Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Design and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Group Composition
3.2. PWS Cohort in Comparison with Healthy Children of the PLUTO Cohort
3.2.1. Prenatal Characteristics
3.2.2. Labor Characteristics
3.2.3. Birth Measurements
3.3. PWS Cohort in Comparison with the (Dutch) General Population Statistics
3.3.1. Prenatal Characteristics
3.3.2. Labor Characteristics
3.4. Comparison between Genetic PWS Subtypes
3.4.1. Prenatal and Labor Characteristics
3.4.2. Birth Measurements
3.4.3. Neonatal Characteristics
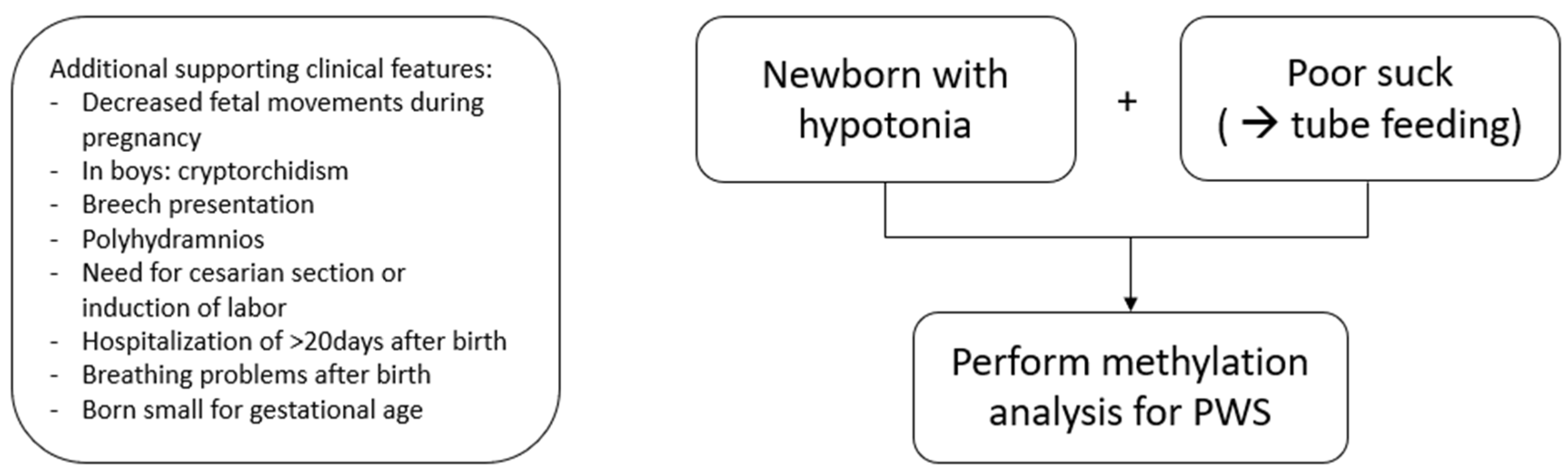
3.5. When to Perform Genetic Testing for PWS in Newborns and Infants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, V.A.; Cassidy, S.B.; Butler, M.G.; Hanchett, J.M.; Greenswag, L.R.; Whitman, B.Y.; Greenberg, F. Prader-Willi Syndrome: Consensus Diagnostic Criteria. Pediatrics 1993, 91, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F. Prader-Willi syndrome and the hypothalamus. Acta Paediatr. 1997, 86, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lionti, T.; Reid, S.M.; White, S.M.; Rowell, M.M. A population-based profile of 160 Australians with Prader-Willi syndrome: Trends in diagnosis, birth prevalence and birth characteristics. Am. J. Med Genet. Part A 2014, 167, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Vogels, A.; Ende, J.V.D.; Keymolen, K.; Mortier, G.; Devriendt, K.; Legius, E.; Fryns, J.-P. Minimum prevalence, birth incidence and cause of death for Prader–Willi syndrome in Flanders. Eur. J. Hum. Genet. 2003, 12, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.; Mahmoud, R.; Cassidy, S.B.; Kimonis, V. Comparison of perinatal factors in deletion versus uniparental disomy in Prader–Willi syndrome. Am. J. Med. Genet. Part A 2018, 176, 1161–1165. [Google Scholar] [CrossRef]
- Dobrescu, A.-I.; Chirita-Emandi, A.; Andreescu, N.; Farcas, S.; Puiu, M. Does the Genetic Cause of Prader-Willi Syndrome Explain the Highly Variable Phenotype? Maedica 2016, 11, 191–197. [Google Scholar]
- Ciana, G.; Fertz, M.C.; Pecile, V.; DeMarini, S. Premature Birth with Complicated Perinatal Course Delaying Diagnosis of Prader-Willi Syndrome. Case Rep. Pediatr. 2011, 2011, 981941. [Google Scholar] [CrossRef]
- Festen, D.A.M.; Wevers, M.; Lindgren, A.C.; Böhm, B.; Otten, B.J.; Wit, J.M.; Duivenvoorden, H.J.; Hokken-Koelega, A.C.S. Mental and motor development before and during growth hormone treatment in infants and toddlers with Prader–Willi syndrome. Clin. Endocrinol. 2007, 68, 919–925. [Google Scholar] [CrossRef]
- Bakker, N.E.; Kuppens, R.J.; Siemensma, E.P.C.; Tummers-de Lind Van Wijngaarden, R.F.A.; Festen, D.A.M.; Bindels-de Heus, G.C.B.; Bocca, G.; Haring, D.A.J.P.; Hoorweg-Nijman, J.J.G.; Houdijk, E.C.A.M.; et al. Eight Years of Growth Hormone Treatment in Children With Prader-Willi Syndrome: Maintaining the Positive Effects. J. Clin. Endocrinol. Metab. 2013, 98, 4013–4022. [Google Scholar] [CrossRef] [Green Version]
