2017, 34, 1503–1507. [Google Scholar] [CrossRef]
- Dwyer, K.H.; Merz, A.; Lewis, E.F.; Claggett, B.L.; Crousillat, D.R.; Lau, E.S.; Silverman, M.B.; Peck, J.; Rivero, J.; Cheng, S.; et al. Pulmonary Congestion by Lung Ultrasound in Ambulatory Patients with Heart Failure with Reduced or Preserved Ejection Fraction and Hypertension. J. Card. Fail. 2018, 24, 219–226. [Google Scholar] [CrossRef]
- Torino, C.; Tripepi, R.; Loutradis, C.; Sarafidis, P.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrol. Dial. Transplant. 2021, 36, 2321–2326. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Franci, B.; Evangelista, I.; Lucani, B.; Nuti, R.; Pellicori, P. Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin. Res. Cardiol. 2020, 109, 1423–1433. [Google Scholar] [CrossRef]
- Brainin, P.; Claggett, B.; Lewis, E.F.; Dwyer, K.H.; Merz, A.A.; Silverman, M.B.; Swamy, V.; Biering-Sørensen, T.; Rivero, J.; Cheng, S.; et al. Body mass index and B-lines on lung ultrasonography in chronic and acute heart failure. ESC Heart Fail. 2020, 7, 1201–1209. [Google Scholar] [CrossRef]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 2005–2011. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart. Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Platz, E.; Merz, A.; Jhund, P.; Vazir, A.; Campbell, R.; Mcmurray, J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Miglioranza, M.H.; Picano, E.; Badano, L.; Sant’Anna, R.; Rover, M.; Zaffaroni, F.; Sicari, R.; Kalil, R.K.; Leiria, T.L.; Gargani, L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int. J. Cardiol. 2017, 240, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.; Lee, M.; Merz, A.; Silverman, M.; Swamy, V.; et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC Heart Fail. 2019, 7, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Pugliese, N.R.; Frassi, F.; Frumento, P.; Poggianti, E.; Mazzola, M.; De Biase, N.; Landi, P.; Masi, S.; Taddei, S.; et al. Prognostic value of lung ultrasound in patients hospitalized for heart disease irrespective of symptoms and ejection fraction. ESC Heart Fail. 2021, 8, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Šrajer, L.L.; Marko, K.; Hojs, N.V.; Piko, N.; Bevc, S.; Hojs, R.; Ekart, R. Lung ultrasound, hemoglobin, and NT-proBNP in peritoneal dialysis patients. Clin. Nephrol. 2021, 96, 85–88. [Google Scholar] [CrossRef]
- Saad, M.M.; Kamal, J.; Moussaly, E.; Karam, B.; Mansour, W.; Gobran, E.; Abbasi, S.H.; Mahgoub, A.; Singh, P.; Hardy, R.; et al. Relevance of B-Lines on Lung Ultrasound in Volume Overload and Pulmonary Congestion: Clinical Correlations and Outcomes in Patients on Hemodialysis. Cardiorenal Med. 2018, 8, 83–91. [Google Scholar] [CrossRef]
- Pellicori, P.; Shah, P.; Cuthbert, J.; Urbinati, A.; Zhang, J.; Kallvikbacka-Bennett, A.; Clark, A.L.; Cleland, J.G. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur. J. Heart Fail. 2019, 21, 904–916. [Google Scholar] [CrossRef]
