Influence of Thoracic Trauma on Fracture Healing in Long Bones—A Retrospective Analysis
Abstract
:1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
3.1. Comorbidities and Medications
3.2. Trauma Mechanism and Length of Hospital Stay
3.3. Fracture Management
3.4. Thoracic Trauma
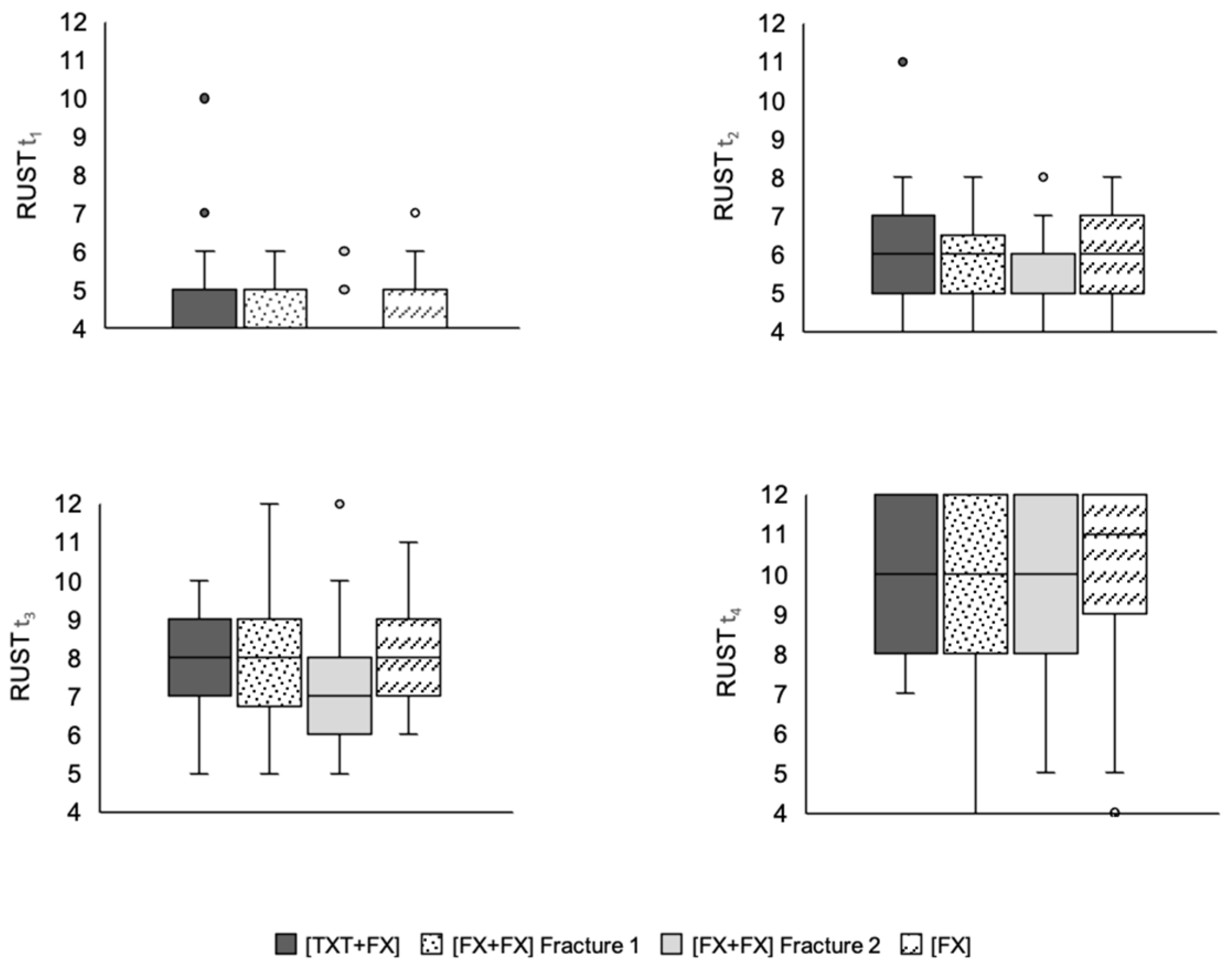
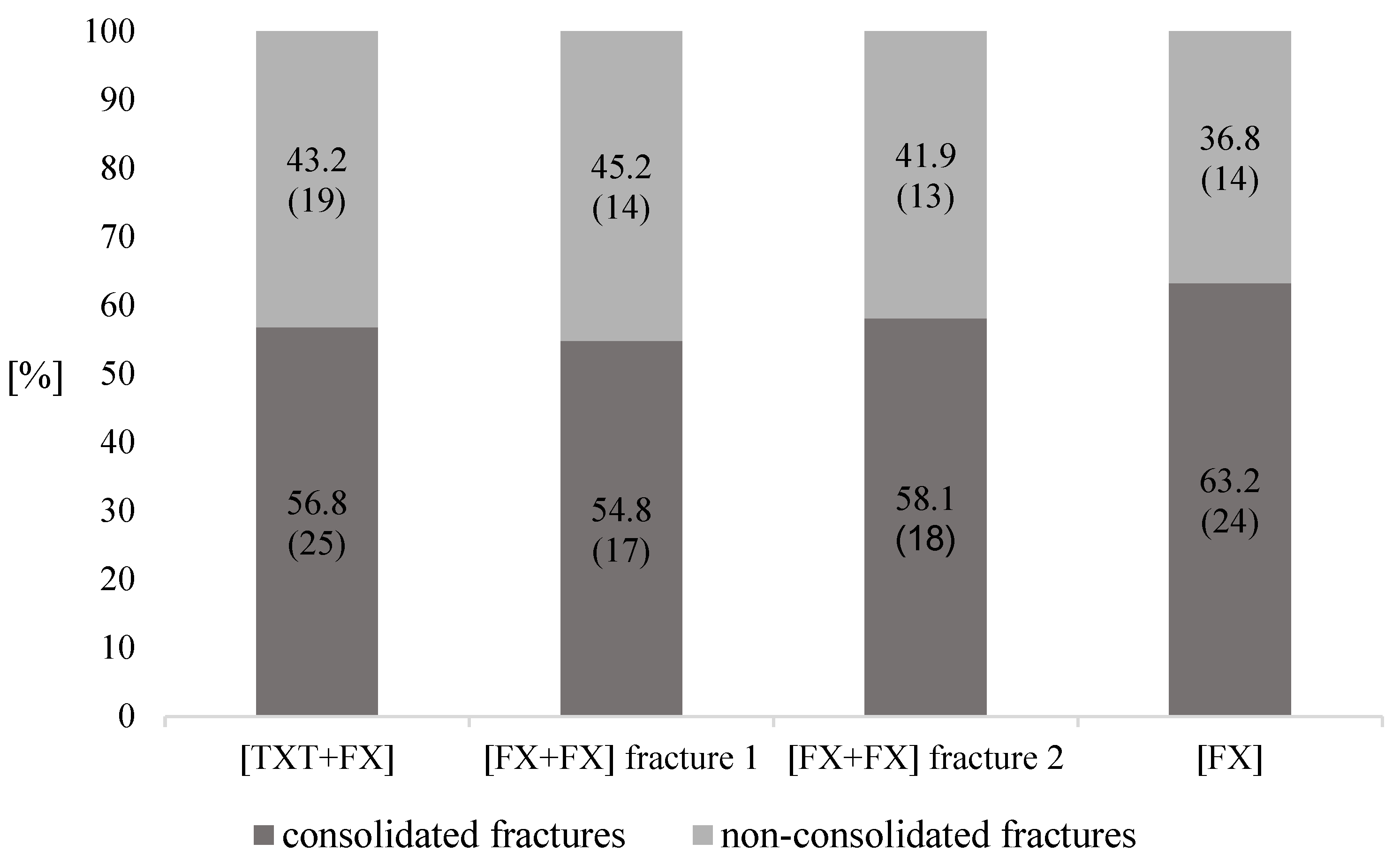
3.5. Fracture Consolidation
3.6. Thoracic Trauma Scores
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AISChest | abbreviated injury scale chest |
| ANOVA | analysis of variance |
| ASA | American Society of Anesthesiologists classification of physical status |
| BMI | body mass index |
| CT | computed tomography |
| FDA | U.S. Food and Drug Administration |
| GCS | glasgow coma scale |
| ICU | intensive care unit |
| IRB | institutional review board |
| ISS | injury severity score |
| PCS | pulmonary contusion score |
| RUST | radiographic union scale in tibial fractures |
| TBI | traumatic brain injury |
| TTS | thoracic trauma severity score |
References
- Calori, G.M.; Mazza, E.L.; Mazzola, S.; Colombo, A.; Giardina, F.; Romano, F.; Colombo, M. Non-unions. Clin Cases Miner Bone Metab. 2017, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018, 42, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Kern, S.; El Khassawna, T.; Ismat, A.; Malhan, D.; Alt, V.; Heiss, C.; Raschke, M.J. Do Systemic Factors Influence the Fate of Nonunions to Become Atrophic? A Retrospective Analysis of 162 Cases. BioMed Res. Int. 2019, 2019, 6407098. [Google Scholar] [CrossRef] [PubMed]
- Marzi, I. Focus on non-union of fractures. Eur. J. Trauma Emerg. Surg. 2019, 45, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-Y.; Wang, T.-C.; Tsai, Y.-H.; Huang, K.-C. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J. Bone Jt. Surg. 2012, 94, 227–230. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Cadosch, D.; Frey, S.P.; Skirving, A.P.; Filgueira, L.; Zellweger, R. Serum-mediated osteogenic effect in traumatic brain-injured patients. ANZ J. Surg. 2009, 79, 449–455. [Google Scholar] [CrossRef]
- Boes, M.; Kain, M.; Kakar, S.; Nicholls, F.; Cullinane, D.; Gerstenfeld, L.; Einhorn, T.A.; Tornetta, P., III. Osteogenic Effects of Traumatic Brain Injury on Experimental Fracture-Healing. J. Bone Jt. Surg. 2006, 88, 738–743. [Google Scholar] [CrossRef]
