The Impact of Pancreatic Head Resection on Blood Glucose Homeostasis in Patients with Chronic Pancreatitis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Data Acquisition
2.2. Surgical Procedures
2.3. Blood Glucose Homeostasis
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
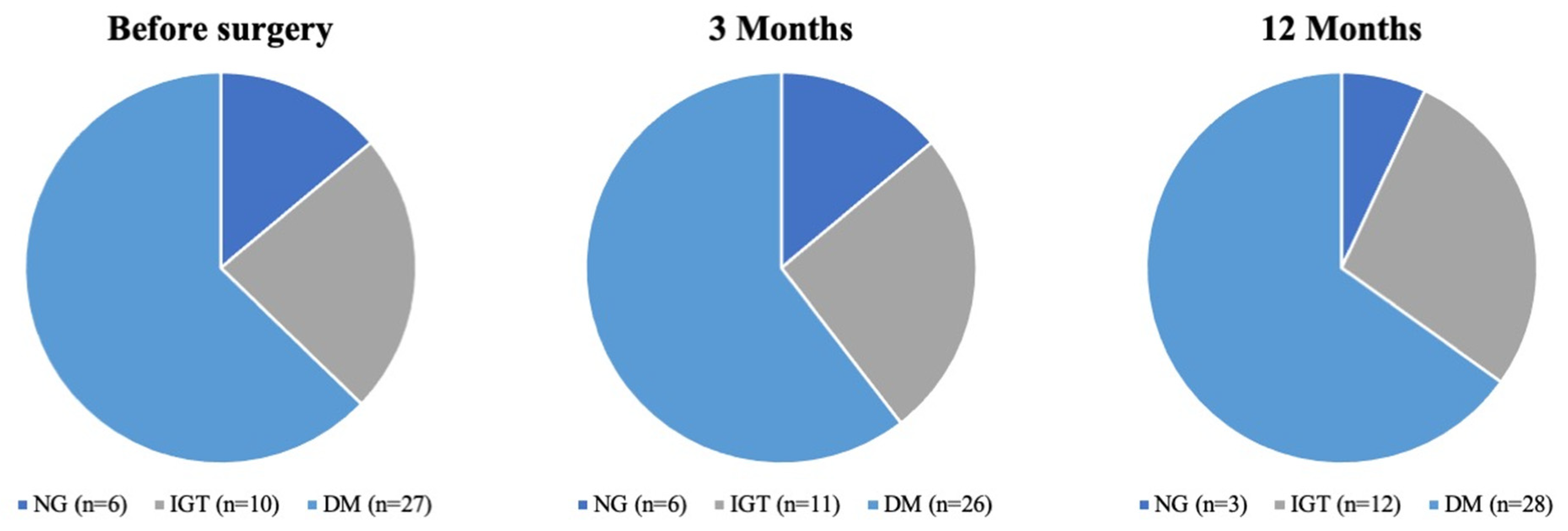
3.2. Impact of Pancreatic Resection on Blood Glucose Homeostasis in Normoglycemic Patients
3.3. Impact of Pancreatic Resection on Blood Glucose Homeostasis in Patients with IGT
3.4. Impact of Pancreatic Resection on Blood Glucose Homeostasis in Diabetic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| BMI | body mass index |
| CDC | Clavien-Dindo classification |
| cPD | classic pancreaticoduodenectomy |
| CP | chronic pancreatitis |
| DM | diabetes mellitus |
| DPPHR | duodenum-preserving pancreatic head resection |
| HOMA2 | updated Homeostasis Model Assessment |
| IGT | impaired glucose tolerance |
| IQR | interquartile range |
| NG | normoglycemic |
| OGTT | oral glucose tolerance test |
| PPPD | pylorus-preserving pancreaticoduodenectomy |
References
- Kahl, S.; Zimmermann, S.; Genz, I.; Glasbrenner, B.; Pross, M.; Schulz, H.U.; Mc Namara, D.; Schmidt, U.; Malfertheiner, P. Risk factors for failure of endoscopic stenting of biliary strictures in chronic pancreatitis: A prospective follow-up study. Am. J. Gastroenterol. 2003, 98, 2448–2453. [Google Scholar] [CrossRef]
- Diener, M.K.; Huttner, F.J.; Kieser, M.; Knebel, P.; Dorr-Harim, C.; Distler, M.; Grutzmann, R.; Wittel, U.A.; Schirren, R.; Hau, H.M.; et al. Partial pancreatoduodenectomy versus duodenum-preserving pancreatic head resection in chronic pancreatitis: The multicentre, randomised, controlled, double-blind ChroPac trial. Lancet 2017, 390, 1027–1037. [Google Scholar] [CrossRef]
