Abstract
Aim: Fibrates have proven efficacy in cardiovascular risk reduction and are commonly used, in addition to statins, to control hypertriglyceridaemia. Their use is often limited due to reduction in glomerular filtration rate at treatment initiation. However, recent studies suggest benign changes in kidney function and improvement of proteinuria, an established early marker of microvascular disease and kidney disease progression. We summarize the evidence from existing trials and provide a summary of effects of fibrates, alone or in combination, on kidney disease progression and proteinuria. Methods and Results: Systematic review and meta-analysis of randomized, controlled trials (PROSPERO CRD42020187764). Out of 12,243 potentially eligible studies, 29 were included in qualitative and quantitative analysis, with a total of 20,176 patients. Mean creatinine increased by 1.05 (95% CI (0.63 to 1.46)) units in patients receiving fibrates vs. comparator, and this was similar in all other subgroups. eGFR showed a bigger decrease in the fibrates arm (SMD −1.99; 95% CI (−3.49 to −0.48)) when all studies were pooled together. Notably, short-term serum creatinine and eGFR changes remained constant in the long-term. Pooled estimates show that fibrates improve albuminuria progression, RR 0.86; 95% CI (0.76 to 0.98); albuminuria regression, RR 1.19; 95% CI (1.08 to 1.310). Conclusions: Fibrates improve albuminuria in patients with and without diabetes when used to treat hyperlipidaemia. The modest creatinine increase should not be a limiting factor for fibrate initiation in people with preserved renal function or mild CKD. The long-term effects on kidney disease progression warrant further study.
1. Introduction
Chronic kidney disease (CKD) is a common disease with increasing prevalence. More than 20 million Americans are affected, with approximately 500,000 of them being diagnosed with end stage kidney disease (ESKD) [1]. Patients with CKD are at increased risk of developing cardiovascular disease (CVD) which is the leading cause of death in this population [2]. CVD mortality accounts for up to 50% of deaths in patients who progress to ESKD [3].
Fibrates, which are peroxisome proliferator-activated receptor (PPAR) a-activators, are agents used for the treatment of dyslipidaemia. Specifically, they lower triglyceride and low-density lipoprotein (LDL) levels, while they increase high density lipoprotein (HDL) levels [4]. Patients with CKD have a distinct lipid profile characterised by elevated triglyceride-rich lipoproteins and low HDL levels, which are associated with subclinical atherosclerosis, coronary artery disease and mortality [5].
Until recently, data from various studies had raised concerns that fibrates might have nephrotoxic effects because of the increase in serum creatinine levels and the decrease in glomerular filtration rate (GFR) when fibrates were administered. Although these initial changes in creatinine levels and estimated GFR are true, newer data from well-designed randomised controlled trials (RCTs) show that these changes do not affect the actual kidney function [6,7,8]. In fact, the FIELD [6], the ACCORD [7] and the DAIS [8] trials showed a beneficial effect of fibrates on albuminuria in diabetic patients; an established marker of early microvascular disease and a predictor of adverse CVD outcomes and kidney disease progression.
A meta-analysis previously examined the effect of fibrates on CVD outcomes in people with kidney disease demonstrating efficacy and safety in this patient group [9]. However, the authors did not focus on long-term renal outcomes and did not take into account fibrate–statin co-administration; a common practice in patients with dyslipidaemia. Although the effect of co-administration was examined by Guo et al. [10], their study did not examine the combined effect of the two drugs in kidney disease. Therefore, little is known about the combined effect of the two drugs in kidney disease and kidney disease progression in such patients. Moreover, a quantitative estimate of the change in kidney function expected with fibrate initiation in the short and long-term is unknown. More importantly, the effect of fibrates on kidney disease progression has not been systematically studied in patients at risk of CKD or those with established CKD.
The objective of this systematic review and meta-analysis was to summarize evidence from existing trials and provide a summary of effects of fibrates, alone or in combination treatment, on kidney disease and kidney disease progression. The primary endpoints examined in our study were: (a) the effect of fibrates on serum creatinine and kidney function, (b) their effect on proteinuria or albuminuria and (c) their effect on development of ESKD.
2. Methods
The study was conducted in accordance with the guidelines from the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement [11].
2.1. Protocol and Registration
A predefined protocol was drafted and registered in the Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020187764; it can also be found as a publication in a pre-prints platform [12].
