Incidence of Tick-Borne Encephalitis during the COVID-19 Pandemic in Selected European Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. COVID-19 Incidence
- I—incidence per 100,000 inhabitants;
- k—number of recorded cases,
- p—total number of inhabitants.
2.2. TBE Incidence
2.3. Statistical Analysis
3. Results
3.1. TBE Incidence in 2015–2019
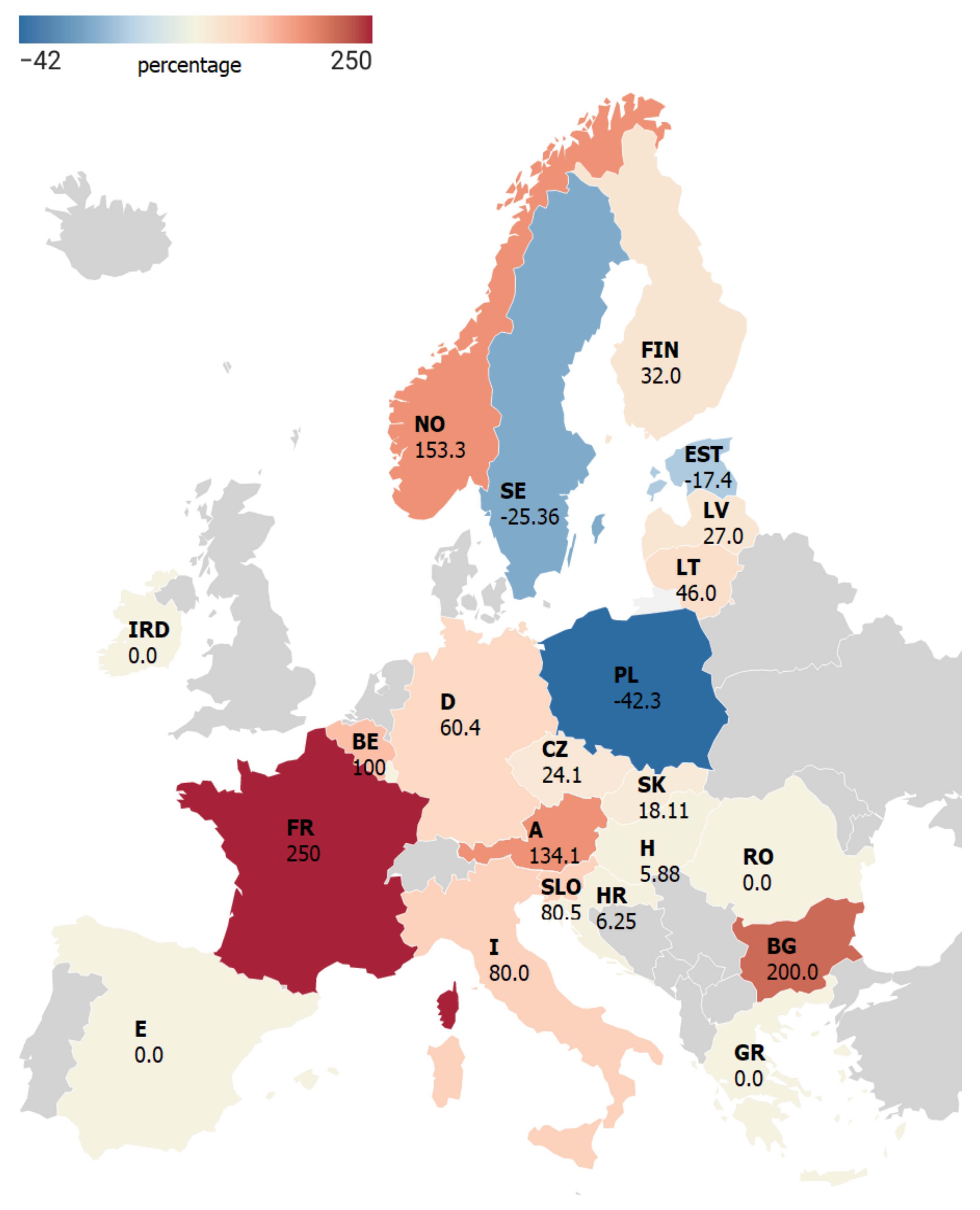
3.2. TBE Incidence in the Pandemic Year 2020
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Cham, Germany, 2018; ISBN 9783319637594. [Google Scholar]
- Wilhelmsson, P.; Lindblom, P.; Fryland, L.; Nyman, D.; Jaenson, T.G.; Forsberg, P.; Lindgren, P.-E. Ixodes ricinus ticks removed from humans in Northern Europe: Seasonal pattern of infestation, attachment sites and duration of feeding. Parasites Vectors 2013, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Opalińska, P.; Wierzbicka, A.; Asman, M.; Rączka, G.; Dyderski, M.K.; Nowak-Chmura, M. Fivefold higher abundance of ticks (Acari: Ixodida) on the European roe deer (Capreolus capreolus L.) forest than field ecotypes. Sci. Rep. 2021, 11, 10649. [Google Scholar] [CrossRef] [PubMed]
- Mihalca, A.D.; Sándor, A.D. The role of rodents in the ecology of Ixodes ricinus and associated pathogens in Central and Eastern Europe. Front. Cell. Infect. Microbiol. 2013, 3, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick-Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Golovljova, I.; Vene, S.; Jaenson, T.G. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasites Vectors 2014, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Rosef, O.; Paulauskas, A.; Radzijevskaja, J. Prevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in questing Ixodes ricinus ticks in relation to the density of wild cervids. Acta Veter. Scand. 2009, 51, 47. [Google Scholar] [CrossRef] [Green Version]
- Kirczuk, L.; Piotrowski, M.; Rymaszewska, A. Detection of tick-borne pathogens of the genera Rickettsia, Anaplasma and Francisella in Ixodes ricinus ticks in Pomerania (Poland). Pathogens 2021, 10, 901. [Google Scholar] [CrossRef]
- Grochowska, A.; Milewski, R.; Pancewicz, S.; Dunaj, J.; Czupryna, P.; Milewska, A.J.; Róg-Makal, M.; Grygorczuk, S.; Moniuszko-Malinowska, A. Comparison of tick-borne pathogen prevalence in Ixodes ricinus ticks collected in urban areas of Europe. Sci. Rep. 2020, 10, 6975. [Google Scholar] [CrossRef]