- Carrel, A.L.; Myers, S.E.; Whitman, B.Y.; Eickhoff, J.; Allen, D.B. Long-Term Growth Hormone Therapy Changes the Natural History of Body Composition and Motor Function in Children with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 1131–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donze, S.H.; Damen, L.; Mahabier, E.F.; Hokken-Koelega, A.C. Cognitive functioning in children with Prader–Willi syndrome during 8 years of growth hormone treatment. Eur. J. Endocrinol. 2020, 182, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Donze, S.H.; Damen, L.; Mahabier, E.F.; Hokken-Koelega, A.C.S. Improved Mental and Motor Development During 3 Years of GH Treatment in Very Young Children With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 3714–3719. [Google Scholar] [CrossRef] [Green Version]
- Magill, L.; Laemmer, C.; Woelfle, J.; Fimmers, R.; Gohlke, B. Early start of growth hormone is associated with positive effects on auxology and metabolism in Prader-Willi-syndrome. Orphanet J. Rare Dis. 2020, 15, 283. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Ambroziak, A.; Wysocka-Mincewicz, M.; Doleżal-Ołtarzewska, K.; Zygmunt-Górska, A.; Wędrychowicz, A.; Żak, T.; Noczyńska, A.; Birkholz-Walerzak, D.; Stawerska, R.; Hilczer, M.; et al. Effects of Recombinant Human Growth Hormone Treatment, Depending on the Therapy Start in Different Nutritional Phases in Paediatric Patients with Prader–Willi Syndrome: A Polish Multicentre Study. J. Clin. Med. 2021, 10, 3176. [Google Scholar] [CrossRef]
- Corripio, R.; Tubau, C.; Calvo, L.; Brun, C.; Capdevila, N.; Larramona, H.; Gabau, E. Safety and effectiveness of growth hormone therapy in infants with Prader-Willi syndrome younger than 2 years: A prospective study. J. Pediatr. Endocrinol. Metab. 2019, 32, 879–884. [Google Scholar] [CrossRef]
- Bacheré, N.; Diene, G.; Delagnes, V.; Molinas, C.; Moulin, P.; Tauber, M. Early Diagnosis and Multidisciplinary Care Reduce the Hospitalization Time and Duration of Tube Feeding and Prevent Early Obesity in PWS Infants. Horm. Res. Paediatr. 2007, 69, 45–52. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Q.; Ma, B.; Mao, S.; Dai, Y.; Zhu, M.; Zou, C. Perinatal features of Prader-Willi syndrome: A Chinese cohort of 134 patients. Orphanet J. Rare Dis. 2020, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Mahmoud, R.; Gold, J.-A.; Miller, J.L.; Roof, E.; Tamura, R.; Dykens, E.; Butler, M.G.; Driscoll, D.J.; Kimonis, V. Multicentre study of maternal and neonatal outcomes in individuals with Prader-Willi syndrome. J. Med. Genet. 2018, 55, 594–598. [Google Scholar] [CrossRef]