- Claes, L.; Ignatius, A.; Lechner, R.; Gebhard, F.; Kraus, M.; Baumgärtel, S.; Recknagel, S.; Krischak, G.D. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop. 2011, 82, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Recknagel, S.; Bindl, R.; Kurz, J.; Wehner, T.; Ehrnthaller, C.; Knöferl, M.W.; Gebhard, F.; Huber-Lang, M.; Claes, L.; Ignatius, A. Experimental blunt chest trauma impairs fracture healing in rats. J. Orthop. Res. 2011, 29, 734–739. [Google Scholar] [CrossRef]
- Claes, L.; Gebhard, F.; Ignatius, A.; Lechner, R.; Baumgärtel, S.; Kraus, M.; Krischak, G.D. The effect of a combined thoracic and soft-tissue trauma on blood flow and tissue formation in fracture healing in rats. Arch. Orthop. Trauma Surg. 2017, 137, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Mangum, L.H.; Avila, J.J.; Hurtgen, B.J.; Lofgren, A.L.; Wenke, J.C. Burn and thoracic trauma alters fracture healing, systemic inflammation, and leukocyte kinetics in a rat model of polytrauma. J. Orthop. Surg. Res. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recknagel, S.; Bindl, R.; Brochhausen, C.; Göckelmann, M.; Wehner, T.; Schoengraf, P.; Huber-Lang, M.; Claes, L.; Inatius, A. Systemic inflammation induced by a thoracic trauma alters the cellular composition of the early fracture callus. J. Trauma Acute Care Surg. 2013, 74, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, S.; Bindl, R.; Kurz, J.; Wehner, T.; Schoengraf, P.; Ehrnthaller, C.; Qu, H.; Gebhard, F.; Huber-Lang, M.; Lambris, J.D.; et al. C5aR-antagonist significantly reduces the deleterious effect of a blunt chest trauma on fracture healing. J. Orthop. Res. 2012, 30, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Kemmler, J.; Bindl, R.; McCook, O.; Wagner, F.; Gröger, M.; Wagner, K.; Scheuerle, A.; Radermacher, P.; Ignatius, A. Exposure to 100% Oxygen Abolishes the Impairment of Fracture Healing after Thoracic Trauma. PLoS ONE 2015, 10, e0131194. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, E.E.; Simon, M.; Vinta, S.R.; Zehm, C.F.; Shabot, S.M.; Minhajuddin, A.; Abouleish, A.E. Adding Examples to the ASA-Physical Status Classification Improves Correct Assignment to Patients. Anesthesiology 2017, 126, 614–622. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B., Jr. The Injury Severity Scor: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma Acute Care Surg. 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Sternbach, G.L. The Glasgow Coma Scale. J. Emerg. Med. 2000, 19, 67–71. [Google Scholar] [CrossRef]
