A Visuoperceptual Measure for Videofluoroscopic Swallow Studies (VMV): A Pilot Study of Validity and Reliability in Adults with Dysphagia
Abstract
:1. Introduction
- inter- and intra-rater reliability
- structural validity
- internal consistency
- hypothesis testing for construct validity
2. Methods
2.1. Participants
2.2. Equipment and Materials
2.3. Protocol
2.4. Manual
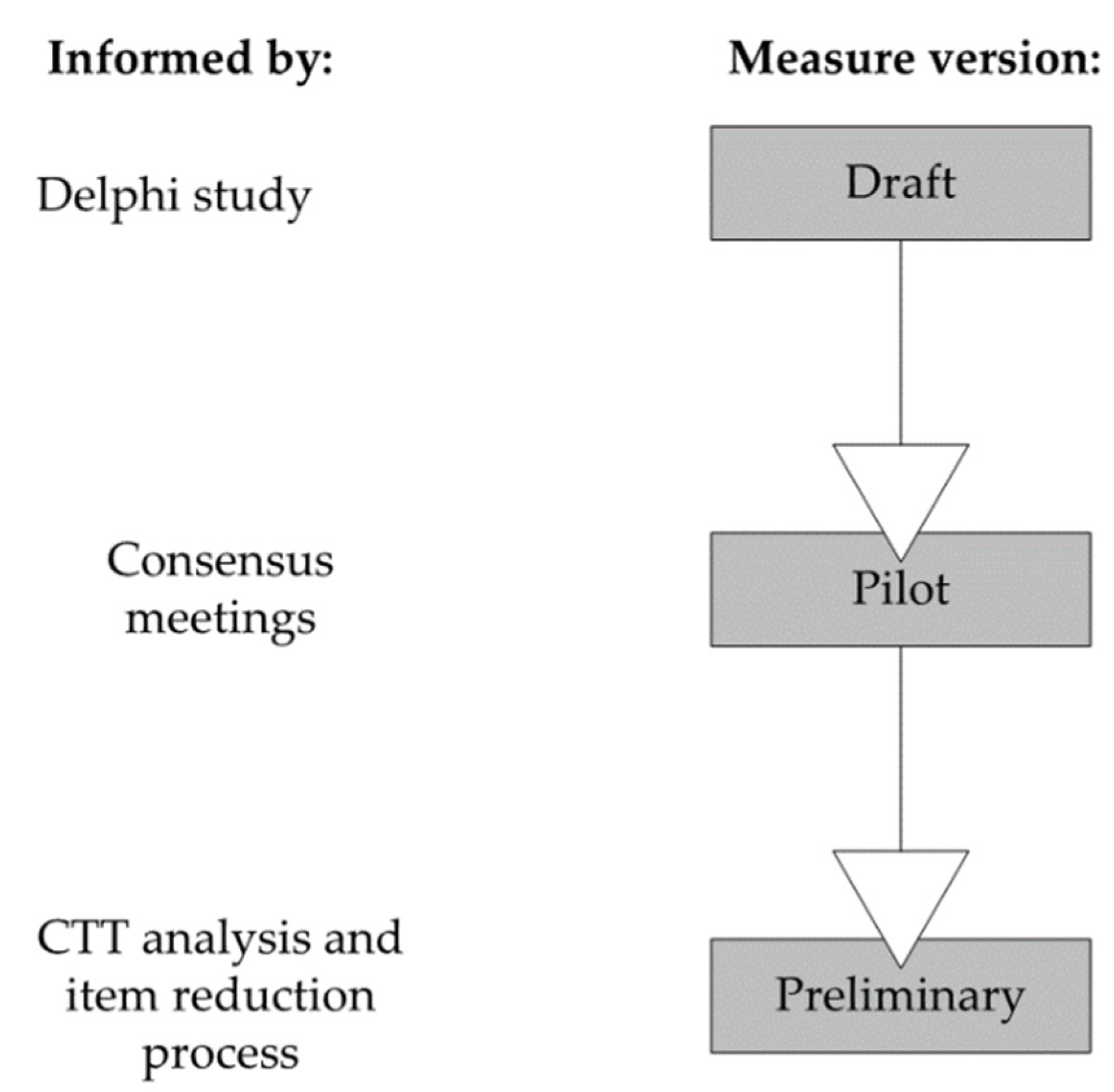
2.5. Raters, Consensus Meetings, Training and Rating
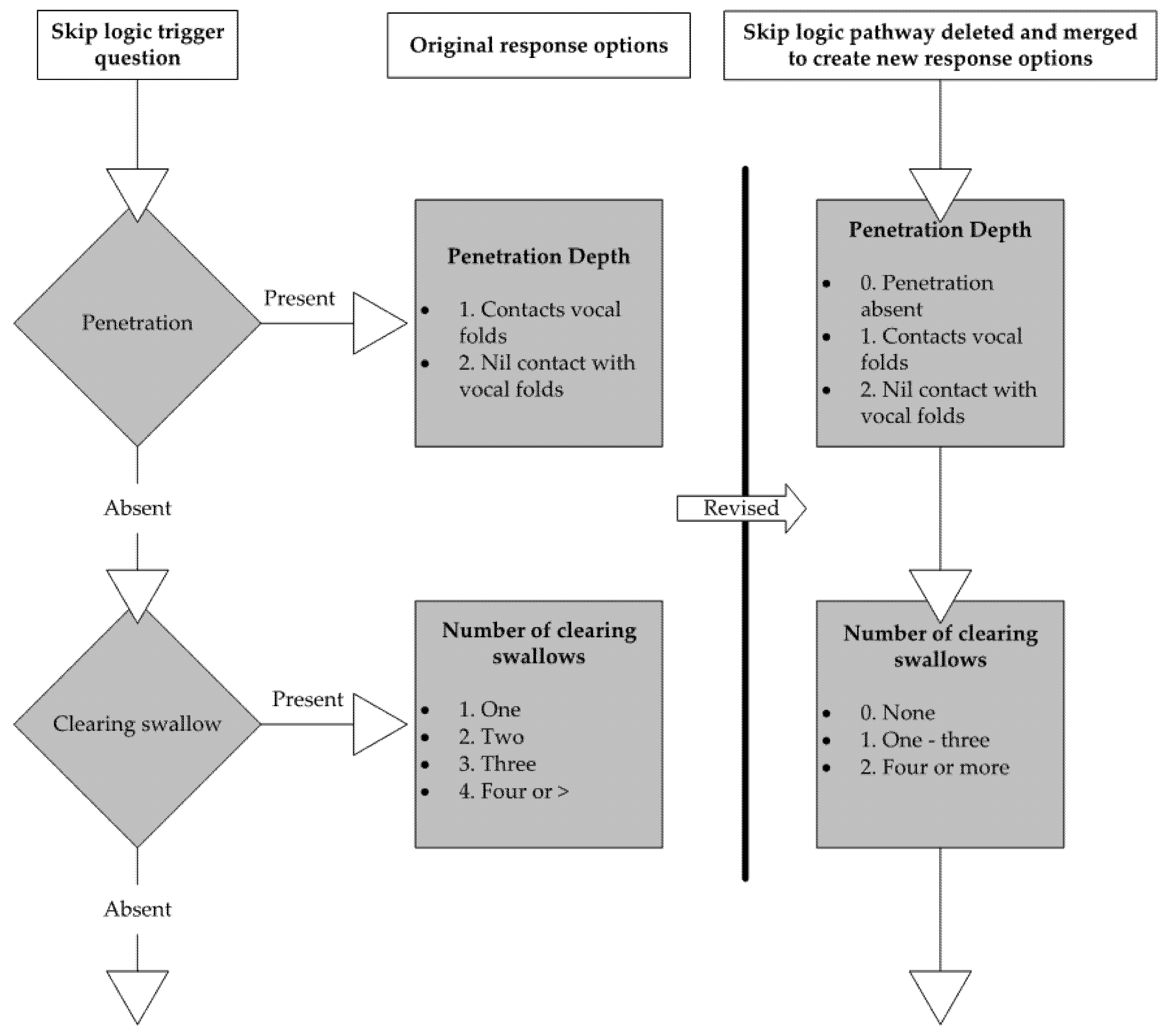
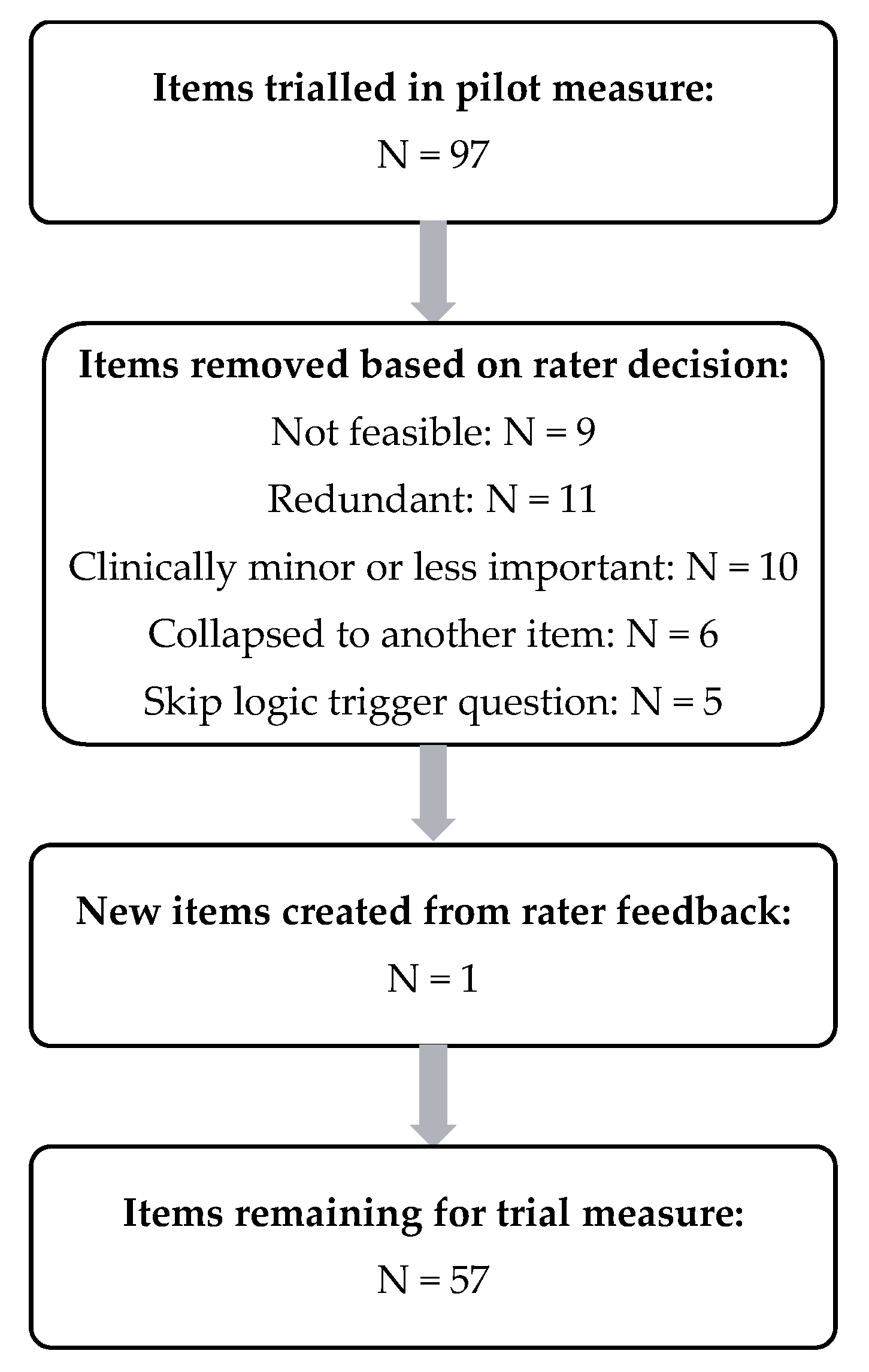
2.6. Item Reduction
2.7. Psychometric Properties
- Reliability: The amount of variance in scores which are reflective of true differences in participant function rather than errors in the measure or process of rating [8]. This study included analysis of inter-rater (differences in scores between raters) and intra-rater (differences within a single rater’s scores applied to repeated measures of the same participant).