- Salvatoni, A.; Moretti, A.; Grugni, G.; Agosti, M.; Azzolini, S.; Bonaita, V.; Cianci, P.; Corica, D.; Crinò, A.; DelVecchio, M.; et al. Anthropometric characteristics of newborns with Prader–Willi syndrome. Am. J. Med. Genet. Part A 2019, 179, 2067–2074. [Google Scholar] [CrossRef]
- Bar, C.; Diene, G.; Molinas, C.; Bieth, E.; Casper, C.; Tauber, M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J. Rare Dis. 2017, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecka-Ambroziak, A.; Wysocka-Mincewicz, M.; Doleżal-Ołtarzewska, K.; Zygmunt-Górska, A.; Żak, T.; Noczyńska, A.; Birkholz-Walerzak, D.; Stawerska, R.; Hilczer, M.; Obara-Moszyńska, M.; et al. Correlation of Genotype and Perinatal Period, Time of Diagnosis and Anthropometric Data before Commencement of Recombinant Human Growth Hormone Treatment in Polish Patients with Prader–Willi Syndrome. Diagnostics 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Murakami, N.; Nagai, T.; Ogata, T. Maternal age effect on the development of Prader–Willi syndrome resulting from upd(15)mat through meiosis 1 errors. J. Hum. Genet. 2011, 56, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Festen, D.A.M.; Wijngaarden, R.D.L.V.; Van Eekelen, M.; Otten, B.J.; Wit, J.M.; Duivenvoorden, H.J.; Hokken-Koelega, A.C.S. Randomized controlled GH trial: Effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin. Endocrinol. 2008, 69, 443–451. [Google Scholar] [CrossRef]
- de Fluiter, K.S.; van Beijsterveldt, I.A.L.P.; Breij, L.M.; Acton, D.; Hokken-Koelega, A.C.S. Association Between Fat Mass in Early Life and Later Fat Mass Trajectories. JAMA Pediatr. 2020, 174, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.; Steegers, E.A.P.; Biharie, A.A.; MacKenbach, J.P.; Hofman, A.; Jaddoe, V.W.V. Explaining differences in birth outcomes in relation to maternal age: The Generation R Study. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 500–509. [Google Scholar] [CrossRef]
- Euro-Peristat Project. European Perinatal Health Report. Health and Care of Pregant Women and Babies in Europe in 2010. Available online: www.europeristat.com (accessed on 3 June 2021).
- Durmuş, B.; Arends, L.R.; Ay, L.; Hokken-Koelega, A.C.; Raat, H.; Hofman, A.; Steegers, E.A.P.; Jaddoe, V.W.V. Parental anthropometrics, early growth and the risk of overweight in pre-school children: The Generation R Study. Pediatr. Obes. 2012, 8, 339–350. [Google Scholar] [CrossRef]
- Andersen, A.-M.N.; Wohlfahrt, J.; Christens, P.; Olsen, J.; Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 2000, 320, 1708–1712. [Google Scholar] [CrossRef] [Green Version]
- Nederlandse Richtlijn voor Obstetrie en Gynaecologie: Hypertensieve Aandoeningen in de Zwangerschap. Richtlijnen NVOG 2005. Available online: https://richtlijnendatabase.nl/richtlijn/hypertensieve_aandoeningen_in_de_zwangerschap/hypertensieve_aandoeningen_-_startpagina.html (accessed on 3 June 2021).