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Slovák, M.; Drexler, J.F.; Klempa, B. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick-borne Dis. 2020, 11, 101414. [Google Scholar] [CrossRef]
- Nosek, J. The ecology, bionomics, behaviour and public healh importance of Dermacentor marginatus and D. reticulatus ticks. Wiad. Parazytol. 1972, 18, 721–725. [Google Scholar] [PubMed]
- Heyman, P.; Cochez, C.; Hofhuis, A.; Van der Giessen, J.; Sprong, H.; Porter, S.R.; Losson, B.; Saegerman, C.; Donoso-Mantke, O.; Niedrig, M.; et al. A clear and present danger: Tick-borne diseases in Europe. Expert Rev. Anti-Infect. Ther. 2010, 8, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1800201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogovič, P.; Stupica, D.; Rojko, T.; Lotrič-Furlan, S.; Avšič-Županc, T.; Kastrin, A.; Lusa, L.; Strle, F. The long-term outcome of tick-borne encephalitis in Central Europe. Ticks Tick-Borne Dis. 2018, 9, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Tokarevich, N.K.; Tronin, A.A.; Blinova, O.V.; Buzinov, R.V.; Boltenkov, V.P.; Yurasova, E.D.; Nurse, J. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob. Health Action 2011, 4, 8448. [Google Scholar] [CrossRef]
- Kozlova, I.; Verkhozina, M.; Demina, T.; Dzhioev, Y.; Tkachev, S.; Karan, L.; Doroshchenko, E.; Lisak, O.; Suntsova, O.; Paramonov, A.; et al. Genetic and biological properties of original TBEV strains group circulating in Eastern Siberia. Encephalitis 2013, 283, 95–112. [Google Scholar]
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick-Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Garlicki, A. Choroby Infekcyjne Ośrodkowego Układu Nerwowego. In Choroby Zakaźne i Pasożytnicze; Boroń-Kaczmarska, A., Wiercińska-Drapało, A., Eds.; PZWL: Warszawa, Poland, 2017. [Google Scholar]
- Bogovic, P. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef]
- Kohlmaier, B.; Schweintzger, N.A.; Sagmeister, M.G.; Švendová, V.; Kohlfürst, D.S.; Sonnleitner, A.; Leitner, M.; Berghold, A.; Schmiedberger, E.; Fazekas, F.; et al. Clinical characteristics of patients with tick-borne encephalitis (TBE): A European Multicentre Study from 2010 to 2017. Microorganisms 2021, 9, 1420. [Google Scholar] [CrossRef]