- Structural Validity: The degree to which the scores adequately reflect the dimensionality of the construct of interest [8]. For example, the VFSS is expected to be multidimensional due to the complexity of the analysis, illustrated by the number of different constructs being assessed (e.g., movement of the bolus, actions of the anatomical structures) and distinct ways constructs are operationalised (i.e., spatial, volume, temporal).
- Internal consistency: The degree of interrelatedness among the VFSS items. Items which measure the same construct should demonstrate a relationship [8]. For example, the items ‘lip seal’, ‘lingual movement’, ‘glossopalatal seal’ and ‘bolus control’ are likely to show a close relationship and score highly on analysis of inter-relatedness as a group.
- Hypotheses testing for construct validity: The extent to which the scores on the measure agree with hypotheses which are theoretically consistent with the condition and the construct being measured [8]. In the case of the VFSS, for example, it is expected that lingual movement correlates strongly with oral residue, with a weaker correlation between lingual movement and parameters of the UES.
2.8. Statistical Analysis
3. Results
3.1. Functional Health Status and Severity Scores
3.2. Reliability (Intra- and Inter-Rater Reliability)
3.3. Structural Validity
Exploratory Factor Analysis
3.4. Internal Consistency
3.5. Hypothesis Testing for Construct Validity
4. Discussion
Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Philpott, H.; Garg, M.; Tomic, D.; Balasubramanian, S.; Sweis, R. Dysphagia: Thinking outside the box. World J. Gastroenterol. 2017, 23, 6942. [Google Scholar] [CrossRef] [PubMed]
- Langmore, S.E. History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dysphagia: Changes over the years. Dysphagia 2017, 32, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Randall, D.R.; Evangelista, L.M.; Kuhn, M.A.; Belafsky, P.C. Subjective assessment of videofluoroscopic swallow studies. Otolaryngol.-Head Neck Surg. 2017, 156, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Swan, K.; Cordier, R.; Brown, T.; Speyer, R. Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre-endoscopic evaluations of swallowing: A systematic review. Dysphagia 2019, 34, 2–33. [Google Scholar] [CrossRef] [Green Version]
- Mokkink, L.B.; Prinsen, C.A.; Bouter, L.M.; de Vet, H.C.; Terwee, C.B. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz. J. Phys. Ther. 2016, 20, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Clavé, P.; Rofes, L.; Carrión, S.; Ortega, O.; Cabré, M.; Serra-Prat, M.; Arreola, V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. In Stepping Stones to Living Well with Dysphagia; Karger Publishers: Basel, Switzerland, 2012; pp. 57–66. [Google Scholar]