- van Leeuwen, M.; Prins, S.M.; de Valk, H.W.; Evers, I.M.; Visser, G.H.A.; Mol, B.W.J. Gestational diabetes mellitus: Treatment reduces the risk of complications. Ned Tijdschr Geneeskd 2011, 155, A2291. [Google Scholar]
- Nederlandse Vereniging Voor Obstetrie en Gynaecologie. Breken van de Vliezen Voor Het Begin Van De Baring. Richtlijnen NVOG 2002. Richtlijnen NVOG 2002. Available online: https://www.nvog.nl/wp-content/uploads/2017/12/Breken-van-de-vliezen-voor-het-begin-van-de-baring-1.0-18-04-2002.pdf (accessed on 3 June 2021).
- Havnen, G.C.; Truong, M.B.-T.; Do, M.-L.H.; Heitmann, K.; Holst, L.; Nordeng, H. Women’s perspectives on the management and consequences of hyperemesis gravidarum—A descriptive interview study. Scand. J. Prim. Health Care 2019, 37, 30–40. [Google Scholar] [CrossRef]
- Cardwell, M.S. Polyhydramnios. Obstet. Gynecol. Surv. 1987, 42, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Panting-Kemp, A.; Nguyen, T.; Chang, E.; Quillen, E.; Castro, L. Idiopathic polyhydramnios and perinatal outcome. Am. J. Obstet. Gynecol. 1999, 181, 1079–1082. [Google Scholar] [CrossRef]
- Hou, L.; Wang, X.; Hellerstein, S.; Zou, L.; Ruan, Y.; Zhang, W. Delivery mode and perinatal outcomes after diagnosis of oligohydramnios at term in China. J. Matern. Neonatal Med. 2018, 33, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Nederlandse Vereniging Voor Obstetrie en Gynaecologie. Beleid Zwangerschap na 41 Weken. Richtlijnen NVOG 2021. Available online: https://richtlijnendatabase.nl/richtlijn/beleid_zwangerschap_41_weken/beleid_zwangerschap_vanaf_41_weken.html#:~:text=Opbasisvandelandelijke,2%25na42wekenzwangerschap (accessed on 3 June 2021).
- Nederlandse Vereniging Voor Obstetrie en Gynaecologie. Stuitligging. Richtlijnen NVOG 2008. Available online: https://www.nvog.nl/wp-content/uploads/2019/03/Stuitligging-2008.pdf (accessed on 3 June 2021).
- Schönbeck, Y.; Talma, H.; Van Dommelen, P.; Bakker, B.; Buitendijk, S.E.; HiraSing, R.A.; van Buuren, S. The world’s tallest nation has stopped growing taller: The height of Dutch children from 1955 to 2009. Pediatr. Res. 2012, 73, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the Child Born Small for Gestational Age through to Adulthood: A Consensus Statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, M.-M.; Gao, Y.-Y.; Wu, B.-B.; Yan, K.; Qin, Q.; Wang, H.; Zhou, W.; Yang, L. Relationship between phenotype and genotype of 102 Chinese newborns with Prader–Willi syndrome. Mol. Biol. Rep. 2019, 46, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T. Meiosis in oocytes: Predisposition to aneuploidy and its increased incidence with age. Hum. Reprod. Update 2007, 14, 143–158. [Google Scholar] [CrossRef]
- Eggermann, T.; Soellner, L.; Buiting, K.; Kotzot, D. Mosaicism and uniparental disomy in prenatal diagnosis. Trends Mol. Med. 2015, 21, 77–87. [Google Scholar] [CrossRef]