- Czupryna, P.; Moniuszko, A.; Pancewicz, S.A.; Grygorczuk, S.; Kondrusik, M.; Zajkowska, J. Tick-borne encephalitis in Poland in years 1993–2008—Epidemiology and clinical presentation. A retrospective study of 687 patients. Eur. J. Neurol. 2010, 18, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Radzišauskienė, D.; Urbonienė, J.; Kaubrys, G.; Andruškevičius, S.; Jatužis, D.; Matulytė, E.; Žvirblytė-Skrebutienė, K. The epidemiology, clinical presentation, and predictors of severe tick-borne encephalitis in Lithuania, a highly endemic country: A retrospective study of 1040 patients. PLoS ONE 2020, 15, e0241587. [Google Scholar] [CrossRef]
- Amicizia, D.; Domnich, A.; Panatto, D.; Lai, P.L.; Cristina, M.L.; Avio, U.; Gasparini, R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccines Immunother. 2013, 9, 1163–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchar, E.; Zajkowska, J.; Flisiak, R.; Mastalerz-Migas, A.; Rosińska, M.; Szenborn, L.; Wdówik, P.; Walusiak-Skorupa, J. Epidemiology, diagnosis, and prevention of tick-borne encephalitis in Poland and selected european countries—A position statement of the polish group of experts. Med. Pr. 2021, 72, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borde, J.P.; Kaier, K.; Hehn, P.; Böhmer, M.M.; Kreusch, T.M.; Dobler, G. Tick-borne encephalitis virus infections in Germany. Seasonality and in-year patterns. A retrospective analysis from 2001-2018. PLoS ONE 2019, 14, e0224044. [Google Scholar] [CrossRef] [PubMed]
- Audi, A.; Al Ibrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Seasonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front. Public Health 2020, 8, 567184. [Google Scholar] [CrossRef] [PubMed]
- Wosik, J.; Clowse, M.E.B.; Overton, R.; Adagarla, B.; Economou-Zavlanos, N.; Cavalier, J.; Henao, R.; Piccini, J.P.; Thomas, L.; Pencina, M.J.; et al. Impact of the COVID-19 pandemic on patterns of outpatient cardiovascular care. Am. Heart J. 2021, 231, 1–5. [Google Scholar] [CrossRef]
- Piątkowska, K.; Zimmermann, A.; Pilarska, A. Limitation of patients’ rights during the COVID-19 pandemics in Poland. Eur. J. Transl. Clin. Med. 2021, 4, 79–85. [Google Scholar] [CrossRef]
- Wormser, G.P.; Jacobson, E.; Shanker, E.M. Negative impact of the COVID-19 pandemic on the timely diagnosis of tick-borne infections. Diagn. Microbiol. Infec. Dis. 2020, 99, 115226. [Google Scholar] [CrossRef]
- Novak, C.B.; Scheeler, V.M.; Aucott, J.N. Lyme disease in the era of COVID-19: A delayed diagnosis and risk for complications. Case Rep. Infect. Dis. 2021, 2021, 6699536. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Data—COVID-19. Available online: https://ec.europa.eu/eurostat/web/covid-19/data (accessed on 4 November 2021).
