Inflammatory Predictors of Prognosis in Patients with Traumatic Cerebral Haemorrhage: Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatments
2.3. Haematological Variables and White Blood Cells
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Haematological Variables and White Blood Cells
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brazinova, A.; Rehorcikova, V.; Tayloro, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Teadom, A.; Halkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A living systematic review. J. Neurotrauma 2015, 38, 1411–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, G.; Murray, G.; Parker, L.; Jennett, B. Adding up the Glasgow Coma Score. Acta Neurochir. Suppl. 1979, 28, 13–16. [Google Scholar] [PubMed]
- Hagbood, I.R.; Mass, D.K.; Menon, E.W.; Stayeberg, G.; Citerio, F.; Lecky, G.T.; Manley, S.; Hill, V.; Legrand, A. Sorgner: Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A prospective longitudinal observational study. Neurosurgery 2015, 76, 67–80. [Google Scholar]
- Salomond, C.H.; Menon, D.K.; Chatfield, D.A.; Williams, G.B.; Pena, A.; Sahakian, B.J.; Pickard, J.D. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. Neuroimage 2006, 29, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Nockels, R.P.; Pitts, L.H.; Noble, L.J. Breakdown of the blood-brain barrier after fluid percussion brain injury in the rat: Part 2: Effect of hypoxia on permeability to plasma proteins. J. Neurotrauma 1992, 9, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Alonso, O.; Halley, M. Early microvascular and neuronal consequences of traumatic brain injury: A light and electron microscopic study in rats. J. Neurotrauma 1994, 11, 289–301. [Google Scholar] [CrossRef]
- Habgood, M.D.; Bye, M.N.; Dzięgielewska, K.M.; Ek, C.J.; Lane, M.A.; Potter, A.; Morganti-Kossmann, C.; Sauders, N.R. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur. J. Neurosci. 2007, 25, 231–238. [Google Scholar] [CrossRef]