- Dudley, O.; Muscatelli, F. Clinical evidence of intrauterine disturbance in Prader-Willi syndrome, a genetically imprinted neurodevelopmental disorder. Early Hum. Dev. 2007, 83, 471–478. [Google Scholar] [CrossRef]
- Whittington, J.; Butler, J.; Holland, A. Pre-, peri- and postnatal complications in Prader–Willi syndrome in a UK sample. Early Hum. Dev. 2008, 84, 331–336. [Google Scholar] [CrossRef]
- Cox, G.F.; Bürger, J.; Lip, V.; Mau, U.A.; Sperling, K.; Wu, B.-L.; Horsthemke, B. Intracytoplasmic Sperm Injection May Increase the Risk of Imprinting Defects. Am. J. Hum. Genet. 2002, 71, 162–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, M.; Katalinic, A.; Groß, S.; Sutcliffe, A.; Varon, R.; Horsthemke, B. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J. Med. Genet. 2005, 42, 289–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, E.R.; Brueton, L.A.; Bowdin, S.C.; Luharia, A.; Cooper, W.; Cole, T.R.; Macdonald, F.; Sampson, J.R.; Barratt, C.; Reik, W.; et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J. Med. Genet. 2003, 40, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Gross, N.; Rabinowitz, R.; Gross-Tsur, V.; Hirsch, H.J.; Eldar-Geva, T. Prader-Willi syndrome can be diagnosed prenatally. Am. J. Med. Genet. Part A 2014, 167, 80–85. [Google Scholar] [CrossRef]
- Butler, M.G.; Sturich, J.; Myers, S.E.; Gold, J.-A.; Kimonis, V.; Driscoll, D.J. Is gestation in Prader-Willi syndrome affected by the genetic subtype? J. Assist. Reprod. Genet. 2009, 26, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Schaller, F.; Watrin, F.; Sturny, R.; Massacrier, A.; Szepetowski, P.; Muscatelli, F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 2010, 19, 4895–4905. [Google Scholar] [CrossRef]
- Swaab, D.F.; Purba, J.S.; Hofman, M.A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. J. Clin. Endocrinol. Metab. 1995, 80, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Goldkrand, J.W.; Schulte, R.L.; Messer, R.H. Maternal and fetal plasma cortisol levels at parturition. Obstet. Gynecol. 1976, 47, 41–45. [Google Scholar]
- Wijngaarden, R.D.L.V.; Otten, B.; Festen, D.; Joosten, K.; De Jong, F.; Sweep, F.; Hokken-Koelega, A. High Prevalence of Central Adrenal Insufficiency in Patients with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 1649–1654. [Google Scholar] [CrossRef]
- Cizmecioglu, F.M.; Jones, J.H.; Paterson, W.F.; Kherra, S.; Kourime, M.; McGowan, R.; Shaikh, M.G.; Donaldson, M. Neonatal Features of the Prader-Willi Syndrome; The Case for Making the Diagnosis During the First Week of Life. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 264–273. [Google Scholar] [CrossRef]
- Goldstone, A.; Holland, A.J.; Hauffa, B.P.; Hokken-Koelega, A.C.; Tauber, M. Recommendations for the Diagnosis and Management of Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4183–4197. [Google Scholar] [CrossRef]