- Anunciacao, L. An overview of the history and methodological aspects of psychometrics-history and methodological aspects of psychometrics. J. ReAttach Ther. Dev. Divers. 2018, 1, 44–58. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Knol, D.L.; Stratford, P.W.; Alonso, J.; Patrick, D.L.; Bouter, L.M.; De Vet, H.C. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Med. Res. Methodol. 2010, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Mokkink, L.B.; Boers, M.; van der Vleuten, C.P.M.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; De Vet, H.C.; Terwee, C.B. COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: A Delphi study. BMC Med. Res. Methodol. 2020, 20, 293. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Prinsen, C.A.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.; Terwee, C.B. COSMIN Study Design Checklist for Patient-Reported Outcome Measurement Instruments; Amsterdam Public Health Research Institute: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Terwee, C.B.; Prinsen, C.A.; Chiarotto, A.; Westerman, M.J.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; De Vet, H.C.; Mokkink, L.B. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A Delphi study. Qual. Life Res. 2018, 27, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Mokkink, L.B.; De Vet, H.C.; Prinsen, C.A.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Prinsen, C.A.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; De Vet, H.C.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P. How to make more published research true. Rev. Cuba. De Inf. En Cienc. De La Salud (ACIMED) 2015, 26, 187–200. [Google Scholar] [CrossRef] [Green Version]
- El-Den, S.; Schneider, C.; Mirzaei, A.; Carter, S. How to measure a latent construct: Psychometric principles for the development and validation of measurement instruments. Int. J. Pharm. Pract. 2020, 28, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Swan, K.; Cordier, R.; Brown, T.; Speyer, R. Visuoperceptual Analysis of the Videofluoroscopic Study of Swallowing: An International Delphi Study. Dysphagia 2021, 36, 595–613. [Google Scholar] [CrossRef]
- Giraldo-Cadavid, L.F.; Leal-Leaño, L.R.; Leon-Basantes, G.A.; Bastidas, A.R.; Garcia, R.; Ovalle, S.; Abondano-Garavito, J.E. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope 2017, 127, 2002–2010. [Google Scholar] [CrossRef]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.; Chen, J.; Cichero, J.A.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.A.; Troche, M.S. Effects of Verbal Cueing on Respiratory-Swallow Patterning, Lung Volume Initiation, and Swallow Apnea Duration in Parkinson’s Disease. Dysphagia 2020, 35, 460–470. [Google Scholar] [CrossRef]
- Steele, C.M.; Namasivayam-MacDonald, A.M.; Guida, B.T.; Cichero, J.A.; Duivestein, J.; Hanson, B.; Lam, P.; Riquelme, L.F. Creation and initial validation of the international dysphagia diet standardisation initiative functional diet scale. Arch. Phys. Med. Rehabil. 2018, 99, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Bonilha, H.S.; Blair, J.; Carnes, B.; Huda, W.; Humphries, K.; McGrattan, K.; Michel, Y.; Martin-Harris, B. Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia 2013, 28, 528–538. [Google Scholar] [CrossRef]
- Speyer, R.; Kertscher, B.; Cordier, R. Functional health status in oropharyngeal dysphagia. J. Gastroenterol. Hepatol. Res. 2014, 3, 1043–1048. [Google Scholar]