- Rao, N.N.; Stokes, M.B.; Rajwani, A.; Ullah, S.; Williams, K.; King, D.; Macaulay, E.; Russell, C.H.; Olakkengil, S.; Carroll, R.P.; et al. Effects of Arteriovenous Fistula Ligation on Cardiac Structure and Function in Kidney Transplant Recipients. Circulation 2019, 139, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Hetz, P.; Pirklbauer, M.; Müller, S.; Posch, L.; Gummerer, M.; Tiefenthaler, M. Prophylactic Ligature of AV Fistula Prevents High Output Heart Failure after Kidney Transplantation. Am. J. Nephrol. 2020, 51, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Golper, T.A.; Hartle, P.M.; Bian, A. Arteriovenous fistula creation may slow estimated glomerular filtration rate trajectory. Nephrol. Dial. Transplant. 2015, 30, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Weekers, L.; Vanderweckene, P.; Castanares-Zapatero, D.; Bonvoisin, C.; Hamoir, E.; Maweja, S.; Krzesinski, J.-M.; Delanaye, P.; Pottel, H.; Jouret, F. The closure of arteriovenous fistula in kidney transplant recipients is associated with an acceleration of kidney function decline. Nephrol. Dial. Transplant. 2017, 32, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bardowska, K.; Letachowicz, K.; Kamińska, D.; Kusztal, M.; Gołębiowski, T.; Królicki, T.; Zajdel, K.; Mazanowska, O.; Janczak, D.; Krajewska, M. The attitude of kidney transplant recipients towards elective arteriovenous fistula ligation. PLoS ONE 2020, 15, e0234931. [Google Scholar] [CrossRef]
- Trampuž, B.V.; Arnol, M.; Gubenšek, J.; Ponikvar, R.; Ponikvar, J.B. A national cohort study on hemodialysis arteriovenous fistulas after kidney transplantation–Long-term patency, use and complications. BMC Nephrol. 2021, 22, 344. [Google Scholar] [CrossRef]
- Letachowicz, K.; Kusztal, M.; Gołębiowski, T.; Letachowicz, W.; Weyde, W.; Klinger, M. External dilator-assisted banding for high-flow hemodialysis arteriovenous fistula. Ren. Fail. 2016, 38, 1067–1070. [Google Scholar] [CrossRef]
- Bojakowski, K.; Gziut, A.; Góra, R.; Foroncewicz, B.; Kaźmierczak, S.; Kasprzak, D.; Małyszko, J.; Andziak, P. To Close, Observe, or Reconstruct: The Third Way of Managing Dialysis Fistula Aneurysms in Kidney Transplant Recipients. J. Clin. Med. 2021, 10, 4567. [Google Scholar] [CrossRef]
- Iwakura, K.; Onishi, T. A practical guide to the lung ultrasound for the assessment of congestive heart failure. J. Echocardiogr. 2021, 19, 195–204. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Q.; Zhi, G.; Zhang, L.; Huang, D.; Dangsheng, H.; Zhang, M. The application of lung ultrasound in acute decompensated heart failure in heart failure with preserved and reduced ejection fraction. Echocardiography 2017, 34, 1462–1469. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | AVF Present n = 82 | AVF Absent n = 74 | p-Value |
|---|---|---|---|
| Sum of B-lines | 5.5 ± 5.0 | 4.8 ± 4.9 | 0.3507 |
| Age (years) | 56.4 ± 10.9 | 54.1 ± 12.2 | 0.2137 |
