Functional Status Following Pulmonary Rehabilitation: Responders and Non-Responders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurements
2.3. Intervention
2.4. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. General Effects of PR
3.3. Responders, Non-Responders, and Predictors of Response
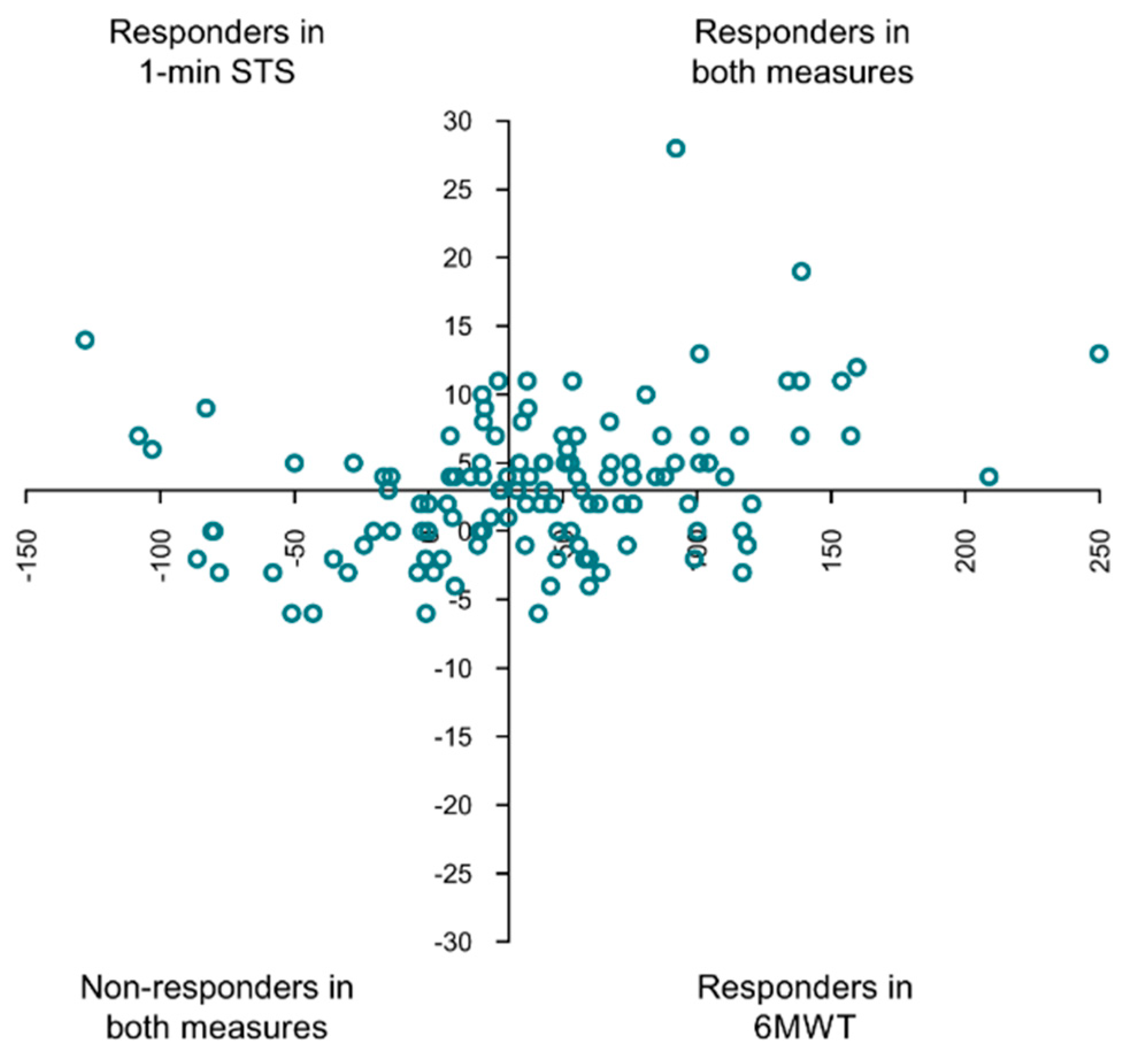
3.4. Comparison of Responders and Non-Responders Classified with the 1 min STS and the 6MWT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, Cd003793. [Google Scholar] [CrossRef]
- Spruit, M.A.; Augustin, I.M.; Vanfleteren, L.E.; Janssen, D.J.; Gaffron, S.; Pennings, H.-J.; Smeenk, F.; Pieters, W.; Bergh, J.J.V.D.; Michels, A.-J.; et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Leidy, N.K. Functional status and the forward progress of merry-go-rounds: Toward a coherent analytical framework. Nurs. Res. 1994, 43, 196–202. [Google Scholar] [CrossRef]
- Souto-Miranda, S.; Marques, A. Triangulated perspectives on outcomes of pulmonary rehabilitation in patients with COPD: A qualitative study to inform a core outcome set. Clin. Rehabil. 2018, 33, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.J.; Puhan, M.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef] [Green Version]
- Reychler, G.; Boucard, E.; Peran, L.; Pichon, R.; Le Ber-Moy, C.; Ouksel, H.; Liistro, G.; Chambellan, A.; Beaumont, M. One minute sit-to-stand test is an alternative to 6MWT to measure functional exercise performance in COPD patients. Clin. Respir. J. 2017, 12, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; de Bisschop, C.; Beaumont, M.; Ouksel, H.; Jean, V.; Dessables, F.; Chambellan, A. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2609–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozalevli, S.; Ozden, A.; Itil, O.; Akkoclu, A. Comparison of the Sit-to-Stand Test with 6min walk test in patients with chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troosters, T.; Gosselink, R.; Decramer, M. Exercise Training in COPD: How to Distinguish Responders from Nonresponders. J. Cardiopulm. Rehabil. 2001, 21, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.R.; McKeough, Z.; Morris, N.; Chang, A.T.; Yerkovich, S.; Seale, H.E.; Paratz, J.D. Metabolic Disease and Participant Age Are Independent Predictors of Response to Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2013, 33, 249–256. [Google Scholar] [CrossRef]
- Scott, A.S.; Baltzan, M.A.; Fox, J.; Wolkove, N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can. Respir. J. 2010, 17, 219–223. [Google Scholar] [CrossRef]
- De Torres, J.P.; Pinto-Plata, V.; Ingenito, E.; Bagley, P.; Gray, A.; Berger, R.; Celli, B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002, 121, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Costi, S.; Luppi, F.; Cirelli, G.; Cilione, C.; Coletti, O.; Fabbri, L.; Clini, E. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008, 63, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2022. Available online: https://goldcopd.org/2022-gold-reports-2 (accessed on 16 November 2021).