- Mommsen, P.; Zeckey, C.; Andruszkow, H.; Weidemann, J.; Frömke, C.; Puljic, P.; van Griensven, M.; Frink, M.; Krettek, C.; Hildebrand, F. Comparison of Different Thoracic Trauma Scoring Systems in Regards to Prediction of Post-Traumatic Complications and Outcome in Blunt Chest Trauma. J. Surg. Res. 2012, 176, 239–247. [Google Scholar] [CrossRef]
- Pape, H.-C.; Remmers, D.; Rice, J.; Ebisch, M.; Krettek, C.; Tscherne, H. Appraisal of Early Evaluation of Blunt Chest Trauma: Development of a Standardized Scoring System for Initial Clinical Decision Making. J. Trauma Inj. Infect. Crit. Care 2000, 49, 496–504. [Google Scholar] [CrossRef]
- Keller, W.K.; Dillihunt, R.C.; Fenner, H.A.; Hobbs, N.M.; Jolley, F.L.; Keeney, A.H.; Weygandt, P.L.; Hames, L.N. Rating the Severity of Tissue Damage. JAMA 1971, 215, 277. [Google Scholar] [CrossRef]
- Litrenta, J.; Tornetta, P.; Mehta, S.; Jones, C.; O’Toole, R.V.; Bhandari, M.; Kottmeier, S.; Ostrum, R.; Egol, K.; Ricci, W.; et al. Determination of Radiographic Healing: An Assessment of Consistency Using RUST and Modified RUST in Metadiaphyseal Fractures. J. Orthop. Trauma 2015, 29, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.E.; Hussein, A.I.; Lybrand, K.E.; Wulff, A.; Simmons, E.; Choi, J.H.; Litrenta, J.; Ricci, W.M.; Nascone, J.W.; O’Toole, R.; et al. Correlation between RUST assessments of fracture healing to structural and biomechanical properties. J. Orthop. Res. 2017, 36, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3, A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.O.; Wong, S.P. A common language effect size statistic. Psychol. Bull. 1992, 111, 361–365. [Google Scholar] [CrossRef]
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Strobel, M.C.; Reinholdt, L.G.; Malcolm, R.D.; Pritchett-Corning, K. Genetic Monitoring of Laboratory Mice and Rats. In Laboratory Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1403–1416. [Google Scholar] [CrossRef]