2.2. Eligibility Criteria
We developed a PICOS model to predefine our inclusion criteria. PICOS stands for population, intervention, comparator, outcome and study type. Specifically, we included adult patients (18 years or older) with chronic kidney disease (eGFR < 60 mL/min) or patients with risk factors for CKD. The intervention was fibrates alone or fibrates plus statin administration with placebo as the comparator in the former and statin in the latter. The outcomes of interest were changes in serum creatinine, renal function (using any GFR estimation formula or measured GFR), CKD progression (GFR loss of more than 5 mL/min per year), development of ESKD, change in proteinuria, development of proteinuria and proteinuria reduction. Progression of albuminuria is defined as a change from normal urine albumin excretion to microalbuminuria or macroalbuminuria or from microalbuminuria to macroalbuminuria. Albuminuria regression is defined as a change from macroalbuminuria or microalbuminuria to normal urine albumin excretion or from macroalbuminuria to normal urine albumin excretion. All included studies were randomised controlled trials.
The exclusion criteria were paediatric patients (younger than 18 years old) or any form of renal replacement therapy (peritoneal dialysis or haemodialysis) transplantation or eGFR < 15 mL/min/1.73 m2.
2.3. Information Sources and Search
MEDLINE, SCOPUS, the Cochrane Library and the Clinical Trials registers (clinicaltrials.gov (accessed on 1 October 2020)); (clinicaltrialsregistry.eu (accessed on 1 October 2020)) electronic databases were searched from inception until July 2020. A combination of relevant Medical Subject Headings (MeSH) terms and relevant short terms were used as keywords with a modified algorithm in each database. An example of the search syntax can be found in the supplementary file.
2.4. Study Selection and Data Collection Process
Two independent reviewers (AH and AK) screened all potentially eligible studies against the inclusion and exclusion criteria and any disagreement was resolved by a third reviewer (AP).
After the screening, extraction of data from all included studies, was done by AH and AK independently in a predefined excel sheet. Data extraction was done for study main characteristics, design, methodology, sample characteristics, intervention, comparator group characteristics and outcomes as listed earlier in the PICOS model.
2.5. Risk of Bias
Two independent reviewers (AH and PK) assessed all included studies for risk of bias using the Cochrane tool for assessing the risk of bias in RCTs [13]. Again, any disagreement was resolved by a third reviewer (AP).
2.6. Summary Measures and Synthesis of Results
Characteristics of included studies were summarised on tables and presented in the form of narrative synthesis in the text. Suitability of studies for inclusion in the meta-analysis was based on clinical, methodological and statistical homogeneity. Where meta-analysis was possible, we analysed data accordingly:
- For continuous outcomes (i.e., eGFR, creatinine), we performed a generalised inverse variance analysis of standardised mean difference between patients in intervention and control group, pre and post administration of intervention/treatment/placebo using a random effects model.
- For categorical outcomes, relative risk was calculated using number of affected patients per outcome of interest from the included studies and a pooled estimate is presented using forest plots. Pooled estimates were calculated with a random-effects model (Der Simonian–Laird method) to account for both within and between study variability. Heterogeneity between synthesised studies were calculated using the I2 statistic and the presence of publication bias was investigated graphically by precision funnel plots. All statistical analyses were performed using STATA (Version 14, StataCorp, College Station, TX, USA).
3. Results
3.1. Study Selection
A total of 12,243 potentially eligible studies were identified using the predefined search algorithm(s). After duplicates removal, the title and abstract of the remaining 9578 studies were screened and 9145 studies were further excluded. The remaining 433 studies were assessed for eligibility and 404 were excluded with reasons as presented in Figure 1. A total of 29 studies [7,8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] were included in the qualitative and the quantitative analysis.

Figure 1.
Flow diagram.
3.2. Study Characteristics
A total of 20,176 patients were included in the eligible studies, 10,249 in the intervention arm and 9841 in the comparator arm while 86 patients received both interventions in the setting of crossover studies. The majority of the studies (n = 21) had examined fenofibrate alone or in combination [7,8,14,15,17,18,20,21,22,24,25,26,27,28,29,30,31,33,34,35,40,41] and 11 of them fenofibrate vs. placebo [8,14,15,17,18,26,27,31,34,35]. Among the included studies more than half of the participants came from the FIELD and the ACCORD studies. Study characteristics are summarised in Table 1.

Table 1.
Study characteristics of included studies.