| Male, n (%) | 56 (68.3%) | 42 (56.7 %) | 0.1397 |
| BMI (kg/m2) | 27.0 ± 4.3 | 26.6 ± 5.4 | 0.5612 |
| Serum creatinine concentration (mg/dL) | 1.45 ± 0.50 | 1.47 ± 0.55 | 0.7658 |
| eGFR (mL/min/1.73m2) | 53.5 ± 14.7 | 53.2 ± 17.1 | 0.9802 |
| Duration between study visit and transplantation (months) | 90 ± 57 | 129 ± 66 | 0.0001 |
| Duration between study visit and RRT initiation (months) | 144 ± 81 | 174 ± 72 | 0.0154 |
| First transplantation, n (%) | 68 (82.9%) | 64 (86.5%) | 0.8753 |
| Diabetes mellitus, n (%) | 19 (23.2%) | 15 (20.3%) | 0.6641 |
| Heart disease, n (%) | 32 (39%) | 20 (27%) | 0.1141 |
| Charlson comorbidity index | 4.5 ± 2.0 | 4.3 ± 1.6 | 0.4179 |
| Smoking, current or previous, n (%) | 38 (46.3%) | 34 (45.9%) | 0.9114 |
| Medications, n (%) | |||
| Steroids, calcineurin inhibitor, mycophenolate, n (%) | 66 (80.5%) | 53 (71.6%) | 0.2663 |
| Antihypertensive, n (%) | 75 (91.5%) | 66 (89.2%) | 0.8346 |
| Statins, n (%) | 33 (40.2%) | 26 (35.1%) | 0.6229 |
| Antiplatelet/anticoagulants, n (%) | 24 (29.3%) | 14 (18.9%) | 0.1879 |
| Characteristics | No Congestion n = 101 | Mild Congestion n = 49 | Moderate Congestion n = 6 | p-Value |
|---|---|---|---|---|
| Sum of B-lines | 2.2 ± 1.5 | 9.4 ± 2.7 | 20.3 ± 4.7 | <0.001 |
| Age (years) | 53.9 ± 12 | 57.1 ± 10.4 | 64.3 ± 4 | 0.0356 |
| Male, n (%) | 59 (58.4%) | 35 (71.4%) | 4 (66.7%) | 0.2965 |
| BMI (kg/m2) | 25.9 ± 4.6 | 28.5 ± 5 | 28.9 ± 4.6 | 0.0048 |
| Serum creatinine concentration, (mg/dL) | 1.39 ± 0.46 | 1.58 ± 0.64 | 1.67 ± 0.33 | 0.044 |
| eGFR (mL/min/1.73 m2) | 55.3 ± 15.5 | 50.7 ± 16.3 | 42.2 ± 10.9 | 0.0503 |
| Duration between study visit and transplantation (months) | 106 ± 64 | 115 ± 677 | 95 ± 43 | 0.65 |
| Duration between study visit and RRT initiation, (months) | 157 ± 82 | 164 ± 73 | 141 ± 54 | 0.68 |
| Prior diabetes mellitus, n (%) | 17 (16.8%) | 13 (26.5%) | 4 (66.7%) | 0.0101 |
| Prior heart disease, n (%) | 22 (21.8%) | 25 (51%) | 5 (83.3%) | <0.0001 |
| Charlson comorbidity index | 4.1 ± 1.7 | 4.7 ± 1.7 | 6.8 ± 1.5 | 0.0005 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Risk Factor | OR | 95% CI | p | OR | 95% CI | p |
| Charlson comorbidity index | 1.21 | (1.03,1.44) | 0.0233 | 1.07 | (0.89,1.29) | 0.4537 |
| Heart disease | 2.45 | (1.31,4.58) | 0.0049 | 1.24 | (0.61,2.50) | 0.5423 |
| Symptoms of volume overload | 7.50 | (3.32,17.0) | <0.0001 | 5.90 | (2.43,14.3) | 0.0001 |
| BMI | 1.09 | (1.03,1.16) | 0.0026 | 1.09 | (1.03,1.17) | 0.0046 |
| eGFR | 0.98 | (0.96,0.99) | 0.0057 | 1.29 | (0.51,3.26) | 0.5721 |
| Uric acid | 1.44 | (1.16,1.78) | 0.0009 | 0.97 | (0.94,1.01) | 0.1132 |
| Cholesterol | 0.993 | (0.988,0.999) | 0.0160 | 0.994 | (0.988,1.000) | 0.0452 |
| Donor age | 1.03 | (1.01,1.05) | 0.0122 | 1.01 | (0.99,1.03) | 0.4657 |
| Risk Factor | n = 61 | n = 18 | n = 32 | n = 45 | p-Value |
|---|---|---|---|---|---|
| Charlson comorbidity index | 3.36 ± 0.88 | 2.83 ± 0.79 | 4.28 ± 1.20 | 6.44 ± 1.49 | <0.0001 |