- Gubbels, E.; Cuyx, S.; Hermans, C.; Panis, B. Hypotonie bij neonaten. Ned Tijdschr Geneeskd 2019, 163, 1–8. [Google Scholar]
- Laugel, V.; Cossée, M.; Matis, J.; De Saint-Martin, A.; Echaniz-Laguna, A.; Mandel, J.-L.; Astruc, D.; Fischbach, M.; Messer, J. Diagnostic approach to neonatal hypotonia: Retrospective study on 144 neonates. Eur. J. Pediatr. 2007, 167, 517–523. [Google Scholar] [CrossRef]
| PWS Cohort (n = 244) | PLUTO Cohort (n = 365) | p-Value * | Population Statistics | p-Value ** | |
|---|---|---|---|---|---|
| Maternal and Pregnancy Characteristics | |||||
| Maternal age (years) | 33.0 (30.0; 37.0) | 32.0 (29.0; 36.0) | 0.078 | 30.2 [26] | <0.001 |
| High maternal age (>35 years) | 92 (40.5) | 115 (31.7) | 0.028 | 21.6 [27] | <0.001 |
| Paternal age (years) | 34.0 (32.0; 39.0) | 34.0 (31.0; 38.0) | 0.112 | 33.0 [28] | <0.001 |
| High paternal age (>35 years) | 108 (49.1) | 137 (45.1) | 0.362 | NF | NA |
| Maternal pre-pregnancy BMI (kg/m2) | 0.008 | NF | NA | ||
| BMI < 20 | 7 (5.8) | 39 (11.4) | |||
| BMI 20–25 | 86 (71.7) | 188 (55.0) | |||
| BMI 25–30 | 23 (19.2) | 78 (22.8) | |||
| BMI 30–35 | 4 (3.3) | 25 (7.3) | |||
| BMI > 35 | 0 (0.0) | 12 (3.5) | |||
| Parity | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 0.599 | NF | NA |
| History of miscarriage | 50 (22.0) | 88 (24.1) | 0.560 | 15–20 [29] | 0.044 |
| Medically assisted reproduction | 10 (4.5) | 25 (6.8) | 0.250 | 2.8 [27] | 0.088 |
| Hypertension/pre-eclampsia | 13 (5.8) | 30 (8.2) | 0.075 | 1–3 [30] | <0.001 |
| Gestational diabetes | 6 (2.7) | 14 (3.8) | 0.457 | 2–5 [31] | 0.317 |
| Premature rupture of membranes | 19 (8.5) | 19 (5.2) | 0.113 | 1–8 [32] | <0.001 |
| Hyperemesis | 14 (6.3) | 19 (5.2) | 0.584 | 0.3–3 [33] | <0.001 |
| Amniotic fluid | |||||
| Polyhydramnios | 57 (27.3) | 2 (0.5) | <0.001 | 0.2–3.9 [34,35] | <0.001 |
| Oligohydramnios | 16 (7.7) | 1 (0.3) | NA | <1.0–4.4 [36] | <0.001 |
| Labor Characteristics | |||||
| Term | <0.001 # | ||||
| Preterm <37 weeks | 42 (17.6) | # | 7.9 [27] | <0.001 | |
| Full term 37–41 weeks | 131 (54.8) | 316 (86.6) | 74.4 | <0.001 | |
| Late/Post-term >41 weeks | 66 (27.6) | 49 (13.4) | 17.7 [37] | <0.001 | |
| Mode of delivery | <0.001 | ||||
| Vaginal delivery | 102 (46.8) | 212 (58.6) | 73 [27] | <0.001 | |
| Assisted delivery | 15 (6.9) | 45 (12.4) | 10 [27] | 0.418 | |
| Caesarean section (CS) | 101 (46.3) | 105 (29.0) | 17 [27] | <0.001 | |
| Type of CS | 0.594 | 0.260 | |||
| Primary | 40 (41.2) | 45 (45.0) | 45.0 [27] | ||
| Secondary | 57 (58.8) | 55 (55.0) | 55.0 [27] | ||
| Induction of labor | 86 (38.6) | 44 (12.1) | <0.001 | 21.4 [27] | <0.001 |
| Breech presentation | 70 (31.0) | 17 (4.7) | <0.001 | 3–4 [38] | <0.001 |
| Birth Measurements | |||||
| Male | 130 (53.3) | 210 (57.5) | 0.300 | NF | NA |