3.3. Risk of Bias within Studies
Risk of bias was assessed with the Cochrane tool [13] for assessing risk of bias in randomised control trials and the results can be seen in Table 2 and Table 3. As expected, because of the strict methodology RCTs have to follow, the majority of studies had low risk of bias in most of the domains. Some studies examined the results without blinding the assessors and this was considered a high risk of bias. Also, when not enough information was provided in the manuscript, some domains were characterised as not clear.

Table 2.
Risk of bias using for each included study.

Table 3.
Risk of bias presented as percentage across all included studies.
3.4. Synthesis of Results
Standardised mean differences were used to assess the effect of continuous data and relative risk to assess outcomes that were reporting number of patients. All forest plots were done using the random effects models. A summary of all pooled effect estimates can be found in Table 4.

Table 4.
Summary of effect estimates for each outcome.
3.5. Creatinine Change
Creatinine appears to have an increase of 1.05 (95% CI (0.63–1.46)) mg/dL when comparing the mean change of patients receiving fibrates vs. the mean change of patients receiving the comparator. In this analysis the comparison was made for studies using either fibrates alone vs. placebo or fibrates plus statins vs. that statin. The results are presented in Figure 2.
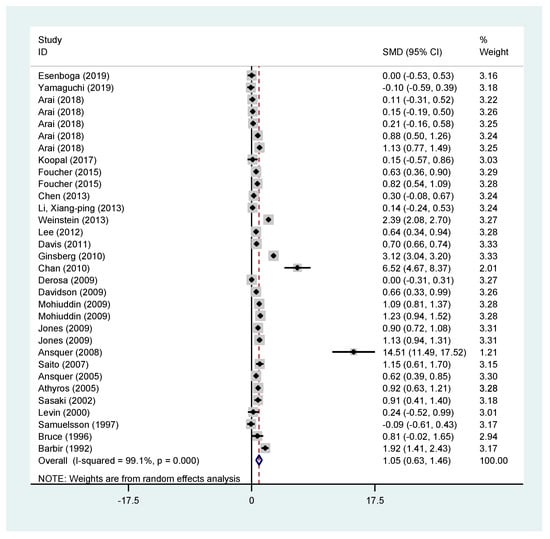
Figure 2.
Pooled effects of creatinine from all included studies.
Creatinine seems to increase in all arms where fibrates were used, whether this was fenofibrate (all studies: standardised mean difference (SMD) 1.34; 95% CI (0.82–1.86), fenofibrate vs. placebo: SMD 1.22; 95% CI (0.74–1.89), fenofibrate plus statin vs. statin: SMD 1.07; 95% CI (0.34–1.79)) or bezafibrate vs. a comparator, SMD 0.68; 95% CI (0.01–1.34). Results can be found in the supplementary file.
Creatinine change was also assessed in patients with diabetes with similar results (all studies SMD 1.49 95% CI (0.29–2.71) and in studies examining fenofibrates vs. placebo: SMD 0.86; 95% CI (0.35–1.37)). Results can be found in the supplementary file.
Similarly, the short-term effect (3 months or less) of fibrates in creatinine change was examined. For all studies, the SMD of creatinine in the fibrate groups was 0.97 95% CI (0.67–1.26). For studies using fibrates alone the SMD was 1.23 95% CI (0.88–1.58), whilst for studies examining fenofibrate vs. placebo the SMD was 2.73 95% CI (1.53–3.94) and for studies examining the effect of the combination of fenofibrate and a statin 1.02 (0.70–1.34). Short-term effect of creatinine change in studies examining bezafibrates was not statistically significant (all studies: SMD 0.65 95% CI (−0.11 to 1.42) and bezafibrate vs. placebo: SMD 0.79 95% CI (−0.17 to 1.75)). However, all the above results had significant heterogeneity. Results can be found in the supplementary file.
3.6. eGFR
eGFR showed a bigger decrease in the fibrates arm (SMD −1.99; 95% CI (−3.49 to −0.48)) when all studies were pooled together. The same appears for all the subgroup analyses [fenofibrate vs. placebo or fenofibrate plus statin vs. statin: SMD −2.69; 95% CI (−4.47 to −0.91), fenofibrate alone vs. placebo: SMD −2.53; 95% CI (−4.46 to −0.60), fenofibrate plus statin vs. statin: SMD −2.98; 95% CI (−8.00 to 2.05)] with only the latter being statistically non-significant. The results are presented in Figure 3.