- Hindorff, L.A.; Bonham, V.L.; Brody, L.C.; Ginoza, M.E.C.; Hutter, C.M.; Manolio, T.A.; Green, E.D. Prioritizing diversity in human genomics research. Nat. Rev. Genet. 2018, 19, 175–185. [Google Scholar] [CrossRef]
- Röhrig, B.; Du Prel, J.-B.; Wachtlin, D.; Blettner, M. Types of Study in Medical Research. Dtsch. Aerzteblatt Online 2009, 106, 262–268. [Google Scholar] [CrossRef]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38, S3–S9. [Google Scholar] [CrossRef]
- Özkan, S.; Nolte, P.A.; van den Bekerom, M.P.J.; Bloemers, F.W. Diagnosis and management of long-bone nonunions: A nationwide survey. Eur. J. Trauma Emerg. Surg. 2019, 45, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.; Abdou, S.; Stranix, J.T.; Levine, J.P.; McLaurin, T.; Tejwani, N.; Thanik, V.; Leucht, P. Comparing Radiographic Progression of Bone Healing in Gustilo IIIB Open Tibia Fractures Treated with Muscle Versus Fasciocutaneous Flaps. J. Orthop. Trauma 2018, 32, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Cekic, E. Reliability of the Radiographic Union Score for Tibial Fractures. Acta Orthop. Traumatol. Turc. 2014, 48, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.S.; de Cotias, R.B.; Azi, M.L.; Teixeira, A.A.d.A. Reliability of the radiographic union scale in tibial fractures (RUST). Rev. Bras. Ortop. 2017, 52, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Macri, F.; Marques, L.F.; Backer, R.C.; Santos, M.J.; Belangero, W.D. Validation of a standardised gait score to predict the healing of tibial fractures. J. Bone Jt. Surg. 2012, 94, 544–548. [Google Scholar] [CrossRef]
- Jones, A.L.; Bucholz, R.W.; Bosse, M.J.; Mirza, S.K.; Lyon, T.R.; Webb, L.X.; Lawrance, X.; Valentin-Opran, A. Recombinant Human BMP-2 and Allograft Compared with Autogenous Bone Graft for Reconstruction of Diaphyseal Tibial Fractures with Cortical Defects. J. Bone Jt. Surg. 2006, 88, 1431–1441. [Google Scholar] [CrossRef]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Tornetta, P.; Sprague, S.; Schemitsch, E.H. A Lack of Consensus in the Assessment of Fracture Healing Among Orthopaedic Surgeons. J. Orthop. Trauma 2002, 16, 562–566. [Google Scholar] [CrossRef]
- Cook, G.E.; Bates, B.D.; Tornetta, P.; McKee, M.D.; Morshed, S.; Slobogean, G.P.; Schemitsch, E.H. Assessment of Fracture Repair. J. Orthop. Trauma 2015, 29, S57–S61. [Google Scholar] [CrossRef]