- Eurostat. Database—Population and Demography. Available online: https://ec.europa.eu/eurostat/web/population-demography/demography-population-stock-balance/database (accessed on 4 November 2021).
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 4 November 2021).
- Eurostat. Health Personnel, Nursing and Caring Professionals. Available online: https://ec.europa.eu/eurostat/databrowser/view/HLTH_RS_PRSNS__custom_88217/bookmark/table?lang=en&bookmarkId=f209f632-69ec-4656-829e-f6e562c9888a (accessed on 4 November 2021).
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e0008318. [Google Scholar] [CrossRef] [PubMed]
- Stefanoff, P.; Orlíková, H.; Príkazský, V.; Beneš, Č.; Rosińska, M. Cross-border surveillance differences: Tick-borne encephalitis and Lyme borreliosis in the Czech Republic and Poland, 1999-2008. Central Eur. J. Public Health 2014, 22, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotrbova, K.; Lunackova, J. Seroprevalence of tick-borne encephalitis and Lyme borreliosis in a defined Czech population. Int. J. Infect. Dis. 2019, 79, 135. [Google Scholar] [CrossRef] [Green Version]
- Kiffner, C.; Zucchini, W.; Schomaker, P.; Vor, T.; Hagedorn, P.; Niedrig, M.; Rühe, F. Determinants of tick-borne encephalitis in counties of southern Germany, 2001–2008. Int. J. Health Geogr. 2010, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zając, Z.; Kulisz, J.; Bartosik, K.; Woźniak, A.; Dzierżak, M.; Khan, A. Environmental determinants of the occurrence and activity of Ixodes ricinus ticks and the prevalence of tick-borne diseases in eastern Poland. Sci. Rep. 2021, 11, 15472. [Google Scholar] [CrossRef] [PubMed]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Hofmann, H.; Kunz, C. Early serological diagnosis of virus infections. Wien. Klin. Wochenschr. 1973, 85, 490–493. [Google Scholar]
- Korotkov, Y.S.; Nikitin, A.Y.; Antonova, A.M. Role of climatic factors in long-term dynamics of tick-borne encephalitis morbidity in the city of Irkutsk. Byull. Vostochno-Sibirskogo Nauchnogo Tsentr. Sibirsk. Otdel. Ross. Akad. Med. Nauk. 2007, 3, 121–125. [Google Scholar]
- Lindgren, E. Climate and tickborne encephalitis. Conserv. Ecol. 1998, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Dörrbecker, B.; Dobler, G.; Spiegel, M.; Hufert, F.T. Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med. Infect. Dis. 2010, 8, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Kozuch, O.; Zuffová, E.; Elecková, E.; Hails, R.S.; Nuttall, P.A. Tick-Borne Encephalitis Virus Transmission between Ticks Cofeeding on Specific Immune Natural Rodent Hosts. Virology 1997, 235, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, C.; Bastic, V.; Maeder, G.; Patalas, E.; Gern, L. Microclimate and the zoonotic cycle of tick-borne encephalitis virus in Switzerland. J. Med. Entomol. 2011, 48, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Canham, C.D.; Oggenfuss, K.; Winchcombe, R.J.; Keesing, F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006, 4, 40145. [Google Scholar] [CrossRef]
- Tutiempo Network, S.L. Weather in Europe. Available online: https://en.tutiempo.net/europe.html (accessed on 5 November 2021).