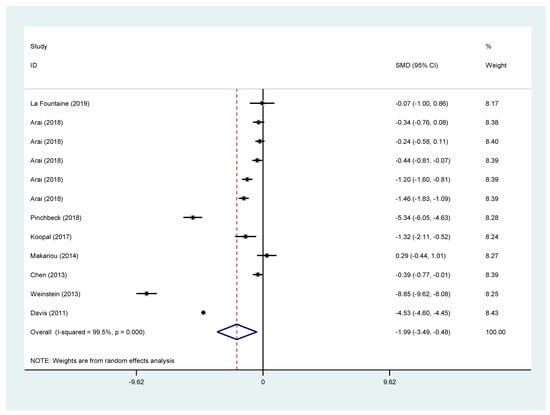
Figure 3.
Pooled effects for eGFR change from all included studies.
Similarly, the short-term effect (3 months or less) of fibrates in eGFR change was examined. For all studies, the SMD of eGFR in the fibrate groups was −1.88 95% CI (−3.02 to −0.73). For studies using fenofibrate the was SMD −2.64 95% CI (−4.55 to −0.72), for studies examining fenofibrate vs. placebo −2.38 95% CI (−4.20 to −0.57) and for studies examining the effect of the combination of fenofibrates and statins −2.98 95% CI (−8.00 to 2.05) with the latter being statistically not significant. Likewise, all these results were affected by significant heterogeneity. Results can be found in the supplementary file.
3.7. Albuminuria
Data reporting on albuminuria were analysed as progression, regression and urinary albumin excretion mean changes.
Pooled estimates show that patients receiving fibrates were less likely to have albuminuria progression (RR 0.86; 95% CI (0.76–0.98)) and more likely to have albuminuria regression (RR 1.19; 95% CI (1.08–1.31)). Results are presented in Figure 4A,B.
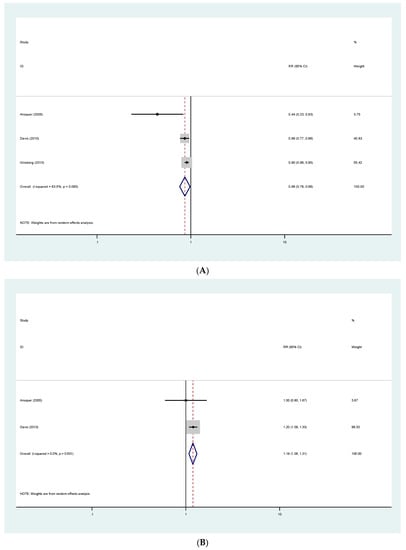
Figure 4.
(A) Effects in proteinuria progression. (B) Effects in proteinuria regression.
3.8. Chronic Kidney Disease and End Stage Kidney Disease
Most of the studies included patients with normal kidney function. Only one study, focused exclusively on patients with CKD stage III [24] showing that a combination of fenofibric acid and a statin was safe in these patients for at least 16 weeks. In addition, two studies included patients with range of eGFR 10–70 mL/min/min, showing that GFR remained similar between patients receiving fenofibrate and patients receiving the control and that urinary protein excretion did not change after 12 months of follow up [36]. A result confirmed also by Levin et al. [35] where despite the initial increase in the serum creatinine, the change from baseline of creatinine for the two groups was similar and protein excretion was also similar six months after, at the end of the study.
Data from the available two studies [26,36] show some evidence that fibrates might reduce ESKD progression, but this was not statistically significant (RR 0.85; 95% CI (0.49–1.49)), perhaps due to the small number of studies/participants. Results can be found in the supplementary file.
3.9. Publication Bias across Studies
The presence of publication bias was investigated graphically by precision funnel plots and it can be found in the supplementary file. In general, many of the studies failed to identify precisely the pooled effect estimated that we calculated in this meta-analysis. There was no evidence of large publication bias for any of the outcomes studied.
4. Discussion
Fibrates are commonly used drugs in patients with CVD with established results [42]. However, the effects of these drugs in kidney function, kidney disease progression and in patients with established CKD are not well examined. Their use is often limited due to concerns of nephrotoxicity.
In this systematic review and meta-analysis, we provide information about the safety of fibrates when used alone or in combination with statins. This adds to the previous meta-analysis by Min Jun et al. [9] which examined mainly the effect of fibrates on CVD and reported the effects of fibrates when used as a single treatment.
Additionally, our results show that both short-term (3 months or less) and long-term creatinine change have similar values, therefore creatinine might have a rise initially, but remains relatively constant afterwards. Similarly, short term changes in eGFR remain relatively similar with long-term use of fibrates.
