COVID-19 and Intracranial Hemorrhage: A Multicenter Case Series, Systematic Review and Pooled Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pandemic Registry
2.2. Systematic Review
2.2.1. Eligibility Criteria
2.2.2. Search Strategy
2.3. Statistical Analysis
3. Results
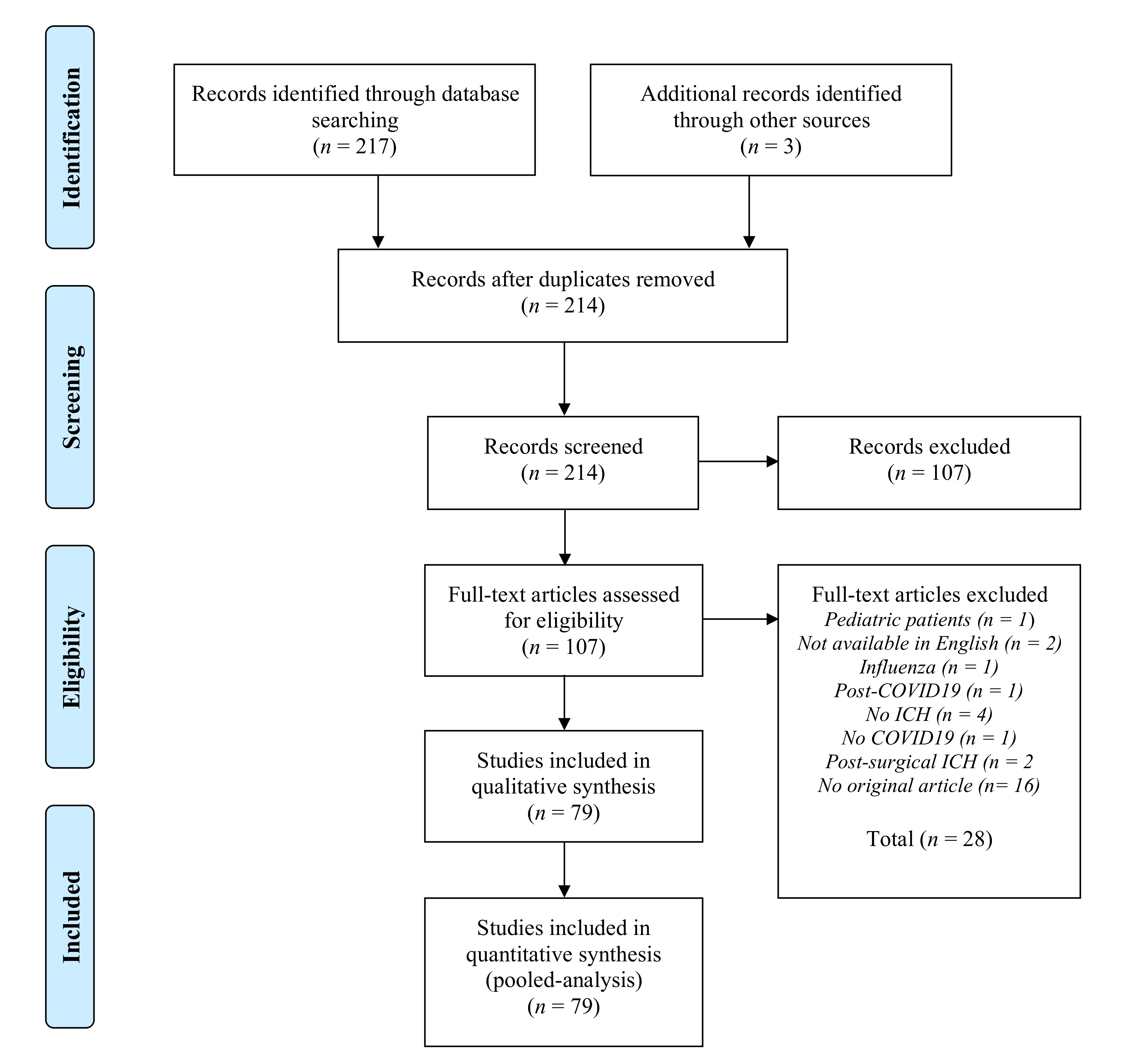
3.1. Literature Screening and Article Selection
3.2. Baseline Characteristics of Individual-Level Patient Data
3.3. Pooled Analysis of Aggregate Data
4. Discussion
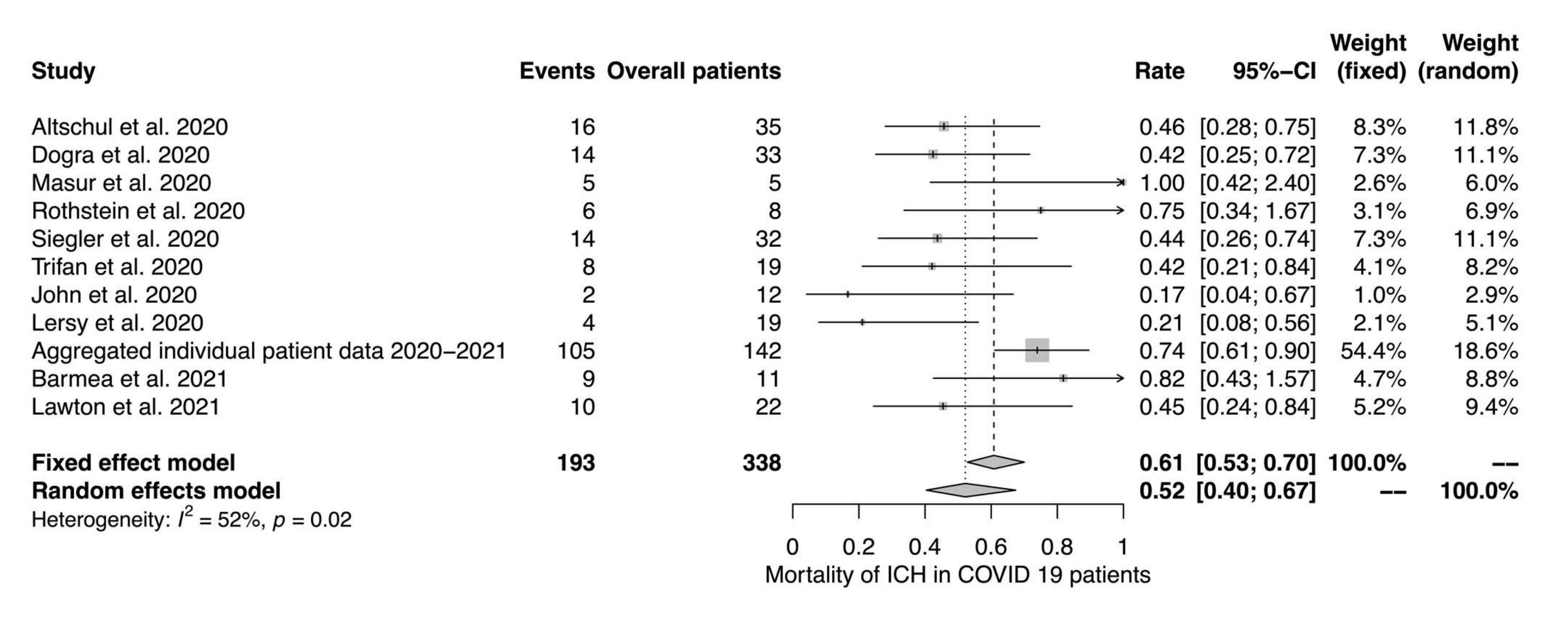
4.1. Prevalence and Mortality
4.2. Predictors of Clinical Outcome
4.3. Pathophysiology
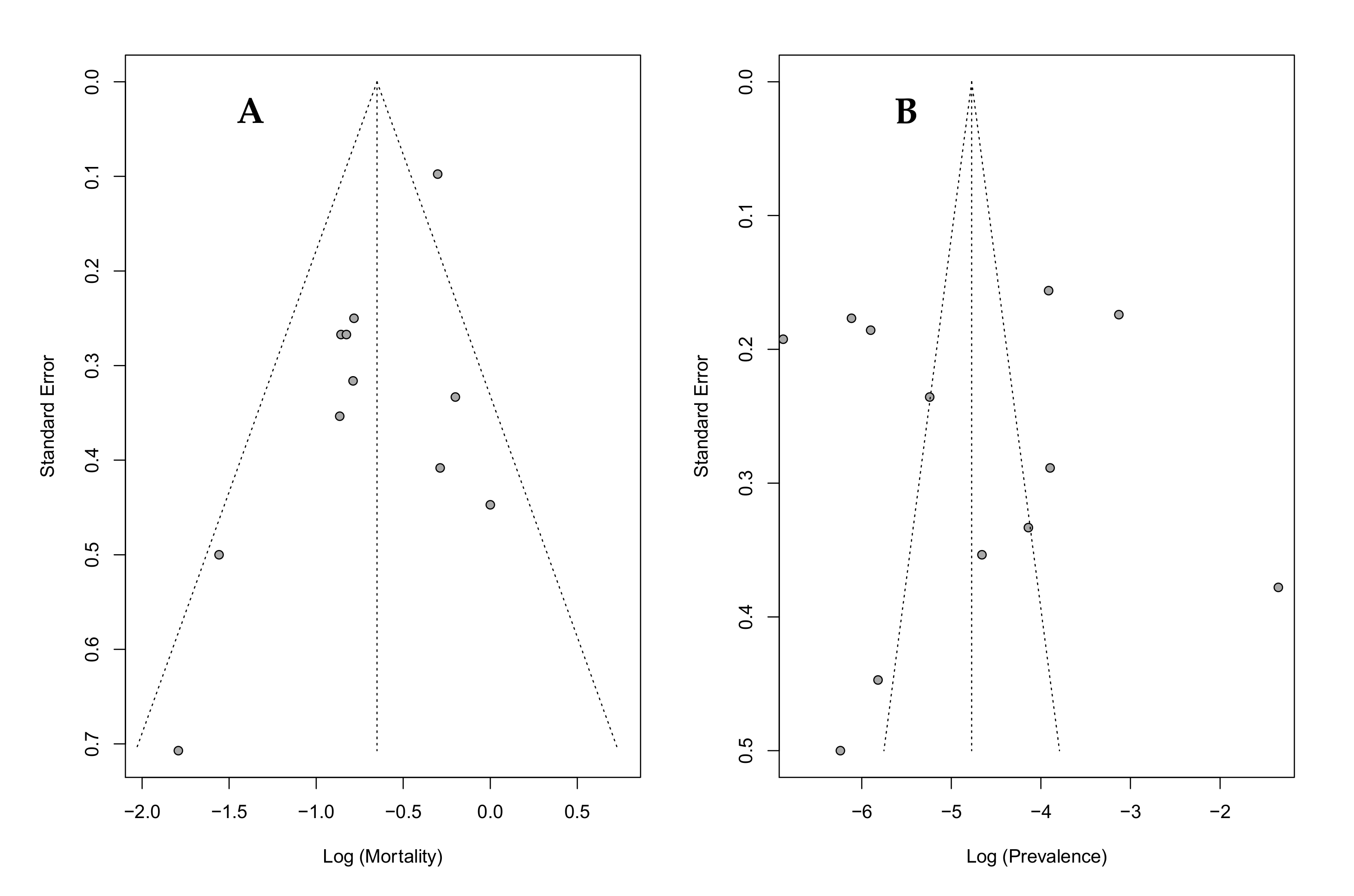
4.4. Limitations
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A Systematic Review of Neurological Symptoms and Complications of COVID-19. J. Neurol. 