- Diener, M.K.; Rahbari, N.N.; Fischer, L.; Antes, G.; Buchler, M.W.; Seiler, C.M. Duodenum-preserving pancreatic head resection versus pancreatoduodenectomy for surgical treatment of chronic pancreatitis: A systematic review and meta-analysis. Ann. Surg. 2008, 247, 950–961. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Ehehalt, F.; Sturm, D.; Rosler, M.; Distler, M.; Weitz, J.; Kersting, S.; Ludwig, B.; Schwanebeck, U.; Saeger, H.D.; Solimena, M.; et al. Blood Glucose Homeostasis in the Course of Partial Pancreatectomy—Evidence for Surgically Reversible Diabetes Induced by Cholestasis. PLoS ONE 2015, 10, e0134140. [Google Scholar] [CrossRef]
- Hirata, K.; Nakata, B.; Amano, R.; Yamazoe, S.; Kimura, K.; Hirakawa, K. Predictive factors for change of diabetes mellitus status after pancreatectomy in preoperative diabetic and nondiabetic patients. J. Gastrointest. Surg. 2014, 18, 1597–1603. [Google Scholar] [CrossRef]
- You, D.D.; Choi, S.H.; Choi, D.W.; Heo, J.S.; Ho, C.Y.; Kim, W.S. Long-term effects of pancreaticoduodenectomy on glucose metabolism. ANZ J. Surg. 2012, 82, 447–451. [Google Scholar] [CrossRef]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran Cao, H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J.Y.; Kim, E.J.; Kang, M.J.; Kwon, W.; Chang, Y.R.; Han, I.W.; Kim, S.W. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br. J. Surg. 2013, 100, 1064–1070. [Google Scholar] [CrossRef]
- Gloor, B.; Friess, H.; Uhl, W.; Buchler, M.W. A modified technique of the Beger and Frey procedure in patients with chronic pancreatitis. Dig. Surg. 2001, 18, 21–25. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S14–S31. [Google Scholar] [CrossRef]
- Solimena, M.; Schulte, A.M.; Marselli, L.; Ehehalt, F.; Richter, D.; Kleeberg, M.; Mziaut, H.; Knoch, K.P.; Parnis, J.; Bugliani, M.; et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia 2018, 61, 641–657. [Google Scholar] [CrossRef]