Even though fibrates administration results in an increase in serum creatinine, the fact that progression of albuminuria is reducing [6,7,8] and regression is increasing [6,8] is reassuring. We cannot comment on the effect of fibrates on ESKD development [26,36] as our data do not support statistically significant effects. It is important to note the relatively short follow-up of studies. It is also worth mentioning that, creatinine elevation was fully reversible in the FIELD study [6] eight weeks after the completion of the trial but with sustained CVD benefits, and eGFR returned to baseline values in the ACCORD study [7] after fenofibrate discontinuation. Both studies did not demonstrate any added major adverse event compared to the control. In addition, in a subgroup of participants in the ACCORD trial, Chauhan et al. demonstrated that the rise in serum creatinine is not accompanied by an increase of urinary biomarkers representing tubular injury, inflammation or fibrosis, providing further support for benign change in kidney function [43].
The mechanism by which serum creatinine is increased once fibrates are administered is not yet fully understood. In part, it can be explained by the involvement of the peroxisome proliferator receptors, that generate vasodilatory prostaglandins resulting in an increase in kidney blood filtration which increases serum creatinine and decreases GFR [44]. Moreover, emerging evidence, suggest that alterations in the PPAR pathway at the molecular level are both early and late events in CKD and CKD progression mouse models [45,46] and fenofibrate treatment exerts nephroprotective effects thought the attenuation of inflammatory and fibrotic pathways [41,47,48]. Enhancing binding at the PPARa receptor is a promising development, influencing downstream gene and physiological effects of PPRAa activation. The peroxisome proliferator-activated receptor alpha modulator pemafibrate has a structure which increases its selectivity for PPRAa and consequently its potency by >2500-fold, compared to fenofibrate, and has a better benefit–risk profile. The results of the PROMINENT study, a phase III placebo-controlled RCT, on the effect of pemafibrate on CVD events in high-risk type 2 diabetics, including those with mild to moderate renal impairment are awaited (ClinicalTrials.gov: NCT03071692).
In our meta-analysis, the most commonly used fibrate was fenofibrate. Fenofibrates were found to be effective and safe in the FIELD study, even for patients with reduced eGFR [49]. In fact, patients with eGFR of 30–59 mL/min/1.73 m2 were the ones with the greatest CVD reduction and at the same time, no further ESKD progressions were observed. This effect was also observed in the VA-HIT trial [50] where fibrates were effective in reducing CVD in patients with mild to moderate renal insufficiency.
Furthermore, because of the CVD risk improvement fibrates induce, it is possible that the reason behind the preservation of kidney function eventually, and the reduction in the progression of microalbuminuria, is attributed to the benefit they have at the vascular level, at least in patients with type 2 diabetes [8].
Although, we found high heterogeneity and publication bias in pooled estimates relating to kidney function, the effect of fibrates in progression and regression of proteinuria, two of the main outcomes of our study, had 63.5% and 0% heterogeneity, respectively. These were analyses with 14,385 and 2152 patients accordingly, therefore we decided to proceed to a meta-analysis, providing robust quantitative estimates on the effect of fibrates on albuminuria, a marker commonly used as a surrogate outcome for kidney disease progression and early CVD.
Limitations
We observed large heterogeneity within and between the studies on eGFR and creatinine. Nonetheless, the direction of the effect estimate is in keeping with a priori expectation. An explanation for this heterogeneity is that almost all the included studies were not examining kidney related variables as a primary outcome, therefore the information obtained was related to patients with different kidney status and characteristics. Furthermore, different fibrates and different statins were used in the included studies even with sometimes different dosages, which could also be a reason for this heterogeneity. In addition, since kidney related outcomes were not the primary endpoint in most of the studies, the small number of participants in many of the studies introduced power issues within each study estimate. Additionally, the observation period for most of the studies was less than three months which might also have added to this heterogeneity. Thus, large multicentred randomised controlled trials are still needed to examine this topic, recruiting patients with similar kidney statuses. Last but not least, the effect of fibrates in patients with eGFR less than 30 was not examined so results cannot be extrapolated to this population.
5. Conclusions
Our study is in agreement with a previous meta-analysis and expands further on the effect of fibrates alone, or in combination with a statin, on kidney function and proteinuria. The modest increase in creatinine at treatment initiation remains unchanged throughout the treatment course and is reversible upon cessation of fibrate treatment. Patients with CKD are commonly older people presenting with co-existing diabetes and a distinct lipid profile phenotype which renders fibrates a promising agent to study for the treatment of CVD and kidney disease progression in kidney disease patients. Data on the safety of fibrates in CKD are lacking. Importantly, our analysis provides evidence that fibrates not only reduce albuminuria progression but also increase albuminuria regression in patients with and without diabetes when used to treat hyperlipidaemia. Longer-term studies are needed on the effect of fibrates in delaying ESKD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11030768/s1. Supplementary file: Pooled effects.