- Steele, G.M.; Randolph, S.E. An experimental evaluation of conventional control measures against the sheep tick, Ixodes ricinus (L.) (Acari: Ixodidae). I. A unimodal seasonal activity pattern. Bull. Entomol. Res. 1985, 75, 489–500. [Google Scholar] [CrossRef]
- Dorko, E.; Hockicko, J.; Rimárová, K.; Bušová, A.; Popaďák, P.; Popaďáková, J.; Schréter, I. Milk outbreaks of tick-borne encephalitis in Slovakia, 2012–2016. Cent. Eur. J. Public Health 2018, 26, S47–S50. [Google Scholar] [CrossRef] [Green Version]
- Alix-Garcia, J.; Munteanu, C.; Zhao, N.; Potapov, P.V.; Prishchepov, A.V.; Radeloff, V.C.; Krylov, A.; Bragina, E. Drivers of forest cover change in Eastern Europe and European Russia, 1985–2012. Land Use Policy 2016, 59, 284–297. [Google Scholar] [CrossRef]
- Eurostat. Database—Forestry. Available online: https://ec.europa.eu/eurostat/web/forestry/data/database (accessed on 5 November 2021).
- Hellenbrand, W.; Kreusch, T.; Böhmer, M.; Wagner-Wiening, C.; Dobler, G.; Wichmann, O.; Altmann, D. Epidemiology of tick-borne encephalitis (TBE) in Germany, 2001–2018. Pathogens 2019, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Kříž, B.; Fialová, A.; Šebestová, H.; Daniel, M.; Malý, M. Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Pro Epidemiol. Mikrobiol. Ces. Lek. Spol. JE Purkyne 2018, 67, 134–140. [Google Scholar]
- Rubel, F.; Brugger, K. Operational TBE incidence forecasts for Austria, Germany, and Switzerland 2019–2021. Ticks Tick-Borne Dis. 2021, 12, 101579. [Google Scholar] [CrossRef]
- Kerlik, J.; Avdičová, M.; Štefkovičová, M.; Tarkovská, V.; Pántiková Valachová, M.; Molčányi, T.; Mezencev, R. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: Analysis of tick-borne encephalitis outbreaks in Slovakia during 2007–2016. Travel Med. Infect. Dis 2018, 26, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zavadska, D.; Odzelevica, Z. TBE in Latvia. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press Pte Ltd.: Singapore, 2020; 248p. [Google Scholar]
- Mickiene, A. TBE in Lithuania. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press Pte Ltd.: Singapore, 2020; 256p. [Google Scholar]