2021, 268, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D’Agostini, S.; Gigli, G.L.; Bnà, C.; Vogrig, A. Stroke in Patients with SARS-CoV-2 Infection: Case Series. J. Neurol. 2020, 267, 2185–2192. [Google Scholar] [CrossRef]
- Benussi, A.; Pilotto, A.; Premi, E.; Libri, I.; Giunta, M.; Agosti, C.; Alberici, A.; Baldelli, E.; Benini, M.; Bonacina, S.; et al. Clinical Characteristics and Outcomes of Inpatients with Neurologic Disease and COVID-19 in Brescia, Lombardy, Italy. Neurology 2020, 95, e910–e920. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A Global Threat to the Nervous System. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie-Empfehlungen zur Stationären Therapie von Patienten mit COVID-19. Pneumologie 2021, 75, 88–112. [Google Scholar] [PubMed]
- Beyrouti, R.; Best, J.G.; Chandratheva, A.; Perry, R.J.; Werring, D.J. Characteristics of Intracerebral Haemorrhage Associated with COVID-19: A Systematic Review and Pooled Analysis of Individual Patient and Aggregate Data. J. Neurol. 2021, 268, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, P.; Junior, C.G.; Moro, E.; Cavallieri, F.; Zedde, M. COVID-19 and Cerebrovascular Diseases: A Systematic Review and Perspectives for Stroke Management. Front. Neurol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Deutsches Zentrum für Infektionsforschung. Lean European Open Survey on SARS-CoV-2 Infected Patients-Lean European Open Survey on SARS-CoV-2 Infected Patients (LEOSS). 2020. Available online: https://leoss.net/ (accessed on 13 April 2021).