- Winkler, C.; Jolink, M.; Knopff, A.; Kwarteng, N.A.; Achenbach, P.; Bonifacio, E.; Ziegler, A.G. Age, HLA, and Sex Define a Marked Risk of Organ-Specific Autoimmunity in First-Degree Relatives of Patients With Type 1 Diabetes. Diabetes Care 2019, 42, 1684–1691. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Frulloni, L.; Garg, P.; Greer, J.B.; Schneider, A.; Yadav, D.; Shimosegawa, T. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology 2016, 16, 218–224. [Google Scholar] [CrossRef]
- Sasikala, M.; Talukdar, R.; Pavan Kumar, P.; Radhika, G.; Rao, G.V.; Pradeep, R.; Subramanyam, C.; Nageshwar Reddy, D. beta-Cell dysfunction in chronic pancreatitis. Dig. Dis. Sci. 2012, 57, 1764–1772. [Google Scholar] [CrossRef]
- Laramee, P.; Wonderling, D.; Cahen, D.L.; Dijkgraaf, M.G.; Gouma, D.J.; Bruno, M.J.; Pereira, S.P. Trial-based cost-effectiveness analysis comparing surgical and endoscopic drainage in patients with obstructive chronic pancreatitis. BMJ Open 2013, 3, e003676. [Google Scholar] [CrossRef][Green Version]
- Willner, A.; Bogner, A.; Mussle, B.; Teske, C.; Hempel, S.; Kahlert, C.; Distler, M.; Weitz, J.; Welsch, T. Disease duration before surgical resection for chronic pancreatitis impacts long-term outcome. Medicine 2020, 99, e22896. [Google Scholar] [CrossRef]
- Cahen, D.L.; Gouma, D.J.; Nio, Y.; Rauws, E.A.; Boermeester, M.A.; Busch, O.R.; Stoker, J.; Lameris, J.S.; Dijkgraaf, M.G.; Huibregtse, K.; et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N. Engl. J. Med. 2007, 356, 676–684. [Google Scholar] [CrossRef]
- Issa, Y.; Kempeneers, M.A.; Bruno, M.J.; Fockens, P.; Poley, J.W.; Ahmed Ali, U.; Bollen, T.L.; Busch, O.R.; Dejong, C.H.; van Duijvendijk, P.; et al. Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial. JAMA 2020, 323, 237–247. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, J.P.; Pottakat, B.; Kamalanathan, S.; Mohan, P.; Kate, V.; Kar, S.S.; Selviambigapathy, J. Effect of Frey’s procedure on islet cell function in patients with chronic calcific pancreatitis. Hepatobiliary Pancreat Dis. Int. 2018, 17, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, J.R.; Bloechle, C.; Knoefel, W.T.; Kuechler, T.; Binmoeller, K.F.; Broelsch, C.E. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized trial. Ann. Surg. 1995, 221, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, J.R.; Bloechle, C.; Broering, D.C.; Knoefel, W.T.; Kuechler, T.; Broelsch, C.E. Extended drainage versus resection in surgery for chronic pancreatitis: A prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann. Surg. 1998, 228, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.; Adam, U.; Makowiec, F.; Riediger, H.; Wellner, U.; Tittelbach-Helmrich, D.; Hopt, U.T. Short- and long-term results of duodenum preservation versus resection for the management of chronic pancreatitis: A prospective, randomized study. Surgery 2012, 152 (Suppl. 1), S95–S102. [Google Scholar] [CrossRef]
- Muller, M.W.; Friess, H.; Martin, D.J.; Hinz, U.; Dahmen, R.; Buchler, M.W. Long-term follow-up of a randomized clinical trial comparing Beger with pylorus-preserving Whipple procedure for chronic pancreatitis. Br. J. Surg. 2008, 95, 350–356. [Google Scholar] [CrossRef]
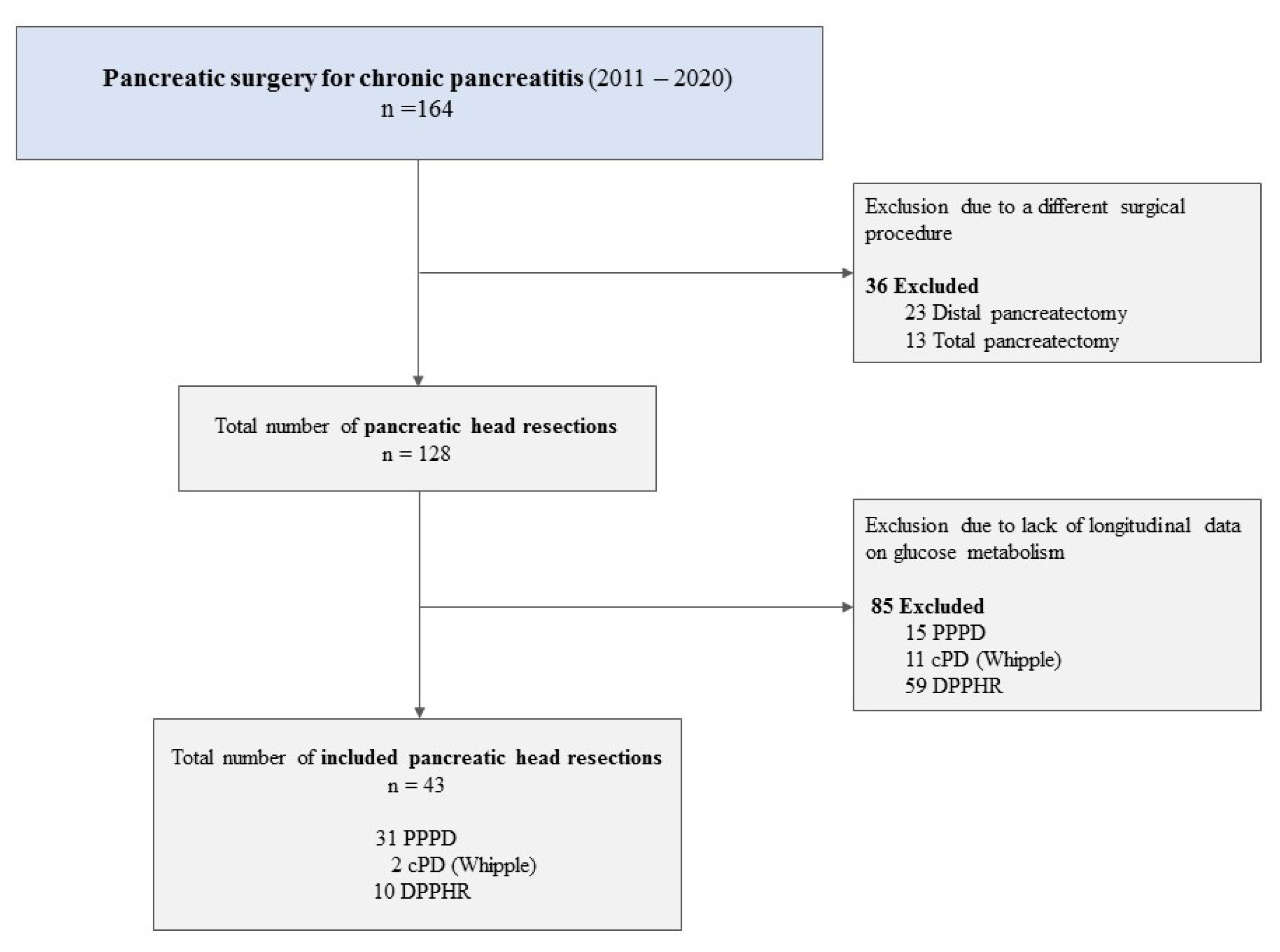
| Variable | Overall n = 43 | NG n = 6 | IGT n = 10 | DM n = 27 | p-Value 1 |
|---|---|---|---|---|---|
| Male sex [n/(%)] | 38 (88.6) | 5 (83.3) | 8 (80) | 25 (92.6) | 0.52 |
| Median age [years] (IQR) | 53 (47–57) | 46 (43–51) | 56 (51–64) | 53 (49–56) | 0.03#,* |
| BMI [kg/m2] (IQR) | 22.2 (20.7–25) | 21.5 (21.3–23.5) | 22.1 (20.7–23.5) | 22.4 (19.6–24.8) | 0.98 # |
| ASA score | 0.43 | ||||
| I | 1 (2.3) | - | 1 (10) | - | |
| II | 23 (53.5) | 4 (66.7) | 5 (50) | 14 (51.9) | |
| III | 19 (44.2) | 2 (33.3) | 4 (40) | 13 (48.1) | |
| Alcohol abuse [n/(%)] | 35 (81.4) | 6 (100) | 8 (80) | 21 (77.8) | 0.45 |
| Nicotine abuse [n/(%)] | 34 (79) | 6 (100) | 7 (70) | 21 (77.8) | 0.35 |
| Hypertension [n/(%)] | 21 (48.8) | 1 (16.7) | 5 (50) | 15 (55.6) | 0.23 |
| Exocrine insufficiency [n/(%)] | 14 (32.6) | 2 (33.3) | 1 (10) | 11 (40.7) | 0.21 |
| Type of surgery [n/(%)] | 0.5 | ||||