- Radzišauskienė, D.; Žagminas, K.; Ašoklienė, L.; Jasionis, A.; Mameniškienė, R.; Ambrozaitis, A.; Jančorienė, L.; Jatužis, D.; Petraitytė, I.; Mockienė, E. Epidemiological patterns of tick-borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: A retrospective study. Eur. J. Neurol. 2018, 25, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capligina, V.; Seleznova, M.; Akopjana, S.; Freimane, L.; Lazovska, M.; Krumins, R.; Kivrane, A.; Namina, A.; Aleinikova, D.; Kimsis, J.; et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasites Vectors 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Zavadska, D.; Odzelevica, Z.; Karelis, G.; Liepina, L.; Litauniece, Z.A.; Bormane, A.; Lucenko, I.; Perevoscikovs, J.; Bridina, L.; Veide, L.; et al. Tick-borne encephalitis: A 43-year summary of epidemiological and clinical data from Latvia (1973 to 2016). PLoS ONE 2018, 13, e0204844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schley, K.; Malerczyk, C.; Beier, D.; Schiffner-Rohe, J.; Von Eiff, C.; Häckl, D.; Süß, J. Vaccination rate and adherence of tick-borne encephalitis vaccination in Germany. Vaccine 2021, 39, 830–838. [Google Scholar] [CrossRef]
- Erber, W.; Schmitt, H.-J. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick-Borne Dis. 2018, 9, 768–777. [Google Scholar] [CrossRef]
- Bogovic, P.; Lotric-Furlan, S.; Strle, F. What tick-borne encephalitis may look like: Clinical signs and symptoms. Travel Med. Infect. Dis. 2010, 8, 246–250. [Google Scholar] [CrossRef]
- Moniuszko-Malinowska, A.; Pancewicz, S.; Czupryna, P. Has COVID-19 influenced on tick-borne epidemiology? Prz. Epidemiol. 2020, 74, 740–741. [Google Scholar] [CrossRef]
- Pańczuk, A. Lyme borreliosis in the Lublin province during the COVID-19 pandemic. Health Probl. Civiliz. 2021, 15, 291–297. [Google Scholar] [CrossRef]
- Krawczuk, K.; Czupryna, P.; Pancewicz, S.; Ołdak, E.; Moniuszko-Malinowska, A. Comparison of tick-borne encephalitis between children and adults—analysis of 669 patients. J. NeuroVirology 2020, 26, 565–571. [Google Scholar] [CrossRef]

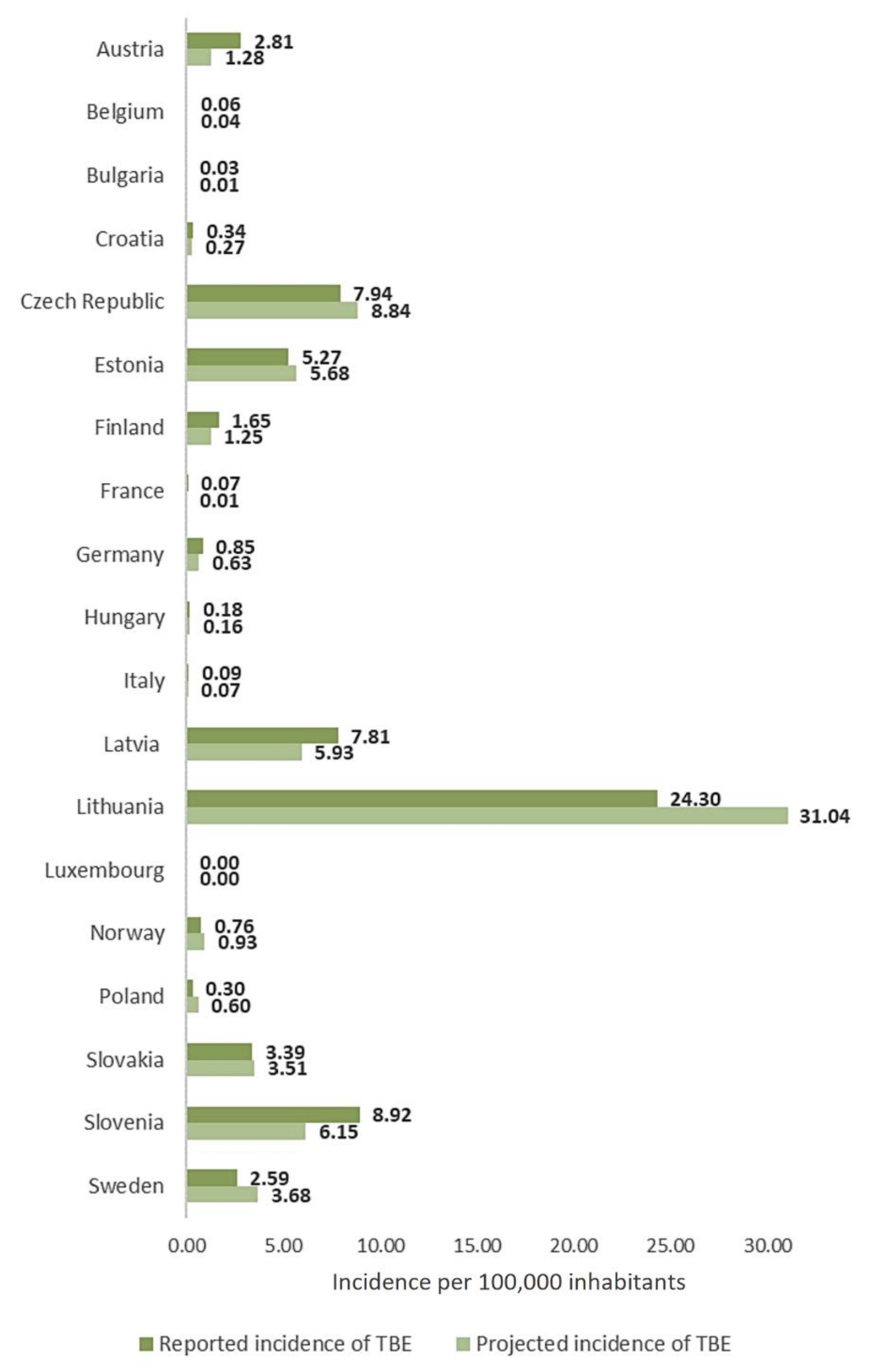
| Country | Health Care Personnel per 100,000 | Incidence of COVID-19 per 100,000 in 2020 | Number of Cases and Incidence of TBE per 100,000 Inhabitants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |||||||||
| N | Inc. | N | Inc. | N | Inc. | N | Inc. | N | Inc. | N | Inc. | |||