| Birth weight SDS | −1.06 (−1.78; −0.34) | 0.26 (−0.49; 1.00) | <0.001 | NF | NA |
| Birth length SDS | −0.27 (−1.32; 0.72) | 0.73 (−0.18; 1.45) | <0.001 | NF | NA |
| Birth head circumference SDS | 0.74 (−0.11; 1.60) | 0.53 (−0.53; 1.22) | 0.076 | NF | NA |
| Small for gestational age | 50 (21.1) | 9 (2.5) | <0.001 | NF | NA |
| Deletion (n = 117) | mUPD (n = 106) | ICD (n = 10) | p-Value | |
|---|---|---|---|---|
| Maternal and Pregnancy Characteristics | ||||
| Maternal age (years) | 31.0 (28.0; 34.0) | 36.0 (32.8; 39.0) | 28.0 (24.0; 33.5) | <0.001 |
| High maternal age (>35 years) | 25 (22.5) | 60 (61.2) | 2 (22.2) | <0.001 |
| Paternal age (years) | 33.0 (30.3; 36.0) | 37.0 (33.0; 40.0) | 30.5 (28.3; 40.0) | <0.001 |
| High paternal age (>35 years) | 36 (33.3) | 65 (67.7) | 2 (25.0) | <0.001 |
| Maternal pre-pregnancy BMI (kg/m2) | 0.043 | |||
| BMI < 20 | 6 (9.5) | 1 (1.9) | 0 (0.0) | |
| BMI 20–25 | 43 (68.3) | 39 (75.0) | 3 (75.0) | |
| BMI 25–30 | 14 (22.2) | 9 (17.3) | 0 (0.0) | |
| BMI 30–35 | 0 (0.0) | 3 (5.8) | 1 (25.0) | |
| BMI > 35 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Parity | 1 (1.0; 2.0) | 1 (1.0;2.0) | 2 (1.0;2.3) | 0.967 |
| History of miscarriage | 27 (24.1) | 22 (22.7) | 0 (0.0) | 0.249 |
| Medically assisted reproduction | 5 (4.6) | 3 (3.2) | 1 (11.1) | 0.514 |
| Hypertension/pre-eclampsia | 6 (5.1) | 4 (4.3) | 1 (11.1) | 0.771 |
| Gestational diabetes | 1 (0.9) | 5 (5.3) | 0 (0.0) | 0.141 |
| Premature rupture of membranes | 9 (8.1) | 8 (8.5) | 1 (11.1) | 0.951 |
| Hyperemesis | 6 (5.4) | 7 (7.4) | 1 (11.1) | 0.716 |
| Amniotic fluid | ||||
| Polyhydramnios | 27 (25.5) | 26 (30.6) | 2 (22.2) | 0.687 |
| Oligohydramnios | 7 (6.6) | 6 (7.1) | 2 (22.2) | 0.228 |
| Decreased fetal movements | 71 (73.2) | 72 (83.7) | 3 (60.0) | 0.147 |
| Labor Characteristics | ||||
| Term (weeks) | 0.183 | |||
| Preterm <37 | 15 (13.0) | 22 (21.2) | 3 (30.0) | |
| Full term 37–41 | 63 (54.8) | 58 (55.8) | 3 (30.0) | |
| Late/Post-term >41 | 37 (32.2) | 24 (23.1) | 4 (40.0) | |
| Mode of delivery | 0.258 | |||
| Vaginal delivery | 54 (50.0) | 39 (41.9) | 6 (75.0) | |
| Assisted delivery | 8 (7.4) | 6 (6.5) | 1 (12.5) | |
| Caesarean section | 46 (42.6) | 48 (51.6) | 1 (12.5) | |
| Type of caesarean section | 0.487 | |||
| Primary | 18 (40.9) | 19 (40.4) | 1 (100) | |
| Secondary | 26 (59.1) | 28 (59.6) | 0 (0.0) | |
| Induction of labor | 43 (39.1) | 35 (36.8) | 3 (33.3) | 0.909 |
| Breech presentation | 34 (30.6) | 32 (33.0) | 1 (11.1% | 0.703 |
| Birth Characteristics | ||||
| Male | 64 (54.7) | 57 (53.8) | 7 (70.0) | 0.320 |
| Birth weight SDS | −1.16 (−1.71; −0.33) | −1.09 (−2.10; −0.41) | −0.30 (−1.34; 0.03) | 0.220 |
| Birth length SDS | −0.47 (−1.47; 0.70) | −0.24 (−1.06; 0.86) | 0.15 (−0.21; 0.81) | 0.505 |
| Birth head circumference SDS | 0.95 (0.11; 1.65) | 0.52 (−0.13; 1.36) | 1.08 (0.01; 2.73) | 0.171 |