- Riley, R.D.; Simmonds, M.C.; Look, M.P. Evidence Synthesis Combining Individual Patient Data and Aggregate Data: A Systematic Review Identified Current Practice and Possible Methods. J. Clin. Epidemiol. 2007, 60, e1–e431. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Prisma Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Piroth, L.; Cottenet, J.; Mariet, A.S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the Characteristics, Morbidity, and Mortality of COVID-19 and Seasonal Influenza: A Nationwide, Population-Based Retrospective Cohort Study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, Case Fatality, and Functional Outcome of Intracerebral Haemorrhage over Time, According to Age, Sex, and Ethnic Origin: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Lim, Z.J.; Subramaniam, A.; Ponnapa Reddy, M.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef]
- Abadie, V.; Jacquin, A.; Daubail, B.; Vialatte, A.L.; Lainay, C.; Durier, J.; Osseby, G.V.; Giroud, M.; Bejot, Y. Prevalence and Prognostic Value of Headache on Early Mortality in Acute Stroke: The Dijon Stroke Registry. Cephalalgia Int. J. Headache 2014, 34, 887–894. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case Characteristics, Resource Use, and Outcomes of 10 021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Melmed, K.R.; Cao, M.; Dogra, S.; Zhang, R.; Yaghi, S.; Lewis, A.; Jain, R.; Bilaloglu, S.; Chen, J.; Czeisler, B.M.; et al. Risk Factors for Intracerebral Hemorrhage in Patients with COVID-19. J. Thromb. Thrombolysis 2020, 51, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Rosand, J.; Eckman, M.H.; Knudsen, K.A.; Singer, D.E.; Greenberg, S.M. The Effect of Warfarin and Intensity of Anticoagulation on Outcome of Intracerebral Hemorrhage. Arch. Intern. Med. 2004, 164, 880–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; González, E.; Redondo-Peñas, I.; Perona-Moratalla, A.B.; Del Valle-Pérez, J.A.; et al. Neurologic Manifestations in Hospitalized Patients with COVID-19: The ALBACOVID Registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.N.; Dettinger, J.S.; Berchtold-Herz, M.; Utzolino, S.; Bemtgen, X.; Zotzmann, V.; Schmid, B.; Biever, P.M.; Bode, C.; Müller-Peltzer, K.; et al. Intracerebral Hemorrhage in COVID-19 Patients with Pulmonary Failure: A Propensity Score-Matched Registry Study. Neurocrit. Care 2021, 34, 739–747. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Del Sorbo, L.; Fan, E. Intracranial Hemorrhage in Adults on ECMO. Perfusion 2018, 33, 42–50. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The Vasculature Unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.I.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lersy, F.; Willaume, T.; Brisset, J.C.; Collange, O.; Helms, J.; Schneider, F.; Chammas, A.; Willaume, A.; Meyer, N.; Anheim, M.; et al. Critical Illness-Associated Cerebral Microbleeds for Patients with Severe COVID-19: Etiologic Hypotheses. J. Neurol. 