| Austria | 1612.14 | 4040.5 | 79 | 0.92 | 96 | 1.10 | 123 | 1.40 | 170 | 1.93 | 106 | 1.20 | 250 | 2.81 |
| Belgium | 1296.33 | 5587.7 | 1 | 0.01 | 1 | 0.01 | 3 | 0.03 | 3 | 0.03 | 4 | 0.03 | 7 | 0.06 |
| Bulgaria | nd. | 2922.9 | 2 | 0.03 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 1 | 0.01 | 2 | 0.03 |
| Croatia | nd. | 5218.7 | 26 | 0.62 | 6 | 0.14 | 10 | 0.24 | 22 | 0.54 | 13 | 0.32 | 14 | 0.34 |
| Czech Republic | 1400.43 | 6716.5 | 349 | 3.31 | 565 | 5.35 | 677 | 6.40 | 712 | 6.71 | 773 | 7.26 | 849 | 7.94 |
| Estonia | 994.67 | 2104.5 | 115 | 8.75 | 80 | 6.08 | 84 | 6.38 | 85 | 6.44 | 82 | 6.19 | 70 | 5.27 |
| Finland | nd. | 652.9 | 68 | 1.24 | 61 | 1.11 | 82 | 1.49 | 79 | 1.43 | 69 | 1.25 | 91 | 1.65 |
| France | 1754.85 | 3880.3 | 10 | 0.02 | 15 | 0.02 | 2 | 0.01 * | 25 | 0.04 | 4 | 0.01 | 46 | 0.07 |
| Germany | 1262.44 | 2117.0 | 221 | 0.27 | 353 | 0.43 | 486 | 0.59 | 583 | 0.70 | 444 | 0.53 | 705 | 0.85 |
| Greece | nd. | 1300.1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 2 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Hungary | 1002.61 | 3314.6 | 22 | 0.22 | 14 | 0.14 | 14 | 0.14 | 30 | 0.31 | 17 | 0.17 | 18 | 0.18 |
| Ireland | 1258.12 | 1831.9 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| Italy | nd. | 3555.8 | 5 | 0.01 | 48 | 0.08 | 24 | 0.04 | 39 | 0.06 | 37 | 0.05 | 55 | 0.09 |
| Latvia | nd. | 2164.2 | 141 | 7.10 | 91 | 4.62 | 178 | 9.13 | 100 | 5.17 | 118 | 6.15 | 149 | 7.81 |
| Lithuania | 1628.41 | 5069.8 | 336 | 11.5 | 633 | 21.91 | 474 | 16.64 | 384 | 13.67 | 711 | 25.45 | 679 | 24.3 |
| Luxembourg | nd. | 7367.5 | 1 | 0.18 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | 1906.19 | 919.6 | 9 | 0.17 | 12 | 0.23 | 16 | 0.30 | 26 | 0.49 | 35 | 0.66 | 41 | 0.76 |
| Poland | nd. | 3422.0 | 115 | 0.30 | 211 | 0.56 | 196 | 0.52 | 148 | 0.39 | 197 | 0.52 | 114 | 0.3 |
| Romania | 946.67 | 3294.8 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 4 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Slovakia | 793.44 | 3288.3 | 80 | 1.48 | 169 | 3.11 | 75 | 1.38 | 156 | 2.87 | 161 | 2.95 | 185 | 3.39 |
| Slovenia | nd. | 5789.2 | 62 | 3.01 | 83 | 4.02 | 102 | 4.94 | 153 | 7.40 | 111 | 5.33 | 187 | 8.92 |
| Spain | nd. | 4068.9 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 * | 0 | 0.00 |