| PPPD | 31 (72.1) | 5 (83.3) | 9 (90) | 17 (63) | |
| cPD | 2 (4.7) | 2 (7.4) | |||
| DPPHR | 10 (23.2) | 1 (16.7) | 1 (10) | 8 (29.6) | |
| Length of hospital stay [days] (IQR) | 13 (12–17) | 13 (12–13) | 15 (13–21) | 13 (12–16) | 0.06 # |
| Complications CDC > 2 [n/(%)] | 6 (14) | 1 (16.7) | 3 (30) | 2 (7.4) | 0.21 |
| Variable | Before Surgery | 3 Months | 12 Months | p-Value * |
|---|---|---|---|---|
| BMI kg/m2 (IQR) | 21.5 (21.3–23.5) | 23.2 (22.1–24.4) | 23 (20.9–23.9) | 0.21 |
| Fasting glucose mmol/L (IQR) | 5.25 (4.65–5.36) | 5.29 (5.23–5.42) | 5.76 (5.43–5.87) | 0.36 |
| HbA1c % (IQR) | 5.2 (5.1 -5.4) | 5.7 (5.6–5.8) | 5.5 (5.2–6.0) | 0.64 |
| Insulin nmol/L (IQR) | 0.03 (0.02–0.05) | 0.03 (0.03–0.04) | 0.03 (0.01–0.05) | 0.58 |
| C-peptide nmol/L (IQR) | 0.44 (0.28–0.48) | 0.5 (0.41–0.68) | 0.85 (0.59–0.99) | 0.27 |
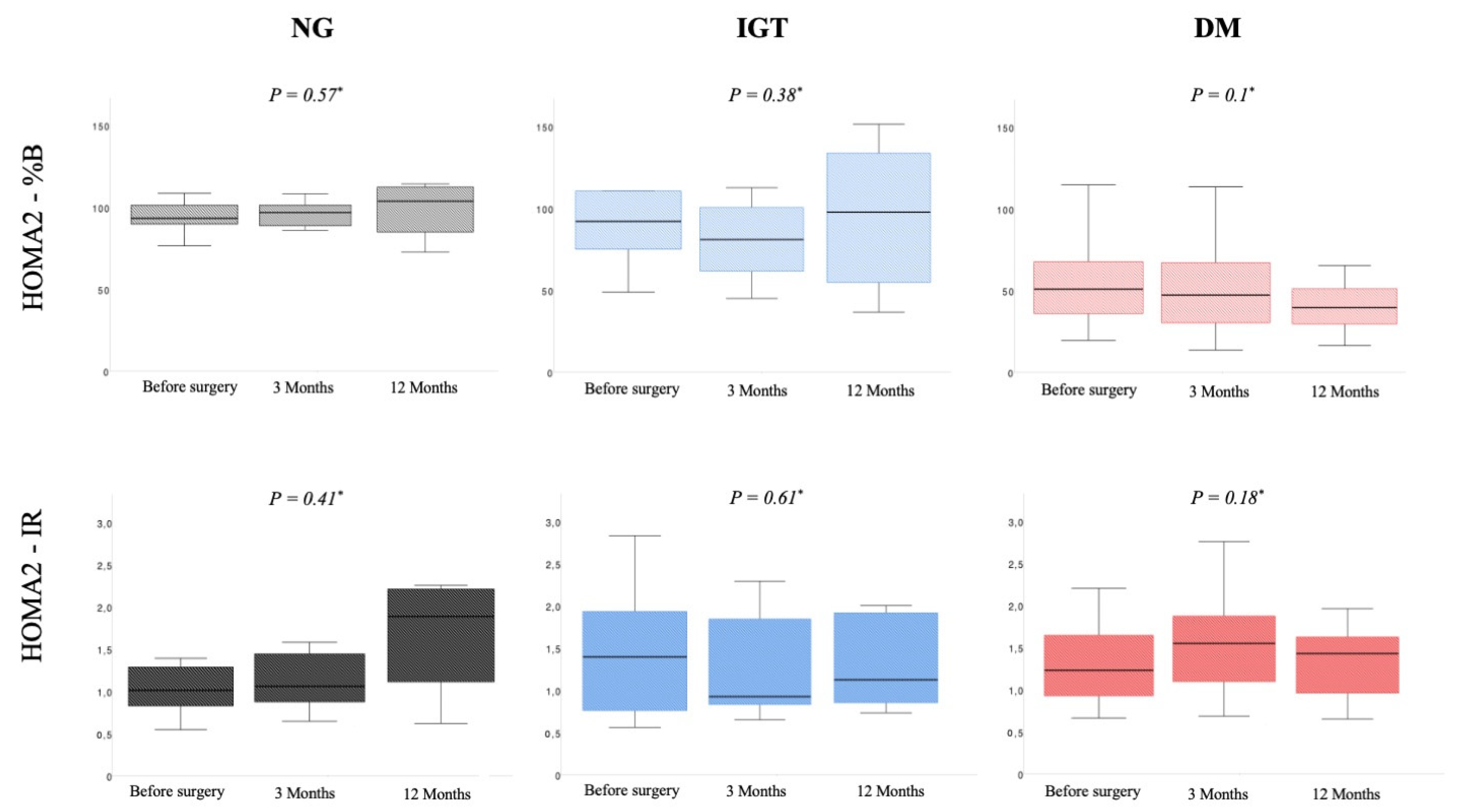
| HOMA2-%B (IQR) | 91.9 (88.1–100.4) | 93.1 (82.8–98.8) | 109 (93.8–118) | 0.57 |
| HOMA2-%S (IQR) | 93.5 (74–113.5) | 89.5 (65.1–108.7) | 52.8 (43.8–94.6) | 0.44 |
| HOMA2-IR (IQR) | 1.07 (0.88–1.55) | 1.12 (0.92–1.54) | 1.95 (1.35–2.28) | 0.41 |
| Variable | Before Surgery | 3 Months | 12 Months | p-Value * |
|---|---|---|---|---|
| BMI kg/m2 (IQR) | 22.1 (20.7–23.5) | 21.3 (20.3–23.2) | 22.2 (21–24.4) | 0.04 |
| Fasting glucose mmol/L (IQR) | 5.45 (4.96–6.04) | 5.33 (4.95–6.15) | 5.22 (4.9–6.41) | 0.02 |
| HbA1c % (IQR) | 5.5 (5.4–6.0) | 5.9 (5.3–6.3) | 6.0 (5.8–6.3) | 0.69 |
| Insulin nmol/L (IQR) | 0.04 (0.02–0.07) | 0.03 (0.01–0.06) | 0.03 (0.02–0.05) | 0.64 |
| C-peptide nmol/L (IQR) | 0.6 (0.32–0.86) | 0.35 (0.29–0.73) | 0.54 (0.37–0.83) | 0.54 |
| HOMA2-%B (IQR) | 91.9 (74.7–108.6) | 79.5 (57.8–101.4) | 98.6 (51.4–131) | 0.38 |
| HOMA2-%S (IQR) | 73.9 (50.2–138.4) | 120 (52.4–139.1) | 87.1 (48.4–118.4) | 0.52 |
| HOMA2-IR(IQR) | 1.4 (0.7–2) | 0.8 (0.7–1.9) | 1.2 (0.8–2.1) | 0.61 |
| Variable | Before Surgery | 3 Months | 12 Months | p-Value * |
|---|---|---|---|---|