- Lotocki, G.; Pablo de Rivero Vaccari, J.; Rerez, E.R.; Sanchez-Molano, J.; Furones-Alonso, O.; Bramlett, H.M.; Dietrich, W.D. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of post-traumatic hypothermia. J. Neurotrauma 2009, 26, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Stahel, P.F.; Shohami, E.; Younis, F.M.; Kariya, K.; Otto, V.I.; Lenzlinger, P.M.; Grosjean, M.B.; Eugster, H.P.; Trentz, O.; Kossmann, T.; et al. Experimental closed head injury: Analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000, 20, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Lucas, S.-M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), 232–240. [Google Scholar] [CrossRef] [Green Version]
- Chatzipanteli, K.; Alonso, O.F.; Kraydieh, S.; Dietrich, W.D. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: Biochemical and immunocytochemical studies. J. Cereb. Blodd Flow Metab. 2000, 20, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Alonso, O.; Busto, R.; Globus, M.Y.; Ginsberg, M.D. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994, 87, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Chatzipanteli, K.; Olonso, O.F.; Howard, M.; Dietrich, W.D. The effect of brain temperature on hemoglobin extravasation after traumatic brain injury. J. Neurosurg. 2002, 97, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Schoettle, R.J.; Kochanek, P.M.; Magargee, M.J.; Uhl, M.W.; Nemoto, E.M. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J. Neurotrauma 1990, 7, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- Brennan, J.H.; Bernard, S.; Cameron, P.A.; Rosenfeld, J.V.; Mitra, B. Ethanol and isolated traumatic brain injury. J. Clin. Neurosci. 2015, 22, 1375–1381. [Google Scholar] [CrossRef]
- Chen, J.; Qu, X.; Li, Z.; Zhang, D.; Hou, L. Peak Neutrophil-to-Lymphocyte Ratio Correlates with Clinical Outcomes in Patients with Severe Traumatic Brain Injury. Neurocritical Care 2019, 30, 334–339. [Google Scholar] [CrossRef]
- Zhuang, D.; Sheng, J.; Peng, G.; Li, T.; Cai, S.; Din, F.; Li, L.; Huang, M.; Tian, F.; Li, K.; et al. Neutrophil to lymphocyte ratio predicts early growth of traumatic intracerebral haemorrhage. Ann. Clin. Trans. Neur 2021, 8, 1601–1609. [Google Scholar] [CrossRef]
- Haugdahl Nost, T.; Alcala, K.; Ubarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.W.; Yoo, H.M.; Park, C.H.; Song, K.Y. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Gastrointest. Oncol. 2015, 22, 4363–4370. [Google Scholar] [CrossRef]