- Chrysou, K.; Halat, G.; Hoksch, B.; Schmid, R.A.; Kocher, G.J. Lessons from a large trauma center: Impact of blunt chest trauma in polytrauma patients—still a relevant problem? Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, J.G.; Collinge, J.D.; Wilson, R.F.; Eachempati, S.R. Pulmonary Contusions. J. Trauma: Inj. Infect. Crit. Care 1999, 46, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.H.; Schriger, D.; Green, S. Looking Through the Retrospectoscope: Reducing Bias in Emergency Medicine Chart Review Studies. Ann. Emerg. Med. 2014, 64, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Sakitani, N.; Iwasawa, H.; Nomura, M.; Miura, Y.; Kuroki, H.; Ozawa, J.; Moriyama, H. Mechanical Stress by Spasticity Accelerates Fracture Healing After Spinal Cord Injury. Calcif Tissue Int. 2017, 101, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Majercik, S.; Pieracci, F.M. Chest Wall Trauma. Thorac. Surg. Clin. 2017, 27, 113–121. [Google Scholar] [CrossRef]
- Getz, P.; Mommsen, P.; Clausen, J.-D.; Winkelmann, M. Limited Influence of Flail Chest in Patients with Blunt Thoracic Trauma–A Matched-pair Analysis. In Vivo 2019, 33, 133–139. [Google Scholar] [CrossRef] [Green Version]
| [TXT+FX] | [FX+FX] | [FX] | |
|---|---|---|---|
| patients n | 44 | 31 | 38 |
| age M ± SD (years) | 46.1 ± 19.1 | 48.8 ± 12.7 | 41.4 ± 20.5 |
| BMI M ± SD (kg/m2) | 26.5 ± 4.7 | 27.8 ± 4.6 | 27.6 ± 7.1 |
| women n (percentage) | 12 (27.3) | 6 (19.4) | 14 (36.8) |
| men n (percentage) | 32 (72.7) | 25 (80.6) | 24 (63.2) |
| [TXT+FX] | [FX+FX] | [FX] | p Value | |
|---|---|---|---|---|
| ISS M ± SD | 24.9 ± 9.1 | 13.7 ± 3.6 | 11.9 ± 10.6 | <0.001 * |
| ASA M ± SD | 2.5 ± 0.8 | 2.3 ± 0.7 | 2.4 ± 0.9 | 0.50 |
| GCS M ± SD | 12 ± 5.1 | 13.4 ± 3.8 | 13.4 ± 3.8 | 0.27 |
| [TXT+FX] | [FX+FX] | [FX] | |
|---|---|---|---|
| coronary heart disease n (%) | 3 (6.8) | 1 (2.2) | 4 (10.5) |
| cardiac arrhythmia n (%) | 2 (4.6) | 2 (6.5) | 2 (5.3) |
| heart failure n (%) | 2 (4.6) | 2 (6.5) | 2 (5.3) |
| arterial hypertension n (%) | 11 (25.0) | 11 (35.5) | 9 (23.7) |
| peripheral arterial occlusive disease n (%) | 2 (4.6) | 0 (0) | 0 (0) |
| diabetes mellitus n (%) | 2 (4.6) | 2 (6.5) | 4 (10.5) |
| osteoporosis n (%) | 2 (4.6) | 3 (9.7) | 0 (0) |
| hypothyreodism n (%) | 7 (15,9) | 1 (2.2) | 3 (7.9) |
| rheumatic diseases n (%) | 1 (2.3) | 0 (0) | 2 (5.3) |