| BMI kg/m2 (IQR) | 22.5 (19.9–25.7) | 22.9 (20.8–25.6) | 23.3 (21.2–25.3) | 0.16 |
| Fasting glucose mmol/L (IQR) | 7.79 (6.27–8.54) | 7.77 (6.62–10.29) | 7.78 (6.34–9.74) | 0.11 |
| HbA1c % (IQR) | 6.8 (6.4–7.9) | 6.9 (6.3–7.7) | 7.4 (6.7–8.3) | 0.36 |
| Insulin nmol/L (IQR) | 0.03 (0.01–0.07) | 0.03 (0.01–0.05) | 0.02 (0.01–0.04) | 0.18 |
| C-peptide nmol/L (IQR) | 0.38 (0.18–0.58) | 0.45 (0.17–0.65) | 0.33 (0.16–0.59) | 0.22 |
| HOMA2-%B (IQR) | 50.3 (34.5–68.3) | 49.3 (31.2–70.4) | 39.8 (28.8–52.6) | 0.1 |
| HOMA2-%S (IQR) | 81.4 (59.8–111.4) | 61.3 (49.8–91.1) | 66.4 (57.5–104.2) | 0.51 |
| HOMA2-IR (IQR) | 1.23 (0.9–1.69) | 1.63 (1.1–2.01) | 1.39 (0.96–1.74) | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hempel, S.; Oehme, F.; Ehehalt, F.; Solimena, M.; Kolbinger, F.R.; Bogner, A.; Welsch, T.; Weitz, J.; Distler, M. The Impact of Pancreatic Head Resection on Blood Glucose Homeostasis in Patients with Chronic Pancreatitis. J. Clin. Med. 2022, 11, 663. https://doi.org/10.3390/jcm11030663
Hempel S, Oehme F, Ehehalt F, Solimena M, Kolbinger FR, Bogner A, Welsch T, Weitz J, Distler M. The Impact of Pancreatic Head Resection on Blood Glucose Homeostasis in Patients with Chronic Pancreatitis. Journal of Clinical Medicine. 2022; 11(3):663. https://doi.org/10.3390/jcm11030663
Chicago/Turabian StyleHempel, Sebastian, Florian Oehme, Florian Ehehalt, Michele Solimena, Fiona R. Kolbinger, Andreas Bogner, Thilo Welsch, Jürgen Weitz, and Marius Distler. 2022. "The Impact of Pancreatic Head Resection on Blood Glucose Homeostasis in Patients with Chronic Pancreatitis" Journal of Clinical Medicine 11, no. 3: 663. https://doi.org/10.3390/jcm11030663
APA StyleHempel, S., Oehme, F., Ehehalt, F., Solimena, M., Kolbinger, F. R., Bogner, A., Welsch, T., Weitz, J., & Distler, M. (2022). The Impact of Pancreatic Head Resection on Blood Glucose Homeostasis in Patients with Chronic Pancreatitis. Journal of Clinical Medicine, 11(3), 663. https://doi.org/10.3390/jcm11030663