- Sahin, F.; Yildiz, P. Serum platelet, MPV, PCT and PDW values, neutrophil to lymphocyte and platelet to lymphocyte ratios in lung cancer diagnosis. Eur. Resp. J. 2015, 46, PA4279. [Google Scholar]
- Tang, J.-N.; Goyal, H.; Yu, S.; Luo, H. Prognostic value of systemic immune-inflammation index (SII) in cancers: A systematic review and meta-analysis. J. Lab. Precis. Med. 2018, 3, 29. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [Green Version]
- Erdal, E.; Inanir, M. Platelet-to-lymphocyte ratio (PLR) and Plateletcrit (PCT) in young patients with morbid obesity. Rev. Assoc. Médica Bras. 2019, 65, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Furuncuiglu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [Green Version]
- Kurtul, M.; Yarlioglues, S.N.; Murat, G.; Ergun, M.; Duran, H.A.; Kasapkara, M.B.; Demircelik, M.; Cetin, A.H. Ocek: Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am. J. Cardiol. 2014, 114, 342–347. [Google Scholar] [CrossRef]
- Schneider, D.J. Factors Contributing to Increased Platelet Reactivity in People with Diabetes. Diabetes Care 2009, 32, 525–527. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ying, A.; Lin, Y.; Yu, J.; Luo, J.; Zeng, Y.; Lin, Y. Neutrophil-to-lymphocyte ratio, hyperglycemia, and outcomes in ischemic stroke patients treated with intravenous thrombolysis. Brain Behav. 2020, 10, e01741. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.; Xiao, Y.; Gu, C.; et al. Normal Reference Intervals of Neutrophil-To-Lymphocyte Ratio, Platelet-To-Lymphocyte Ratio, Lymphocyte-To-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019, 65, 255–265. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the management of severe traumatic brain injury 4th edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Dijkland, S.A.; Foks, K.A.; Polinder, S.; Dippel, D.W.J.; Maas, A.I.R.; Lingsma, H.F.; Steyerberg, E.W. Prognosis in Moderate and Severe Traumatic Brain Injury: A Systematic Review of Contemporary Models and Validation Studies. J. Neurotrauma 2020, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.; Manley, G.T. Classification of traumatic brain injury: Past, present, and future. Handb. Clin. Neurol. 2015, 127, 15–21. [Google Scholar] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 9 October 2021).