2020, 268, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, L.; Bertrand, A.; Laurent, C.; Roze, H.; Chastre, J.; Combes, A.; Luyt, C.E. Diffuse Cerebral Microbleeds after Extracorporeal Membrane Oxygenation Support. Am. J. Respir. Crit. Care Med. 2015, 191, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Jain, R.; Dogra, S.; Krieger, P.; Lewis, A.; Nguyen, V.; Melmed, K.; Galetta, S. Cerebral Microbleeds and Leukoencephalopathy in Critically Ill Patients with COVID-19. Stroke 2020, 51, 2649–2655. [Google Scholar] [CrossRef]
- Kallenberg, K.; Dehnert, C.; Dörfler, A.; Schellinger, P.D.; Bailey, D.M.; Knauth, M.; Bärtsch, P.D. Microhemorrhages in Nonfatal High-Altitude Cerebral Edema. J. Cereb. Blood Flow Metab. 2008, 28, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- Fanou, E.M.; Coutinho, J.M.; Shannon, P.; Kiehl, T.R.; Levi, M.M.; Wilcox, M.E.; Aviv, R.I.; Mandell, D.M. Critical Illness-Associated Cerebral Microbleeds. Stroke 2017, 48, 1085–1087. [Google Scholar] [CrossRef]
- Dixon, L.; McNamara, C.; Gaur, P.; Mallon, D.; Coughlan, C.; Tona, F.; Jan, W.; Wilson, M.; Jones, B. Cerebral Microhaemorrhage in COVID-19: A Critical Illness Related Phenomenon? Stroke Vasc. Neurol. 2020, 5, 315–322. [Google Scholar] [CrossRef]
- Hunt, B.J. Bleeding and Coagulopathies in Critical Care. N. Engl. J. Med. 2014, 370, 847–859. [Google Scholar] [CrossRef] [Green Version]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Poillon, G.; Obadia, M.; Perrin, M.; Savatovsky, J.; Lecler, A. Cerebral Venous Thrombosis Associated with COVID-19 Infection: Causality or Coincidence? J. Neuroradiol. 2021, 48, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Timmons, T.A.; Donnan, G.A.; Whisnant, J.P.; Shelley, C.D.; Smith, T.F. Aneurysmal Subarachnoid Hemorrhage and Viral Infection. Lack of Association. Arch. Neurol. 1986, 43, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Rannikmäe, K.; Woodfield, R.; Anderson, C.S.; Charidimou, A.; Chiewvit, P.; Greenberg, S.M.; Jeng, J.S.; Meretoja, A.; Palm, F.; Putaala, J.; et al. Reliability of Intracerebral Hemorrhage Classification Systems: A Systematic Review. Int. J. Stroke 2016, 11, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Yassi, N.; Shah, D.G.; Ma, M.; Sharma, G.; Putaala, J.; Strbian, D.; Campbell, B.C.; Yan, B.; Tatlisumak, T.; et al. Simultaneous Multiple Intracerebral Hemorrhages (SMICH). Stroke 2017, 48, 581–586. [Google Scholar] [CrossRef]

| Baseline Characteristics | PANDEMIC Registry (n = 34) | All Individual Patient Data (n = 142) | |
|---|---|---|---|
| Age (years), median (IQR) | 64.0 (57.0–76.0) | 61.0 (53.8–71) | |
| Female, n (%) | 5/34 (14.7) | 49/142 (34.5) | |
| Critical disease (LEOSS), n (%) | 22/34 (64.7) | 81/128 (63.3) | |
| ECMO, n (%) | 12/34 (35.3) | 21/101 (20.8) | |
| Anticoagulation, n (%) | 32/34 (94.1) | 86/104 (82.7) | |
| Time from COVID-19 diagnosis to ICH diagnosis (days), median (IQR) | 21.0 (15.5–31.3) | 15 (8.0–22.5) | |
| Non-neurological symptoms | Fever, n (%) | 3/34 (8.8) | 50/119 (42.0) |
| Respiratory symptoms, n (%) | 20/34 (58.8) | 89/116 (76.7) | |