- Woisard, V.; Andrieux, M.; Puech, M. Validation of a self-assessment questionnaire for swallowing disorders (Deglutition Handicap Index). Rev. De Laryngol.-Otol.-Rhinol. 2006, 127, 315–325. [Google Scholar]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Suhr, D.D. Exploratory or confirmatory factor analysis? In Proceedings of the SAS SUGI Proceedings: Statistics, Data Analysis and Data Mining (SUGI 31), San Francisco, CA, USA, 26–29 March 2006. Paper 200-31. [Google Scholar]
- Brady, S.; Donzelli, J. The modified barium swallow and the functional endoscopic evaluation of swallowing. Otolaryngol. Clin. N. Am. 2013, 46, 1009–1022. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213. [Google Scholar] [CrossRef]
- Prinsen, C.A.; Vohra, S.; Rose, M.R.; Boers, M.; Tugwell, P.; Clarke, M.; Williamson, P.R.; Terwee, C.B. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—A practical guideline. Trials 2016, 17, 449. [Google Scholar] [CrossRef] [Green Version]
- Dancey, C.P.; Reidy, J. Statistics without Maths for Psychology; Pearson Education: New York, NY, USA, 2007. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Petrillo, J.; Cano, S.J.; McLeod, L.D.; Coon, C.D. Using classical test theory, item response theory, and Rasch measurement theory to evaluate patient-reported outcome measures: A comparison of worked examples. Value Health 2015, 18, 25–34. [Google Scholar] [CrossRef] [Green Version]
- DeVellis, R.F. Classical test theory. Med. Care 2006, 11, S50–S59. [Google Scholar] [CrossRef]
- Hinkin, T.R. A brief tutorial on the development of measures for use in survey questionnaires. Organ. Res. Methods 1998, 1, 104–121. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Kuhn, M.A. The Clinician’s Guide to Swallowing Fluoroscopy; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Butler, S.G.; Stuart, A.; Castell, D.; Russell, G.B.; Koch, K.; Kemp, S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J. Speech Lang. Hear. Res. 2009, 52, 240–253. [Google Scholar] [CrossRef]
- Nabieh, A.A.; Emam, A.M.; Mostafa, E.M.; Hashem, R.M. Gender Differences in Normal Swallow. Egypt. J. Neck Surg. Otorhinolaryngol. 2018, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Terwee, C.B.; Prinsen, C.A.C.; Garotti, M.R.; Suman, A.; De Vet, H.C.W.; Mokkink, L.B. The quality of systematic reviews of health-related outcome measurement instruments. Qual. Life Res. 2016, 25, 767–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelen, M.O.; Reeve, B.B. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual. Life Res. 2007, 16, 5–18. [Google Scholar] [CrossRef]
| Rater One vs. Two | Rater One vs. Three | Rater Two vs. Three | Average | |
|---|---|---|---|---|
| Thick (L3) | 0.932 | 0.886 | 0.882 | 0.900 |
| Thin (L0) | 0.930 | 0.866 | 0.860 | 0.885 |
| Pudding (L4) | 0.939 | 0.874 | 0.868 | 0.894 |
| Solids (L7) | 0.934 | 0.892 | 0.852 | 0.893 |
| Anterior-Posterior view | 0.868 | 0.910 | 0.842 | 0.873 |
| Total (Average) | 0.921 | 0.886 | 0.861 | 0.889 |
| Item No. | Item Descriptor | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Communalities |
|---|---|---|---|---|---|---|---|
| 1.1 | Number of swallows | 0.120 | 0.867 | 0.126 | −0.060 | −0.105 | 0.797 |
| 1.2 | Lingual motion (liquids) | 0.748 | 0.091 | 0.349 | 0.088 | −0.053 | 0.701 |
| 1.3 | Bolus formation (liquids) | 0.777 | 0.019 | 0.112 | −0.156 | 0.132 | 0.659 |