- Miekisiak, G.; Czyż, M.; Tykocki, T.; Kaczmarczyk, J.; Załuski, R.; Łątka, D. Traumatic brain injury in Poland from 2009–2012: A national study on incidence. Brain Injury 2016, 30, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.; Mai, J.C.; Ecklund, J. Neurosurgical management in traumatic brain injury. Semin. Neurol. 2015, 35, 50–56. [Google Scholar] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Lämmermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmüller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2020, 498, 371–375. [Google Scholar] [CrossRef]
- Sabouri, E.; Majdi, A.; Jangjui, P.; Aghsan, S.R.; Alavi, S.A.N. Neutrophil-to-Lymphocyte Ratio and Traumatic Brain Injury: A Review Study. World Neurosurg 2020, 140, 142–147. [Google Scholar] [CrossRef]
- Siwicka-Gieroba, D.; Malodobry, K.; Biernawska, J.; Robba, C.; Bohatyrewicz, R.; Rola, R.; Dabrowski, W. The Neutrophil/Lymphocyte Count Ratio Predicts Mortality in Severe Traumatic Brain Injury Patients. J. Clin. Med. 2019, 8, 1453. [Google Scholar] [CrossRef] [Green Version]
- Acar, E.; Demir, A.; Alatas, Ö.D.; Beydilli, H.; Yıldırım, B.; Kırlı, U.; Hazer, D.B.; Kılınç, M.R.; Karagöz, Ü.; Derin, S. Evaluation of hematological markers in minor head trauma in the emergency room. Eur. J. Trauma Emerg. Surg. 2016, 42, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Reznik, M.E.; Kalagara, R.; Moody, S.; Drake, J.; Margolis, S.A.; Cizginer, S.; Mahta, A.; Rao, S.S.; Stretz, C.; Wendell, L.C.; et al. Affiliations expand Common biomarkers of physiologic stress and associations with delirium in patients with intracerebral haemorrhage. J. Crit. Care 2021, 64, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Trifan, G.; Testai, F.D. Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral haemorrhage. J. Stroke Cereb. Dis. 2020, 29, 105057. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.-M.; Ho, K.M.; Honeybul, S. Prognostic significance of abnormal hematological parameters in severe traumatic brain injury requiring decompressive craniectomy. J. Neurosurg. 2019, 132, 545–551. [Google Scholar] [CrossRef]
- Herbert, J.P.; Guillotte, A.R.; Hammer, R.D.; Litofsky, N.S. Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences. Brain Sci. 2017, 7, 92. [Google Scholar] [CrossRef]