| Myalgia/arthralgia, n (%) | 3/34 (8.8) | 10/119 (8.4) | |
| Malaise, n (%) | 3/34 (8.8) | 12/119 (10.1) | |
| Neurological symptoms | Focal neurological deficits, n (%) | 6/34 (17.6) | 26/122 (21.3) |
| Altered level of consciousness, n (%) | 20/34 (58.8) | 64/128 (50.0) | |
| Encephalopathy, n (%) | 1/34 (2.9) | 5/123 (4.1) | |
| Headache, n (%) | - | 19/123 (15.5) | |
| Anisocoria, n (%) | 12/34 (35.3) | 29/124 (23.4) | |
| Seizure, n (%) | - | 8/127 (6.3) | |
| ICH | IPH, n (%) | 20/34 (58.8) | 68/142 (47.9) |
| SAH, n (%) | 16/34 (47.1) | 44/142 (31.0) | |
| SDH/EDH, n (%) | 4/34 (11.8) | 8/142 (5.6) | |
| Microbleeds, n (%) | 6/34 (17.6) | 25/130 (19.2) | |
| Lobar microbleeds n (%) | - | 1/25 (4.0) | |
| Deep microbleeds n (%) | 2/6 (33.3) | 2/25 (8.0) | |
| Mixed location microbleeds n (%) | 4/6 (66.6) | 22/25 (88.0) | |
| Not given n (%) | - | - | |
| IVH, n (%) | 4/34 (11.8) | 7/142 (4.9) | |
| HT/PH of IS, n (%) | - | 3/142 (2.1) | |
| SVT with hemorrhage, n (%) | - | 4/142 (2.8) | |
| Other, n (%) | - | - | |
| Multilocular ICH, not further specified, n (%) | - | - | |
| Laboratory values | White blood cells (×109/L), median (IQR) | 20.3 (15.0–26.8) | 15.8 (12.5–22.2) |
| Platelet count (×109/L), median (IQR) | 121.5 (70.5–185) | 176.0 (97.3–261.5) | |
| C-reactive protein (mg/L), median (IQR) | 340.0 (231.0–402.0) | 220.0 (54.5–340.0) | |
| INR, median (IQR) | 1.4 (1.2–1.8) | 1.3 (1.1–1.6) | |
| aPTT (s), median (IQR) | 58.0 (44.0–75.0) | 58 (38.8–68.0) | |
| D-dimer (mg/L), median (IQR) | 17.9 (7.8–23.9) | 6.8 (2.4–18.0) | |
| mRS | 0, n (%) | - | 5/118 (4.2) |
| 1, n (%) | 1/32 (3.1) | 2/118 (1.7) | |
| 2, n (%) | - | 3/118 (2.5) | |
| 3, n (%) | 2/32 (6.3) | 3/118 (2.5) | |
| 4, n (%) | 5/32 (15.6) | 8/118 (6.8) | |
| 5, n (%) | 3/32 (9.4) | 9/118 (7.6) | |
| 6, n (%) | 21/32 (65.6) | 88/118 (74.6) | |
| Death under palliative care, n (%) | 13/24 (54.2) | 34/101 (33.7) | |
| Mortality | Total, n (%) | 21/33 (64) | 88/119 (73.9) |
| IPH, n (%) | 13/20 (65) | 44/68 (64.7) | |
| SAH, n (%) | 12/16 (75.0) | 29/44 (65.9) | |
| SDH/EDH, n (%) | 4/4 (100.0) | 7/8 (87.5) | |
| Microbleeds, n (%) | 3/6 (50.0) | 13/25 (52.0) | |
| Lobar microbleeds n (%) | - | - | |
| Deep microbleeds n (%) | 2/3 | 2/13 | |
| Mixed location microbleeds n (%) | 1/3 | 11/13 | |
| Not given n (%) | - | - | |
| IVH, n (%) | 2/4 (50.0) | 3/7 (42.9) | |
| HT/PH of IS, n (%) | - | 3/3 (100%) | |
| SVT with hemorrhage, n (%) | - | 4/4 (100%) | |
| Other, n (%) | - | - | |
| Multilocular ICH, not further specified, n (%) | - | - | |
| Baseline Characteristics | Favorable Outcome (mRS 0–2) | Non-Favorable Outcome (mRS 3–6) | p-Value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 60.5 (43.25–67.25) | 60.0 (53.0–71.0) | 0.329 | |
| Female, n (%) | 5/10 (50.0) | 38/108 (35.2) | 0.630 | |
| Critical disease (LEOSS), n (%) | 3/10 (20.0) | 72/108 (66.7) | 0.001 | |
| ECMO, n (%) | - | 17/80 (21.3) | 0.147 | |
| Anticoagulation, n (%) | 3/5 (60.0) | 77/88 (87.5) | 0.085 | |