| kidney failure n (%) | 0 (0) | 0 (0) | 1 (2.6) |
| cerebral insult n (%) | 1 (2.3) | 1 (2.2) | 2 (5.3) |
| depression n (%) | 4 (9.1) | 0 (0) | 0 (0) |
| epilepsy n (%) | 0 (0) | 0 (0) | 0 (0) |
| acohol abuse n (%) | 4 (9.1) | 4 (12.9) | 0 (0) |
| hyperparathyreodism n (%) | 0 (0) | 0 (0) | 1 (2.6) |
| hypoparathyreodism n (%) | 0 (0) | 0 (0) | 0 (0) |
| nikotin abuse n (%) | 7 (15.9) | 7 (22.6) | 0 (0) |
| tumor n (%) | 1 (2.3) | 0 (0) | 0 (0) |
| lung disease n (%) | 3 (6.8) | 0 (0) | 3 (7.9) |
| hyperthyreodism n (%) | 0 (0) | 0 (0) | 0 (0) |
| obesity n (%) | 3 (6.8) | 3 (9.7) | 4 (10.5) |
| HIV infection n (%) | 2 (4.6) | 0 (0) | 0 (0) |
| nervous system disease n (%) | 1 (2.3) | 0 (0) | 1 (2.6) |
| drug abuse n (%) | 2 (4.6) | 1 (2.2) | 0 (0) |
| liver disease n (%) | 1 (2.3) | 1 (2.2) | 0 (0) |
| [TXT+FX] | [FX+FX] | [FX] | |
|---|---|---|---|
| high-energy trauma/fall from height ≥ 3 m n (%) | 37 (84.1) | 20 (64.5) | 23 (60.5) |
| low-energy trauma/fall from height < 3 m n (%) | 7 (15.9) | 11 (35.5) | 15 (39.5) |
| Length of hospital stay M ± SD (days) | 21.6 ± 12.1 | 16.8 ± 7.9 | 14.4 ± 11.1 |
| [TXT+FX] | [FX+FX] Fracture 1 | [FX+FX] Fracture 2 | [FX] | |
|---|---|---|---|---|
| plate n | 19 | 9 | 9 | 13 |
| nail n | 8 | 9 | 4 | 9 |
| external fixator n | 0 | 0 | 2 | 0 |
| screws n | 1 | 1 | 1 | 3 |
| cerclage and k-wire n | 1 | 0 | 0 | 3 |
| conservative n | 0 | 0 | 2 | 0 |
| Σ | 29 | 19 | 18 | 28 |
| [TXT+FX] | [FX+FX] Fracture 1 | [FX+FX] Fracture 2 | [FX] | |
|---|---|---|---|---|
| plate n | 4 | 4 | 9 | 6 |
| nail n | 11 | 7 | 2 | 3 |
| external fixator n | 0 | 0 | 0 | 0 |
| screws n | 0 | 1 | 1 | 0 |
| cerclage and k-wire n | 0 | 0 | 0 | 1 |
| conservative n | 0 | 0 | 1 | 0 |
| Σ | 15 | 12 | 13 | 10 |
| Thoracic Injury | Number of Patients |
|---|---|
| hemothorax | 4 |
| pneumothorax | 14 |
| pleural effusion | 4 |
| rib fracture (single or multiple) | 32 |
| cardiac contusion | 1 |
| sternal fracture | 4 |
| lung contusion | 19 |
| chest contusion | 8 |
| lung laceration | 4 |
| [TXT+FX] | [FX+FX] | [FX] | ||
|---|---|---|---|---|
| Fracture 1 | Fracture 2 | |||
| humerus n (percentage) | 3 (12.0) | 3 (17.7) | 0 (0) | 2 (8.3) |
| radius/ulna n (percentage) | 11 (44.0) | 4 (23.5) | 7 (38.9) | 11 (45.8) |
| femur n (percentage) | 6 (24.0) | 5 (29.4) | 1 (5.6) | 5 (20.8) |