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.W.; Quirk, F.; Baveystock, C.M.; Littlejohns, P. A Self-complete Measure of Health Status for Chronic Airflow Limitation: The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.-L.; Mathur, S.; Dechman, G.; Maltais, F.; Camp, P.; Saey, D. Fixed Handheld Dynamometry Provides Reliable and Valid Values for Quadriceps Isometric Strength in People with Chronic Obstructive Pulmonary Disease: A Multicenter Study. Phys. Ther. 2019, 99, 1255–1267. [Google Scholar] [CrossRef]
- Marshall, A.L.; Smith, B.; E Bauman, A.; Kaur, S. Reliability and validity of a brief physical activity assessment for use by family doctors * Commentary. Br. J. Sports Med. 2005, 39, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jácome, C.; Cruz, J.; Oliveira, A.; Marques, A. Validity, Reliability, and Ability to Identify Fall Status of the Berg Balance Scale, BESTest, Mini-BESTest, and Brief-BESTest in Patients With COPD. Phys. Ther. 2016, 96, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Strassmann, A.; Steurer-Stey, C.; Dalla Lana, K.; Zoller, M.; Turk, A.J.; Suter, P.; Puhan, M.A. Population-based reference values for the 1-min sit-to-stand test. Int. J. Public Health 2013, 58, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolen, E.H.; van Hees, H.W.; van Lummel, R.C.; Dekhuijzen, R.; Djamin, R.S.; Spruit, M.A.; van’t Hul, A.J. “Can do” versus “do do”: A Novel Concept to Better Understand Physical Functioning in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.; Rebelo, P.; Paixão, C.; Almeida, S.; Jácome, C.; Cruz, J.; Oliveira, A. Enhancing the assessment of cardiorespiratory fitness using field tests. Physiotherapy 2019, 109, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Jácome, C.; Rebelo, P.; Paixão, C.; Oliveira, A.; Cruz, J.; Freitas, C.; Rua, M.; Loureiro, H.; Peguinho, C.; et al. Improving access to community-based pulmonary rehabilitation: 3R protocol for real-world settings with cost-benefit analysis. BMC Public Heal. 2019, 19, 676. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Gugg, K.; Zwick, R.H. Non-responders to outpatient pulmonary rehabilitation: A retrospective, controlled cohort study. Eur. Respir. J. 2020, 56, 715. [Google Scholar] [CrossRef]
- Stoilkova-Hartmann, A.; Janssen, D.J.; Franssen, F.M.; Wouters, E.F. Differences in change in coping styles between good responders, moderate responders and non-responders to pulmonary rehabilitation. Respir. Med. 2015, 109, 1540–1545. [Google Scholar] [CrossRef]