- Xu, B.; Chandrasekar, A.; Heuvel, F.; Powerski, M.; Nowak, A.; Noack, L.; Omari, J.; Huber-Lang, M.; Roselli, F.; Relja, B. Ethanol Intoxication Alleviates the Inflammatory Response of Remote Organs to Experimental Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 8181. [Google Scholar] [CrossRef]
| Patients GCS > 8 | Patients GCS ≤ 8 | |
|---|---|---|
| Characteristics | ||
| Number of subjects | 75 | 20 |
| Median age (years) | 51 (21–100) | 29 (18–85) |
| Mean ages (years) | 53.9 ± 18.6 | 37.8 ± 19.2 |
| Number of females, n (%) | 12 (16) | 4 (20) |
| Number of males, n (%) | 63 (84) | 16 (80) |
| Surgery within the first 24 h, n (%) | 7 (9.3) | 7 (35) |
| Injury mechanisms, n (%) | ||
| Falls | 34 (45.3) | 6 (30) |
| Traffic accident | 7 (9.3) | 12 (55) |
| Seizures | 12 (16) | 0 (0) |
| Violence | 6 (8) | 1 (5) |
| Others/unknown | 16 (21.3) | 2 (10) |
| Reference Values | Patients GCS > 8 | Patients GCS ≤ 8 | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Med (iqr 25–75%) | Mean ± SD | Med (iqr 25–75%) | p-Value | ||
| RBC (106/µL) | 4.2–6.5 | 4.3 ± 0.8 | 4.4 (3.9–4.7) | 4.3 ± 0.7 | 4.3 (3.8–4.8) | 0.823 |
| HB (g/dL) | 12.0–18.0 | 13.7 ± 1.8 | 14.1 (12.6–14.9) | 13.2 ± 1.8 | 13.4 (12.2–4.6) | 0.308 |
| HCT (%) | 38.0–54.0 | 42.2 ± 10.2 | 42.1 (38.1–44.4) | 39.0 ± 5.2 | 39.7 (35.8–42.7) | 0.111 |
| MCV (fL) | 80.0–97.0 | 93.7 ± 11.5 | 94.8 (90.9–99.0) | 89.3 ± 12.7 | 89.6 (86.8–96.9) | 0.008 |
| MCH (pg/RBC) | 26.0–32.0 | 32.1 ± 2.0 | 31.7 (30.6–33.6) | 33.8 ± 12.6 | 31.1 (29.2–33.0) | 0.124 |
| MCHC (g/dL) | 31.0–36.0 | 33.4 ± 2.6 | 33.6 (32.9–34.4) | 33.6 ± 1.6 | 33.2 (32.4–34.8) | 0.658 |
| RDW (%) | 11.5–14.8 | 13.0 ± 2.1 | 12.2 (11.3–14.0) | 13.3 ± 5.6 | 12.4 (11.0–14.2) | 0.161 |
| Glucose (mg/dL) | 60.0–99.0 | 132.4 ± 40.5 | 124 (107–155) | 164.1 ± 88.2 | 155 (127–195) | p < 0.05 |
| Reference Values | Patients GCS > 8 | Patients GCS ≤ 8 | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Med (iqr 25–75%) | Mean ± SD | Med (iqr 25–75%) | p-Value | ||
| WBC (103/µL) | 4.0–10.2 | 12.1 ± 4.8 | 11.2 (8.7–14.3) | 22.3 ± 3.7 | 16.0 (12.2–22.3) | p < 0.001 |
| Neutrophils (103/µL) | 2.0–6.9 | 9.9 ± 3.1 | 8.7 (6.0–12.0) | 13.5 ± 4.8 | 12.7 (9.5–17.3) | p < 0.01 |
| Lymphocytes (103/µL) | 0.6–3.4 | 1.9 ± 1.2 | 1.6 (1.2–2.2) | 3.4 ± 2.9 | 2.5 (1.3–2.8) | 0.451 |