| Time from COVID-19 diagnosis to ICH diagnosis (days), median (IQR) | 9.5 (1.8–13.5) | 16.0 (10.0–24.5) | 0.012 | |
| Non-neurological symptoms | Fever, n (%) | 5/9 (55.6) | 35/94 (31.8) | 0.281 |
| Respiratory symptoms, n (%) | 7/9 (77.8) | 69/91 (75.8) | 0.896 | |
| Myalgia/arthralgia, n (%) | 0/9 (10.0) | 8/94 (8.5) | 0.362 | |
| Malaise, n (%) | 2/9 (22.2) | 8/94 (8.5) | 0.184 | |
| Neurological symptoms | Focal neurological deficits, n (%) | 2/10 (20.0) | 22/96 (22.9) | 0.834 |
| Altered level of consciousness, n (%) | 3/10 (10.0) | 48/101 (47.5) | 0.289 | |
| Encephalopathy, n (%) | - | 4/97 (3.7) | 0.513 | |
| Headache, n (%) | 4/10 (40.0) | 11/96 (11.5) | 0.014 | |
| Anisocoria, n (%) | - | 28/98 (28.6) | 0.050 | |
| Seizure, n (%) | 1/10 (10.0) | 7/99 (7.1) | 0.735 | |
| ICH | IPH, n (%) | 2/10 (20.0) | 56/108 (51.9) | 0.054 |
| SAH, n (%) | 4/10 (40.0) | 33/108 (30.6) | 0.538 | |
| SDH/EDH, n (%) | - | 7/108 (6.4) | 0.406 | |
| Microbleeds, n (%) | 3/10 (30.0) | 18/102 (17.6) | 0.340 | |
| Lobar microbleeds n (%) | 1/3 (33.3) | - | 0.089 | |
| Deep microbleeds n (%) | - | 2/18 (11.1) | 1.0 | |
| Mixed location microbleeds n (%) | 2/3 (66.6) | 16/18 (88.9) | 0.662 | |
| Not given n (%) | - | - | - | |
| IVH, n (%) | 1/10 (10.0) | 4/108 (3.7) | 0.334 | |
| HT/PH of IS, n (%) | - | 2/108 (1.9) | 0.664 | |
| SVT with hemorrhage, n (%) | - | 4/108 (3.7) | 0.536 | |
| Other, n (%) | - | - | - | |
| Multilocular ICH, not further specified, n (%) | - | - | - | |
| Laboratory values | White blood cells (×109/L), median (IQR) | 17.8 (NA) | 16.0 (10.9–22.5) | 0.758 |
| Platelet count (×109/L), median (IQR) | 103.0 (NA) | 167.5 (94.3–244.5)) | 0.537 | |
| C-reactive protein (mg/L), median (IQR) | 142.0 (49.8–332.6) | 230.0 (55.0–352.5) | 0.457 | |
| INR, median (IQR) | 1.2 (NA) | 1.3 (1.1–1.6) | 0.457 | |
| aPTT (s), median (IQR) | 115.0 (NA) | 56.0 (37.6–68.0) | 0.117 | |
| D-dimer (mg/L), median (IQR) | 7.6 (NA) | 7.8 (2.4–19.7) | 0.550 | |
| Palliative care, n (%) | - | 34/86 (38.4) | 0.016 | |
| Baseline Characteristics | Mean (95%-CI) | I2 (95%-CI) | Number of Studies | |
|---|---|---|---|---|
| Age (years) | 58.8 (54.8; 62.9) | 85.6% (75.9%; 91.4%) | 11 | |
| Female (%) | 34.0 (29.5; 40.4) | 0.0% (0.0%; 36.0%) | 14 | |
| Critical disease (LEOSS) (%) | 23.3 (8.9; 61.2) | 53.8% (0.0%; 83.0%) | 5 | |
| ECMO (%) | 27.5 (5.8; 130.2) | 92.7% (82.0%; 97.0%) | 3 | |
| Anticoagulation (%) | 62.7 (38.2; 103.0) | 82.6% (55.3%; 93.2%) | 4 | |
| Time from COVID-19 diagnosis to ICH diagnosis (days) | 21.5 (14.9; 28.0) | 92.3% (86.0%; 95.8%) | 6 | |
| Non-neurological symptoms | Fever (%) | 36.6 (19.9; 63.5) | 71.0% (17.3%; 89.9%) | 4 |
| Respiratory symptoms (%) | 60.9 (41.2; 90.0) | 64.0% (0.0%; 87.8%) | 4 | |
| Myalgia/arthralgia (%) | 7.0 (3.8; 13.1) | NA | 1 | |
| Malaise (%) | 8.5 (4.8; 14.9) | NA | 1 | |
| Neurological symptoms | Focal neurological deficits (%) | 23.8 (16.8; 33.8) | 16.4% (0.0%; 82.6%) | 5 |
| Altered level of consciousness (%) | 57.3 (39.9; 82.3) | 45.0% (0.0%; 79.8%) | 5 | |