| tibia/fibula n (percentage) | 5 (20.0) | 5 (29.4) | 10 (55.5) | 6 (25.0) |
| AO | [TXT+FX] | [FX+FX] Fracture 1 | [FX+FX] Fracture 2 | [FX] |
|---|---|---|---|---|
| 11 | 3 (6.8) | 3 (9.7) | 0 (0) | 1 (2.6) |
| 12 | 3 (6.8) | 1 (3.2) | 0 (0) | 2 (5.3) |
| 13 | 0 (0) | 0 (0) | 0 (0) | 1 (2.6) |
| 21 | 2 (4.5) | 0 (0) | 2 (6.5) | 4 (10.5) |
| 22 | 1 (2.3) | 3 (9.7) | 4 (12.9) | 3 (7.9) |
| 23 | 9 (20.5) | 4 (12.9) | 5 (16.1) | 7 (18.4) |
| 31 | 0 (0) | 2 (6.5) | 0 (0) | 1 (2.6) |
| 32 | 9 (20.5) | 4 (12.9) | 2 (6.5) | 8 (21.1) |
| 33 | 4 (9.1) | 4 (12.9) | 0 (0) | 2 (5.3) |
| 41 | 5 (11.4) | 1 (3.2) | 5 (16.1) | 5 (13.2) |
| 42 | 7 (15.9) | 6 (19.4) | 10 (32.3) | 1 (2.6) |
| 43 | 1 (2.3) | 1 (3.2) | 1 (3.2) | 2 (5.3) |
| 44 | 0 (0) | 2 (6.5) | 2 (6.5) | 1 (2.6) |
| Σ | 44 (100) | 31 (100) | 31 (100) | 38 (100) |
| [TXT+FX] | p Value | Pearson’s Correlation | |
|---|---|---|---|
| AISChest M ± SD | 5.6 ± 4.3 | 0.34 | 0.15 |
| PCS M ± SD | 2.8 ± 4.4 | 0.03 * | 0.33 |
| TTSmax. M ± SD | 10.4 ± 3.4 | 0.25 | 0.18 |
| TTSmin. M ± SD | 5.4 ± 3.4 | 0.25 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timm, K.; Walter, N.; Heinrich, M.; Knapp, G.; Thormann, U.; El Khassawna, T.; Alt, V.; Heiss, C.; Rupp, M. Influence of Thoracic Trauma on Fracture Healing in Long Bones—A Retrospective Analysis. J. Clin. Med. 2022, 11, 717. https://doi.org/10.3390/jcm11030717
Timm K, Walter N, Heinrich M, Knapp G, Thormann U, El Khassawna T, Alt V, Heiss C, Rupp M. Influence of Thoracic Trauma on Fracture Healing in Long Bones—A Retrospective Analysis. Journal of Clinical Medicine. 2022; 11(3):717. https://doi.org/10.3390/jcm11030717
Chicago/Turabian StyleTimm, Karsten, Nike Walter, Martin Heinrich, Gero Knapp, Ulrich Thormann, Thaqif El Khassawna, Volker Alt, Christian Heiss, and Markus Rupp. 2022. "Influence of Thoracic Trauma on Fracture Healing in Long Bones—A Retrospective Analysis" Journal of Clinical Medicine 11, no. 3: 717. https://doi.org/10.3390/jcm11030717
APA StyleTimm, K., Walter, N., Heinrich, M., Knapp, G., Thormann, U., El Khassawna, T., Alt, V., Heiss, C., & Rupp, M. (2022). Influence of Thoracic Trauma on Fracture Healing in Long Bones—A Retrospective Analysis. Journal of Clinical Medicine, 11(3), 717. https://doi.org/10.3390/jcm11030717