| BMI | 27.5 ± 5.22 | 20.9 ± 2.03 | 29.2 ± 3.54 | 26.6 ± 4.06 | <0.0001 |
| Cholesterol | 251 ± 54.3 | 212 ± 32.8 | 166 ± 17.0 | 222 ± 38.5 | <0.0001 |
| Males | 59% | 56% | 34% | 38% | 0.0555 |
| Age | 51.0 ± 10.1 | 45.6 ± 10.2 | 56.2 ± 10.9 | 64.4 ± 7.5 | <0.0001 |
| Heart disease | 18% | 17% | 47% | 51% | 0.0004 |
| Diabetes | 13% | 0% | 28% | 38% | 0.0013 |
| Symptoms of volume overload | 8% | 11% | 13% | 29% | 0.0261 |
| USBLs | 3.9 ± 3.6 | 2.3 ± 2.3 | 6.5 ± 4.3 | 7.0 ± 6.6 | 0.0003 |
| Presence of AVF | 41% | 44% | 66% | 62% | 0.0539 |
| AVF burden | 80.1 ± 58.9 | 83.3 ± 86.6 | 108.1 ± 69.7 | 120.8 ± 83.3 | 0.0247 |
| Weight | 80.4 ± 16.5 | 61.7 ± 11.3 | 87.3 ± 12.7 | 76.4 ± 12.9 | <0.0001 |
| Waist | 94.2 ± 13.5 | 78.4 ± 10.1 | 102 ± 12.3 | 96.0 ± 12.5 | <0.0001 |
| Serum creatinine | 1.45 ± 0.42 | 1.59 ± 0.76 | 1.43 ± 0.40 | 1.45 ± 0.61 | 0.7558 |
| eGFR | 54.0 ± 16.0 | 50.4 ± 17.7 | 53.7 ± 14.5 | 53.4 ± 16.2 | 0.8715 |
| Platelets | 239 ± 66.6 | 216 ± 37.0 | 205 ± 51.7 | 198 ± 53.9 | 0.0021 |
| Uric acid | 7.12 ± 1.14 | 6.25 ± 1.37 | 7.21 ± 1.36 | 6.90 ± 1.42 | 0.0598 |
| Total protein | 7.22 ± 0.45 | 7.16 ± 0.50 | 7.00 ± 0.48 | 6.94 ± 0.53 | 0.0176 |
| Albumin | 4.33 ± 0.26 | 4.34 ± 0.26 | 4.28 ± 0.32 | 4.15 ± 0.30 | 0.0060 |
| HDL cholesterol | 63.0 ± 17.7 | 61.9 ± 10.6 | 52.5 ± 10.7 | 63.8 ± 15.1 | 0.0061 |
| LDL cholesterol | 153 ± 43.4 | 127 ± 28.4 | 85.6 ± 22.5 | 123 ± 33.0 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letachowicz, K.; Królicka, A.; Tukiendorf, A.; Banasik, M.; Kamińska, D.; Gołębiowski, T.; Kuriata-Kordek, M.; Madziarska, K.; Mazanowska, O.; Krajewska, M. Lung Congestion Severity in Kidney Transplant Recipients Is Not Affected by Arteriovenous Fistula Function. J. Clin. Med. 2022, 11, 842. https://doi.org/10.3390/jcm11030842
Letachowicz K, Królicka A, Tukiendorf A, Banasik M, Kamińska D, Gołębiowski T, Kuriata-Kordek M, Madziarska K, Mazanowska O, Krajewska M. Lung Congestion Severity in Kidney Transplant Recipients Is Not Affected by Arteriovenous Fistula Function. Journal of Clinical Medicine. 2022; 11(3):842. https://doi.org/10.3390/jcm11030842
Chicago/Turabian StyleLetachowicz, Krzysztof, Anna Królicka, Andrzej Tukiendorf, Mirosław Banasik, Dorota Kamińska, Tomasz Gołębiowski, Magdalena Kuriata-Kordek, Katarzyna Madziarska, Oktawia Mazanowska, and Magdalena Krajewska. 2022. "Lung Congestion Severity in Kidney Transplant Recipients Is Not Affected by Arteriovenous Fistula Function" Journal of Clinical Medicine 11, no. 3: 842. https://doi.org/10.3390/jcm11030842
APA StyleLetachowicz, K., Królicka, A., Tukiendorf, A., Banasik, M., Kamińska, D., Gołębiowski, T., Kuriata-Kordek, M., Madziarska, K., Mazanowska, O., & Krajewska, M. (2022). Lung Congestion Severity in Kidney Transplant Recipients Is Not Affected by Arteriovenous Fistula Function. Journal of Clinical Medicine, 11(3), 842. https://doi.org/10.3390/jcm11030842