| Sweden | nd. | 4213.7 | 268 | 2.75 | 238 | 2.42 | 365 | 3.65 | 359 | 3.55 | 355 | 3.47 | 267 | 2.59 |
| Country | Incidence of TBE per 100,000 Inhabitants | Statistical Analysis | ||
|---|---|---|---|---|
| 2015–2019 | 2020 | t (df = 4) | p | |
| Austria | 1.31 | 2.81 | −8.65 | 0.0010 |
| Belgium | 0.02 | 0.06 | −7.76 | 0.0015 |
| Bulgaria | 0.01 | 0.03 | −3.65 | 0.0217 |
| Croatia | 0.37 | 0.34 | 0.35 | 0.7414 |
| Czech Republic | 5.81 | 7.94 | −3.06 | 0.0376 |
| Estonia | 6.77 | 5.27 | 3.00 | 0.0400 |
| Finland | 1.30 | 1.65 | −5.02 | 0.0074 |
| France | 0.02 | 0.07 | −7.84 | 0.0014 |
| Germany | 0.50 | 0.85 | −4.74 | 0.0091 |
| Greece | 0.01 | 0.00 | 1.50 | 0.2080 |
| Hungary | 0.20 | 0.18 | 0.50 | 0.6436 |
| Ireland | 0.00 | 0.00 | 1.00 | 0.3739 |
| Italy | 0.05 | 0.09 | −3.63 | 0.0222 |
| Latvia | 6.43 | 7.81 | −1.73 | 0.1590 |
| Lithuania | 17.83 | 24.30 | −2.50 | 0.0666 |
| Luxembourg | 0.04 | 0.00 | 1.00 | 0.3739 |
| Norway | 0.37 | 0.76 | −4.32 | 0.0124 |
| Poland | 0.46 | 0.30 | 3.24 | 0.0318 |
| Romania | 0.01 | 0.00 | 1.50 | 0.2080 |
| Slovakia | 2.36 | 3.39 | −2.71 | 0.0537 |
| Slovenia | 4.94 | 8.92 | −5.42 | 0.0056 |
| Spain | 0.00 | 0.00 | - | - |
| Sweden | 3.17 | 2.59 | 2.36 | 0.0780 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, Z.; Bartosik, K.; Kulisz, J.; Woźniak, A. Incidence of Tick-Borne Encephalitis during the COVID-19 Pandemic in Selected European Countries. J. Clin. Med. 2022, 11, 803. https://doi.org/10.3390/jcm11030803
Zając Z, Bartosik K, Kulisz J, Woźniak A. Incidence of Tick-Borne Encephalitis during the COVID-19 Pandemic in Selected European Countries. Journal of Clinical Medicine. 2022; 11(3):803. https://doi.org/10.3390/jcm11030803
Chicago/Turabian StyleZając, Zbigniew, Katarzyna Bartosik, Joanna Kulisz, and Aneta Woźniak. 2022. "Incidence of Tick-Borne Encephalitis during the COVID-19 Pandemic in Selected European Countries" Journal of Clinical Medicine 11, no. 3: 803. https://doi.org/10.3390/jcm11030803
APA StyleZając, Z., Bartosik, K., Kulisz, J., & Woźniak, A. (2022). Incidence of Tick-Borne Encephalitis during the COVID-19 Pandemic in Selected European Countries. Journal of Clinical Medicine, 11(3), 803. https://doi.org/10.3390/jcm11030803