Author Contributions
Systemic review of the literature: A.H. and A.K.; statistical analysis and preparation of the first draft of the manuscript: A.H. All authors (A.H., P.K., A.K., A.P.) critically reviewed and revised the final version of the protocol. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1–ITC14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998, 32 (Suppl. 3), S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, B.; Tao, W.; Hao, Z.; Liu, M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst. Rev. 2015, 2015, CD009580. [Google Scholar] [CrossRef]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [Green Version]
- Keech, A.C.; Simes, R.J.; Barter, P.J.; Best, J.; Scott, R.A.P.; Taskinen, M.-R.; Forder, P.M.; Pillai, A.; Davis, T.M.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- The ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R.; Leiter, L.A.; Linz, P.; Friede-Wald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Foucher, C.; Rattier, S.; Taskinen, M.R.; Steiner, G. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: Results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am. J. Kidney Dis. 2005, 45, 485–493. [Google Scholar] [CrossRef]
- Jun, M.; Zhu, B.; Tonelli, M.; Jardine, M.; Patel, A.; Neal, B.; Liyanage, T.; Keech, A.; Cass, A.; Perkovic, V. Effects of fibrates in kidney disease: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 60, 2061–2071. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Meng, F.; Ma, N.; Li, C.; Ding, Z.; Wang, H.; Hou, R.; Qin, Y. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am. J. Cardiol. 2012, 110, 1296–1301. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Kousios, A.; Hadjivasilis, A.; Kouis, P.; Panayiotou, A. The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: Protocol for a Systematic Review; Research Square: Durham, NC, USA, 2020. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esenboga, K.; Çiçek, Ö.F.; Oktay, A.A.; Ayral, P.A.; Gürlek, A. Effect of fenofibrate on serum nitric oxide levels in patients with hypertriglyceridemia. Adv. Clin. Exp. Med. 2019, 28, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Fountaine, M.F.; Cirnigliaro, C.M.; Hobson, J.C.; Lombard, A.T.; Specht, A.F.; Dyson-Hudson, T.A.; Kirshblum, S.C.; Bauman, W.A. A Four Month Randomized Controlled Trial on the Efficacy of Once-daily Fenofibrate Monotherapy in Persons with Spinal Cord Injury. Sci. Rep. 2019, 9, 17166–17169. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shirai, K.; Nagayama, D.; Nakamura, S.; Oka, R.; Tanaka, S.; Watanabe, Y.; Imamura, H.; Sato, Y.; Kawana, H.; et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus. J. Atheroscler. Thromb. 2019, 26, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: A multicenter, placebo-controlled, double-blind, randomized trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinchbeck, J.L.; Moxon, J.V.; Rowbotham, S.E.; Bourke, M.; Lazzaroni, S.; Morton, S.K.; Matthews, E.O.; Hendy, K.; Jones, R.E.; Bourke, B.; et al. Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME-2 Trial. J. Am. Heart Assoc. 2018, 7, e009866. [Google Scholar] [CrossRef] [Green Version]
- Koopal, C.; Marais, A.D.; Westerink, J.; Van Der Graaf, Y.; Visseren, F.L.J. Effect of adding bezafibrate to standard lipid-lowering therapy on post-fat load lipid levels in patients with familial dysbetalipoproteinemia. A randomized placebo-controlled crossover trial. J. Lipid Res. 2017, 58, 2180–2187. [Google Scholar] [CrossRef] [Green Version]
- Foucher, C.; Aubonnet, P.; Reichert, P.; Berli, M.; Schaeffer, A.; Vargas, C.G.C.; Lochocka, A.; Belenky, D.; Koch, H.-F.; Cholib study Investigators. New Fixed-Dose Combinations of Fenofibrate/Simvastatin Therapy Significantly Improve the Lipid Profile of High-Risk Patients with Mixed Dyslipidemia Versus Monotherapies. Cardiovasc. Ther. 2015, 33, 329–337. [Google Scholar] [CrossRef]