| 1.4 | Bolus transport (liquids) | 0.728 | 0.194 | 0.069 | 0.138 | −0.236 | 0.647 |
| 1.5 | Base of tongue retraction | 0.620 | 0.086 | 0.090 | 0.412 | −0.071 | 0.574 |
| 1.6 | Velum elevation | 0.530 | −0.052 | 0.413 | 0.120 | −0.099 | 0.478 |
| 1.7 | Premature Spillage location | 0.239 | 0.056 | 0.169 | 0.781 | 0.145 | 0.719 |
| 1.8 | Location material at swallow initiation | −0.037 | −0.067 | 0.057 | 0.820 | 0.002 | 0.681 |
| 1.9 | Hyoid excursio—superior movement | 0.245 | 0.079 | 0.827 | 0.081 | −0.162 | 0.783 |
| 1.10 | Hyoid excursion—anterior movement | 0.233 | 0.286 | 0.793 | 0.023 | 0.094 | 0.774 |
| 1.11 | Laryngeal excursion | 0.104 | 0.071 | 0.898 | 0.170 | −0.136 | 0.870 |
| 1.12 | Pharyngeal constriction pharyngeal obliterated space | 0.225 | 0.571 | 0.297 | 0.279 | 0.001 | 0.543 |
| 1.13 | Clearing Swallow—Location of residue when swallow triggered | −0.047 | 0.904 | 0.088 | −0.021 | 0.102 | 0.837 |
| 1.14 | Clearing swallows (number) | −0.155 | 0.927 | 0.115 | −0.109 | 0.097 | 0.917 |
| 1.15 | Width of UES opening | −0.032 | 0.124 | −0.072 | −0.015 | 0.922 | 0.872 |
| 1.16 | UES closure impedes flow | −0.185 | 0.037 | −0.113 | 0.110 | 0.909 | 0.886 |
| 1.17 | Oral residue volume | 0.629 | −0.063 | 0.262 | 0.350 | −0.276 | 0.666 |
| 1.18 | Oropharynx residue volume | 0.559 | 0.008 | 0.054 | 0.593 | −0.050 | 0.670 |
| 1.19 | Valleculae residue volume | 0.383 | 0.592 | −0.148 | 0.078 | 0.196 | 0.564 |
| Eigenvalue | 3.525 | 3.282 | 2.704 | 2.139 | 1.989 | ||
| % of Total Variance | 18.55 | 17.27 | 14.23 | 11.26 | 10.47 | ||
| Total variance | 71.78% | ||||||
| Item No. | Item Descriptor | Factor 6 | Factor 7 | Factor 8 | Factor 9 | Communalities |
|---|---|---|---|---|---|---|
| 2.1 | Laryngeal vestibule closure (LVC)—base to arytenoids | 0.248 | 0.754 | 0.160 | 0.191 | 0.691 |
| 2.2 | Epiglottic tilting | 0.148 | 0.716 | 0.036 | 0.259 | 0.604 |
| 2.3 | Laryngeal vestibule closure—base to arytenoids contact relative to UES opening | 0.136 | 0.722 | −0.102 | 0.212 | 0.595 |
| 2.4 | Pharyngeal wall movement | −0.051 | 0.361 | −0.307 | 0.400 | 0.387 |
| 2.5 | Aspiration present on x number of swallows | 0.233 | 0.835 | 0.012 | −0.003 | 0.751 |
| 2.6 | Response to aspiration | 0.978 | 0.171 | 0.086 | 0.052 | 0.995 |
| 2.7 | Cough ability to eject material | 0.952 | 0.166 | 0.066 | 0.097 | 0.948 |
| 2.8 | Cough latency (ordinal) | 0.962 | 0.167 | 0.070 | 0.088 | 0.966 |
| 2.9 | Aspiration occurrence timing | 0.955 | 0.168 | 0.098 | 0.013 | 0.950 |
| 2.10 | Aspiration volume | 0.978 | 0.171 | 0.086 | 0.052 | 0.995 |
| 2.11 | Penetration present on x number of swallows | 0.160 | −0.065 | 0.839 | 0.179 | 0.765 |
| 2.12 | Penetration occurrence timing | 0.147 | 0.026 | 0.907 | 0.053 | 0.848 |
| 2.13 | Penetration depth | 0.018 | 0.478 | 0.757 | 0.217 | 0.849 |
| 2.14 | Response to penetration | 0.167 | 0.772 | 0.485 | 0.143 | 0.881 |
| 2.15 | Permanence of penetration | 0.132 | 0.723 | 0.523 | 0.164 | 0.841 |
| 2.16 | Post. pharyngeal wall of hypopharynx residue volume | 0.138 | 0.161 | 0.089 | 0.674 | 0.507 |
| 2.17 | Pyriform sinus residue volume | 0.052 | 0.088 | 0.153 | 0.872 | 0.794 |
| 2.18 | Laryngeal surface epiglottis residue volume | 0.582 | 0.369 | 0.350 | 0.032 | 0.598 |