| Encephalopathy (%) | 24.4 (7.4; 80.1) | 90.5% (78.7%; 95.8%) | 4 | |
| Headache (%) | 13.9 (8.5; 20.1) | 0% (NA) | 2 | |
| Anisocoria (%) | 20.4 (14.2; 29.4) | NA | 1 | |
| Seizure (%) | 8.4 (4.6; 15.4) | 21.6% (0.0%; 67.1%); | 5 | |
| ICH | IPH (%) | 33.7 (23.2; 48.8) | 63.7% (30.6%; 81.0%) | 11 |
| SAH (%) | 26.6 (16.8; 42.0) | 61.2% (27.2%; 79.3%) | 12 | |
| SDH/EDH (%) | 12.6 (4.4; 35.9) | 84.0% (66.7%; 92.3%) | 6 | |
| Microbleeds (%) | 54.7 (34.4; 87.1) | 84.5% (73.8%; 87.1%) | 11 | |
| Lobar microbleeds | 1.3 (0.3–5.2) | NA | 2 | |
| Deep microbleeds | 8.8 (2.3–28.2) | 72.2 (30.1–89.0) | 5 | |
| Mixed location microbleeds | 35.6 (19.3–65.6) | 82.4 (66.5–90.7) | 8 | |
| Not given | 44.7 (29.5–67.7) | 47.4 (0.0–79.2) | 6 | |
| IVH (%) | 5.9 (3.0; 11.6) | 2.7% (NA) | 2 | |
| HT/PH of IS (%) | 9.2 (2.2; 39.1) | 80.0% (36.7%; 93.7%) | 3 | |
| SVT with hemorrhage (%) | 2.9 (1.2; 7.1) | 0% (NA) | 2 | |
| Other (%) | 46.2 (25.2; 84.8) | 76.4% (50.3%; 88.7%) | 7 | |
| Multilocular ICH, not further specified (%) | 23.0 (6.1; 0.87.0) | 83.8% (59.0%; 93.6%) | 4 | |
| Laboratory values | White blood cells (×109/L) | 13.1 (6.6; 19.5) | 97.4% (95.5%; 98.5%) | 4 |
| Platelet count (×109/L) | 222.9 (193.9; 251.8) | 63.9% (12.7%; 85.1%) | 6 | |
| C-reactive protein (mg/L) | 228.1 (200.1; 256.0) | 0% (NA) | 2 | |
| INR | 1.4 (1.1; 1.6) | 93.7% (87.0%; 96.9%) | 4 | |
| apTT (s) | 45.5 (34.2; 56.7) | 98.3% (97.2%; 98.9%) | 4 | |
| D-dimer (mg/L) | 8.2 (1.8; 14.6) | 98.1% (96.5%; 99.0%) | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidbauer, M.L.; Ferse, C.; Salih, F.; Klingner, C.; Musleh, R.; Kunst, S.; Wittstock, M.; Neumann, B.; Schebesch, K.-M.; Bösel, J.; et al. COVID-19 and Intracranial Hemorrhage: A Multicenter Case Series, Systematic Review and Pooled Analysis. J. Clin. Med. 2022, 11, 605. https://doi.org/10.3390/jcm11030605
Schmidbauer ML, Ferse C, Salih F, Klingner C, Musleh R, Kunst S, Wittstock M, Neumann B, Schebesch K-M, Bösel J, et al. COVID-19 and Intracranial Hemorrhage: A Multicenter Case Series, Systematic Review and Pooled Analysis. Journal of Clinical Medicine. 2022; 11(3):605. https://doi.org/10.3390/jcm11030605
Chicago/Turabian StyleSchmidbauer, Moritz L., Caroline Ferse, Farid Salih, Carsten Klingner, Rita Musleh, Stefan Kunst, Matthias Wittstock, Bernhard Neumann, Karl-Michael Schebesch, Julian Bösel, and et al. 2022. "COVID-19 and Intracranial Hemorrhage: A Multicenter Case Series, Systematic Review and Pooled Analysis" Journal of Clinical Medicine 11, no. 3: 605. https://doi.org/10.3390/jcm11030605
APA StyleSchmidbauer, M. L., Ferse, C., Salih, F., Klingner, C., Musleh, R., Kunst, S., Wittstock, M., Neumann, B., Schebesch, K.-M., Bösel, J., Godau, J., Lochner, P., Adam, E. H., Jahnke, K., Knier, B., Schirotzek, I., Müllges, W., Notz, Q., Dengl, M., ... on behalf of the IGNITE Study Group. (2022). COVID-19 and Intracranial Hemorrhage: A Multicenter Case Series, Systematic Review and Pooled Analysis. Journal of Clinical Medicine, 11(3), 605. https://doi.org/10.3390/jcm11030605