- Makariou, E.S.; Elisaf, M.; Kei, A.; Challa, A.; DiNicolantonio, J.J.; Liberopoulos, E. No effect of switching to high-dose rosuvastatin, add-on nicotinic acid, or addon fenofibrate on serum vitamin D levels in patients with mixed dyslipidemia. Hippokratia 2015, 19, 136–140. [Google Scholar]
- Chen, Y.-P.; Chang, K.-C.; Tseng, W.-K.; Yin, W.-H.; Chen, J.-W.; Lee, Y.-T.; Wu, C.-C. Increased Rosuvastatin Dose versus Concomitant Fenofibrate and Rosuvastatin Therapy to Achieve Lipid Goal in Patients with Diabetes or Atherosclerosis with Metabolic Syndrome. Acta Cardiol. Sin. 2013, 29, 421–428. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27122739 (accessed on 11 December 2020). [PubMed]
- Li, X.P.; Gong, H.R.; Huang, X.S.; Huang, W.Y.; Zhao, S.P. The influence of statin-fibrate combination therapy on lipids profile and apolipoprotein A5 in patients with acute coronary syndrome. Lipids Health Dis. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, D.L.; Williams, L.A.; Carlson, D.M.; Kelly, M.T.; Burns, K.M.; Setze, C.M.; Lele, A.; Stolzenbach, J.C. A randomized, double-blind study of fenofibric acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease. Clin. Ther. 2013, 35, 1186–1198. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, K.I.; Kim, J.Y.; Ahn, Y.K.; Rha, S.-W.; Kim, Y.-J.; Choi, Y.-S.; Choi, S.W.; Jeon, D.W.; Min, P.-K.; et al. Non-lipid effects of rosuvastatin-fenofibrate combination therapy in high-risk Asian patients with mixed hyperlipidemia. Atherosclerosis 2012, 221, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Ting, R.; Best, J.D.; Donoghoe, M.W.; Drury, P.L.; Sullivan, D.R.; Jenkins, A.J.; O’Connell, R.L.; Whiting, M.J.; Glasziou, P.P.; et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011, 54, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C.; Hamilton, S.J.; Rye, K.A.; Chew, G.T.; Jenkins, A.J.; Lambert, G.; Watts, G.F. Fenofibrate concomitantly decreases serum proprotein convertase subtilisin/kexin type 9 and very-low-density lipoprotein particle concentrations in statin-treated type 2 diabetic patients. Diabetes Obes. Metab. 2010, 12, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Salvadeo, S.A.T.; Ferrari, I.; Gravina, A.; Mereu, R.; Palumbo, I.; D’Angelo, A.; Cicero, A. Fenofibrate, simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr. Med. Res. Opin. 2009, 25, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, S.M.; Pepine, C.J.; Kelly, M.T.; Buttler, S.M.; Setze, C.M.; Sleep, D.J.; Stolzenbach, J.C. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: A phase 3, randomized, controlled study. Am. Heart J. 2009, 157, 195–203. [Google Scholar] [CrossRef]
- Jones, P.H.; Davidson, M.H.; Kashyap, M.L.; Kelly, M.T.; Buttler, S.M.; Setze, C.M.; Sleep, D.J.; Stolzenbach, J.C. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: A phase 3 study. Atherosclerosis 2009, 204, 208–215. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Dalton, R.N.; Caussé, E.; Crimet, D.; Le Malicot, K.; Foucher, C. Effect of Fenofibrate on Kidney Function: A 6-Week Randomized Crossover Trial in Healthy People. Am. J. Kidney Dis. 2008, 51, 904–913. [Google Scholar] [CrossRef]
- Saito, Y.; Yamada, N.; Shirai, K.; Sasaki, J.; Ebihara, Y.; Yanase, T.; Fox, J.C. Effect of rosuvastatin 5-20 mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia. Atherosclerosis 2007, 194, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Mikhailidis, D.P.; Papageorgiou, A.A.; Didangelos, T.P.; Peletidou, A.; Kleta, D.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Elisaf, M. Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism 2005, 54, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Yamamoto, K.; Ageta, M. Effects of fenofibrate on high-density lipoprotein particle size in patients with hyperlipidemia: A randomized, double-blind, placebo-controlled, multicenter, crossover study. Clin Ther. 2002, 24, 1614–1626. [Google Scholar] [CrossRef]