| Monocytes (103/µL) | 0.00–0.90 | 0.8 ± 0.5 | 0.6 (0.5–0.9) | 2.0 ± 0.5 | 0.9 (0.6–1.2) | p < 0.05 |
| Platelets (103/µL) | 140–420 | 211 ± 74 | 207 (159–247) | 226 ± 103 | 221 (190–247) | 0.067 |
| NLR (103/µL) | 0.87–4.15 | 5.4 ± 3.3 | 5.3 (3.1–6.4) | 9.6 ± 6.4 | 8.3 (5.3–9.7) | p < 0.001 |
| PLR (103/µL) | 47–198 | 119 ± 44 | 119 (92–144) | 146 ± 71 | 142 (86–162) | 0.340 |
| LMR (103/µL) | 2.45–8.77 | 3.1 ± 1.9 | 3.1 (1.8–3.4) | 2.9 ± 2.3 | 2.6 (1.3–3.2) | 0.287 |
| SII (103/µL) | 142–808 | 995 ± 535 | 977 (588–1216) | 2081 ± 1244 | 1970 (1188–2234) | p < 0.0001 |
| NLR | PLR | LMR | SII | |
|---|---|---|---|---|
| GCS | R = −0.318 p < 0.0001 | R = −0.047 0.621 | R = 0.153 0.105 | R = −0.357 p < 0.0001 |
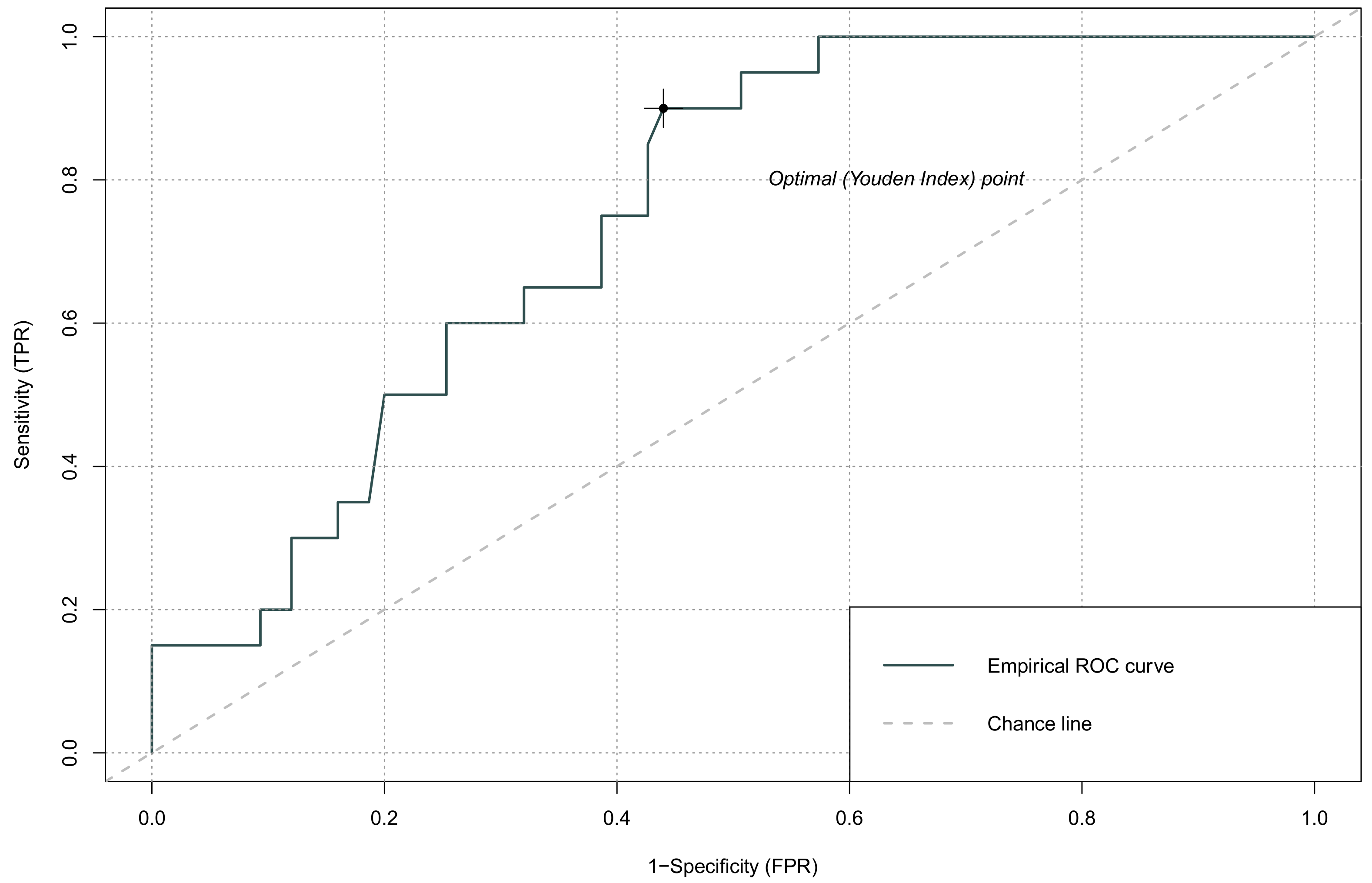
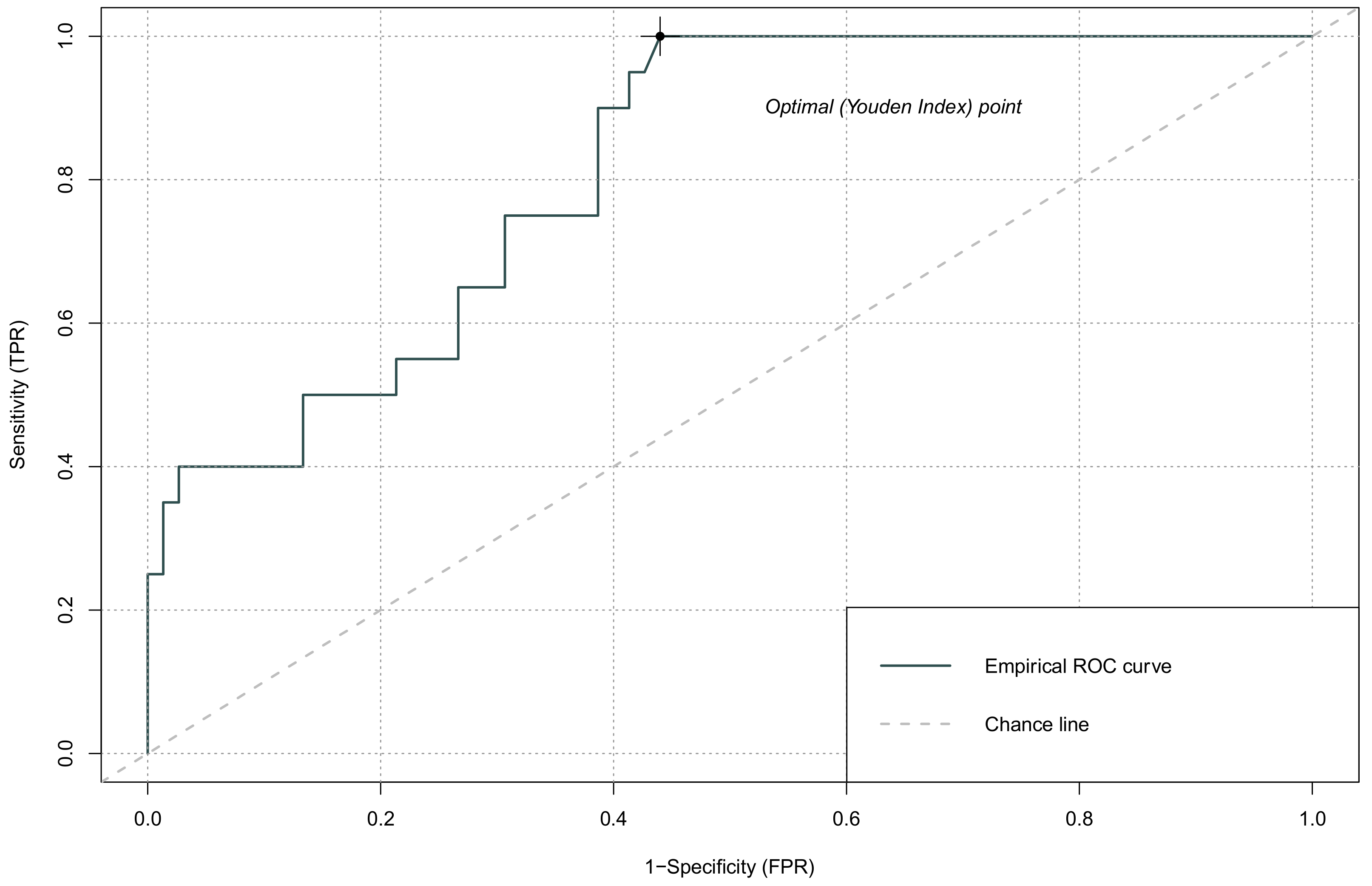
| AUC (95% CI) | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|
| NLR | 0.748 (0.615–0.880) | 0.154 | 0.90 | 0.56 |
| PLR | 0.570 (0.424–0.715) | 0.244 | 0.40 | 0.82 |
| LMR | 0.422 (0.285–0.559) | 0.227 | 0.35 | 0.84 |
| SII | 0.816 (0.696–0.935) | 0.118 | 0.95 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defort, P.; Retkowska-Tomaszewska, N.; Kot, M.; Jarmużek, P.; Tylutka, A.; Zembron-Lacny, A. Inflammatory Predictors of Prognosis in Patients with Traumatic Cerebral Haemorrhage: Retrospective Study. J. Clin. Med. 2022, 11, 705. https://doi.org/10.3390/jcm11030705
Defort P, Retkowska-Tomaszewska N, Kot M, Jarmużek P, Tylutka A, Zembron-Lacny A. Inflammatory Predictors of Prognosis in Patients with Traumatic Cerebral Haemorrhage: Retrospective Study. Journal of Clinical Medicine. 2022; 11(3):705. https://doi.org/10.3390/jcm11030705
Chicago/Turabian StyleDefort, Piotr, Natalia Retkowska-Tomaszewska, Marcin Kot, Paweł Jarmużek, Anna Tylutka, and Agnieszka Zembron-Lacny. 2022. "Inflammatory Predictors of Prognosis in Patients with Traumatic Cerebral Haemorrhage: Retrospective Study" Journal of Clinical Medicine 11, no. 3: 705. https://doi.org/10.3390/jcm11030705
APA StyleDefort, P., Retkowska-Tomaszewska, N., Kot, M., Jarmużek, P., Tylutka, A., & Zembron-Lacny, A. (2022). Inflammatory Predictors of Prognosis in Patients with Traumatic Cerebral Haemorrhage: Retrospective Study. Journal of Clinical Medicine, 11(3), 705. https://doi.org/10.3390/jcm11030705