| 2.19 | Laryngeal vestibule residue volume | 0.164 | 0.799 | −0.010 | 0.043 | 0.667 |
| 2.20 | Clearing Swallow Efficacy | 0.018 | 0.212 | 0.214 | 0.869 | 0.845 |
| Eigenvalue | 5.300 | 4.777 | 2.971 | 2.432 | ||
| % of Total Variance | 26.48 | 23.89 | 14.86 | 12.16 | ||
| Total variance | 77.38% | |||||
| Factors and Description of Items within the Factor | Cronbach’s Alpha |
|---|---|
| Factor 1. Lingual control and motion, velum motion, oral and oropharynx residue | 0.810 |
| Factor 2. Number of swallows, clearing swallows and pharyngeal contraction | 0.861 |
| Factor 3. Hyoid and larynx movement | 0.876 |
| Factor 4. Premature spillage and swallow initiation | 0.698 |
| Factor 5. Upper oesophageal sphincter function | 0.836 |
| Factor 6. Aspiration and underside epiglottis residue | 0.934 |
| Factor 7. Epiglottis movement, aspiration, penetration permanence and response and laryngeal vestibule closure | 0.873 |
| Factor 8. Penetration | 0.853 |
| Factor 9. Pharyngeal wall movement, pharyngeal residue and clearing swallows | 0.714 |
| Total Measure | 0.902 |
| Factor | FOIS | 5-Point Scale | |
|---|---|---|---|
| Correlation coefficient | Correlation coefficient | ||
| 1 | Lingual control and motion, velum motion, oral and oropharynx residue | 0.228 ** | 0.226 ** |
| 2 | Number of swallows, clearing swallows and pharyngeal contraction | 0.289 ** | 0.324 ** |
| 3 | Hyoid and larynx movement | 0.415 ** | 0.432 ** |
| 4 | Premature spillage and swallow initiation | 0.185 ** | 0.264 ** |
| 5 | Upper oesophageal sphincter function | −0.157 * | –0.055 |
| 6 | Aspiration and underside epiglottis residue | 0.199 ** | 0.225 ** |
| 7 | Epiglottis movement, aspiration, penetration permanence and response and Laryngeal Vestibule closure | 0.231 ** | 0.234 ** |
| 8 | penetration | 0.063 | 0.058 |
| 9 | Pharyngeal wall movement, pharyngeal residue and clearing swallows | 0.086 ** | 0.086 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swan, K.; Speyer, R.; Scharitzer, M.; Farneti, D.; Brown, T.; Cordier, R. A Visuoperceptual Measure for Videofluoroscopic Swallow Studies (VMV): A Pilot Study of Validity and Reliability in Adults with Dysphagia. J. Clin. Med. 2022, 11, 724. https://doi.org/10.3390/jcm11030724
Swan K, Speyer R, Scharitzer M, Farneti D, Brown T, Cordier R. A Visuoperceptual Measure for Videofluoroscopic Swallow Studies (VMV): A Pilot Study of Validity and Reliability in Adults with Dysphagia. Journal of Clinical Medicine. 2022; 11(3):724. https://doi.org/10.3390/jcm11030724
Chicago/Turabian StyleSwan, Katina, Renée Speyer, Martina Scharitzer, Daniele Farneti, Ted Brown, and Reinie Cordier. 2022. "A Visuoperceptual Measure for Videofluoroscopic Swallow Studies (VMV): A Pilot Study of Validity and Reliability in Adults with Dysphagia" Journal of Clinical Medicine 11, no. 3: 724. https://doi.org/10.3390/jcm11030724
APA StyleSwan, K., Speyer, R., Scharitzer, M., Farneti, D., Brown, T., & Cordier, R. (2022). A Visuoperceptual Measure for Videofluoroscopic Swallow Studies (VMV): A Pilot Study of Validity and Reliability in Adults with Dysphagia. Journal of Clinical Medicine, 11(3), 724. https://doi.org/10.3390/jcm11030724