- Levin, A.; Duncan, L.; Djurdjev, O.; Shapiro, R.J.; Frohlich, J.; Belanger, A.; Dumas, R.; Ross, S. A randomized placebo-controlled double-blind trial of lipid lowering strategies in patients with renal insufficiency: Diet modification with or without fenofibrate. Clin. Nephrol. 2000, 53, 140–146. [Google Scholar]
- Samuelsson, O.; Attman, P.O.; Knight-Gibson, C.; Kron, B.; Larsson, R.; Mulec, H.; Weiss, L.; Alaupovic, P. Effect of gemfibrozil on lipoprotein abnormalities in chronic renal insufficiency: A controlled study in human chronic renal disease. Nephron 1997, 75, 286–294. [Google Scholar] [CrossRef]
- Bruce, R.; Daniels, A.; Candy, T. Renal function changes in diabetic nephropathy induced by bezafibrate. Nephron 1996, 73, 490. [Google Scholar] [CrossRef]
- Barbir, M.; Hunt, B.; Kushwaha, S.; Kehely, A.; Prescot, R.; Thompson, G.R.; Mitchell, A.; Yacoub, M. Maxepa versus bezafibrate in hyperlipidemic cardiac transplant recipients. Am. J. Cardiol. 1992, 70, 1596–1601. [Google Scholar] [CrossRef]
- Jones, I.R.; Swai, A.; Taylor, R.; Miller, M.; Laker, M.F.; Alberti, K.G.M.M. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care 1990, 13, 855–863. [Google Scholar] [CrossRef]
- Davidson, M.H.; Rooney, M.W.; Drucker, J.; Eugene Griffin, H.; Oosman, S.; Beckert, M. Efficacy and tolerability of atorvastatin/fenofibrate fixed-dose combination tablet compared with atorvastatin and fenofibrate monotherapies in patients with dyslipidemia: A 12-week, multicenter, double-blind, randomized, parallel-group study. Clin. Ther. 2009, 31, 2824–2838. [Google Scholar] [CrossRef]
- Tanaka, T.; Higashijima, Y.; Wada, T.; Nangaku, M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014, 86, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Jakob, T.; Nordmann, A.J.; Schandelmaier, S.; Ferreira-González, I.; Briel, M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst. Rev. 2016, 11, CD009753. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Pattharanitima, P.; Patel, N.; Duffy, A.; Saha, A.; Chaudhary, K.; Debnath, N.; Van Vleck, T.; Chan, L.; Nadkarni, G.N.; et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin. J. Am. Soc. Nephrol. 2019, 14, 656–663. [Google Scholar] [CrossRef]
- Lipscombe, J.; Lewis, G.F.; Cattran, D.; Bargman, J.M. Deterioration in renal function associated with fibrate therapy. Clin Nephrol. 2001, 55, 39–44. Available online: https://europepmc.org/article/med/11200866 (accessed on 4 December 2020). [PubMed]
- Nicolaou, O.; Kousios, A.; Sokratous, K.; Potamiti, L.; Koniali, L.; Neophytou, G.; Papacharalampous, R.; Zanti, M.; Ioannou, K.; Hadjisavvas, A.; et al. Alport syndrome: Proteomic analysis identifies early molecular pathway alterations in Col4a3 knock out mice. Nephrology 2020, 25, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Harzandi, A.; Lee, S.; Bidkhori, G.; Saha, S.; Hendry, B.M.; Mardinoglu, A.; Shoaie, S.; Sharpe, C.C. Acute Kidney Injury Leading to CKD is Associated with a Persistence of Metabolic Dysfunction and Hypertriglyceridemia. iScience 2021, 24, 102046. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Zhang, Y.; Zhang, X.; Wu, J.; Chen, L.; Cha, D.; Su, D.; Hwang, M.-T.; Fan, X.; Davis, L.; et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mic. Kidney Int. 2006, 69, 1511–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.W.; Lim, J.H.; Kim, M.Y.; Shin, S.J.; Chung, S.; Choi, B.S.; Kim, H.W.; Kim, Y.-S.; Park, C.W.; Chang, Y.S. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol. Dial. Transplant. 2012, 27, 2213–2225. [Google Scholar] [CrossRef] [Green Version]
- Ting, R.D.; Keech, A.C.; Drury, P.L.; Donoghoe, M.W.; Hedley, J.; Jenkins, A.J.; Davis, T.M.; Lehto, S.; Celermajer, D.; Simes, R.J.; et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: The FIELD study. Diabetes Care. 2012, 35, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Collins, D.; Robins, S.; Bloomfield, H.; Curhan, G.C. Gemfibrozil for secondary prevention of cardiovascular events in mild to moderate chronic renal insufficiency. Kidney Int. 2004, 66, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).