| Small for gestational age | 17 (14.8) | 31 (30.4) | 1 (10.0) | 0.014 |
| PWS Cohort (n = 244) | Deletion (n = 117) | mUPD (n = 106) | ICD (n = 10) | p-Value * | |
|---|---|---|---|---|---|
| Apgar score at 1 min | 7.0 (5.0; 8.0) | 7.0 (5.0; 8.0) | 7.0 (5.0; 8.0) | 6.5 (5.8; 8.3) | 0.998 |
| Low Apgar score at 1 min | 55 (40.7) | 25 (41.0) | 25 (39.7) | 3 (50.0) | 0.885 |
| Apgar score at 5 min | 8.0 (7.0; 9.0) | 9.0 (7.5; 9.0) | 8.0 (7.0; 9.0) | 8.5 (6.0; 9.0) | 0.642 |
| Low Apgar score at 5 min | 14 (10.1) | 5 (8.2) | 7 (10.8) | 2 (33.3) | 0.162 |
| Cryptorchidism (boys) | 118 (95.9) | 59 (96.7) | 51 (94.4) | 3 (100) | 0.769 |
| Hypotonia | 244 (100.0) | 117 (100.0) | 106 (100.0) | 10 (100.0) | NA |
| Breathing problems | 116 (64.4) | 56 (65.9) | 53 (65.4) | 5 (62.5) | 0.981 |
| Poor sucking leading to tube feeding | 216 (93.9) | 106 (94.6) | 93 (93.0) | 9 (100) | 0.656 |
| Tube feeding duration (weeks) | 15.0 (6.0; 25.0) | 16.0 (6.0; 24.0) | 12.5 (6.0; 24.8) | 19.5 (4.4; 41.0) | 0.667 |
| Duration of hospitalization (days) | 24.0 (16.0; 35.0) | 24.5 (17.0; 35.3) | 22.5 (14.3; 35.0) | 25.0 (20.0; 31.0) | 0.733 |
| Age at diagnosis (weeks) | 7.9 (3.8; 22.8) | 5.7 (3.0; 12.0) | 9.9 (4.5; 26.6) | 23.3 (8.2; 59.5) | 0.001 |
| Characteristics During Pregnancy | Prevalence |
|---|---|
| Decreased fetal movements | 78.5% |
| Caesarian section | 46.3% |
| Induction of labor | 38.6% |
| Breech presentation | 31.0% |
| Polyhydramnios | 27.3% |
| Characteristics During the Neonatal Period | Prevalence |
| Hypotonia | 100% |
| Poor suck leading to tube feeding | 93.9% |
| Cryptorchidism in boys | 95.9% |
| Hospitalization of > 20 days after birth | 65.9% |
| Breathing problems after birth | 64.4% |
| Small for gestational age | 21.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grootjen, L.N.; Uyl, N.E.M.; van Beijsterveldt, I.A.L.P.; Damen, L.; Kerkhof, G.F.; Hokken-Koelega, A.C.S. Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome. J. Clin. Med. 2022, 11, 679. https://doi.org/10.3390/jcm11030679
Grootjen LN, Uyl NEM, van Beijsterveldt IALP, Damen L, Kerkhof GF, Hokken-Koelega ACS. Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome. Journal of Clinical Medicine. 2022; 11(3):679. https://doi.org/10.3390/jcm11030679
Chicago/Turabian StyleGrootjen, Lionne N., Nathalie E. M. Uyl, Inge A. L. P. van Beijsterveldt, Layla Damen, Gerthe F. Kerkhof, and Anita C. S. Hokken-Koelega. 2022. "Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome" Journal of Clinical Medicine 11, no. 3: 679. https://doi.org/10.3390/jcm11030679
APA StyleGrootjen, L. N., Uyl, N. E. M., van Beijsterveldt, I. A. L. P., Damen, L., Kerkhof, G. F., & Hokken-Koelega, A. C. S. (2022). Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome. Journal of Clinical Medicine, 11(3), 679. https://doi.org/10.3390/jcm11030679