- Spruit, M.A.; Pitta, F.; Garvey, C.; ZuWallack, R.L.; Roberts, C.M.; Collins, E.G.; Goldstein, R.; McNamara, R.; Surpas, P.; Atsuyoshi, K.; et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur. Respir. J. 2013, 43, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, K.; Pierce, J.; Papo, S.; Imran, H.; Evans, S.; Wu, W.-C. Efficacy of adding activity of daily living simulation training to traditional pulmonary rehabilitation on dyspnea and health-related quality-of-life. PLoS ONE 2020, 15, e0237973. [Google Scholar] [CrossRef]
- Vaes, A.W.; Delbressine, J.M.; Mesquita, R.; Goertz, Y.M.; Janssen, D.J.; Nakken, N.; Franssen, F.M.; Vanfleteren, L.E.; Wouters, E.F.; Spruit, M.A. Impact of pulmonary rehabilitation on activities of daily living in patients with chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 126, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadflieg, K.; Machado, A.; Haesevoets, S.; Daenen, M.; Thomeer, M.; Ruttens, D.; Spruit, M.A.; Burtin, C. Physical Tests Are Poorly Related to Patient-Reported Outcome Measures during Severe Acute Exacerbations of COPD. J. Clin. Med. 2021, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.; Antons, J.; Vercoulen, J.H.; Goërtz, Y.M.J.; Ebadi, Z.; Burtin, C.; Janssen, D.J.A.; Thong, M.S.Y.; Otker, J.; Coors, A.; et al. Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis. J. Clin. Med. 2019, 8, 1264. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.R.; Morris, N.R.; McKeough, Z.J.; Yerkovich, S.T.; Paratz, J.D. A Simple Clinical Measure of Quadriceps Muscle Strength Identifies Responders to Pulmonary Rehabilitation. Pulm. Med. 2014, 2014, 782702. [Google Scholar] [CrossRef]
- Gephine, S.; Frykholm, E.; Nyberg, A.; Mucci, P.; Van Hees, H.W.H.; Lemson, A.; Klijn, P.; Maltais, F.; Saey, D. Specific Contribution of Quadriceps Muscle Strength, Endurance, and Power to Functional Exercise Capacity in People with Chronic Obstructive Pulmonary Disease: A Multicenter Study. Phys. Ther. 2021, 101, pzab052. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Post | ∆ Pre-Post | Effect Size (d) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 69.0 (65.0–75.0) | ||||
| Sex, n (%) | |||||
| Male | 99 (81.8) | ||||
| Female | 22 (18.2) | ||||
| BMI, kg/m2 | 26.4 ± 4.8 | 26.4 ± 4.6 | −0.0 ± 0.9 | 0.049 | 0.591 |
| Underweight, <21, n (%) | 13 (10.7) | 10 (8.3) | 3 (2.4) | <0.001 * | |
| Obese >30, n (%) | 28 (23.1) | 27 (22.5) | 1 (0.6) | ||
| Smoking status, n (%) | |||||
| Never | 22 (18.2) | ||||
| Former | 82 (67.8) | ||||
| Current | 17 (14.0) | ||||
| Pack-years | 31.2 (10.0–60.0) | ||||
| LTOT, n (%) | 12 (9.9) | ||||
| NIV, n (%) | 16 (13.2) | ||||
| CCI, score | 4.0 (3.0–5.0) | ||||
| FEV1 % predicted | 50.0 (37.0–63.7) | ||||
| GOLD grade, n (%) | |||||
| 1 | 9 (7.4) | ||||
| 2 | 52 (43.0) | ||||
| 3 | 47 (38.8) | ||||
| 4 | 13 (10.7) | ||||
| GOLD group, n (%) | |||||
| A | 32 (26.4) | ||||
| B | 66 (54.5) | ||||
| C | 3 (2.5) | ||||
| D | 20 (16.5) | ||||
| Respiratory-related hospital admissions in the previous 12 months, n | 0.0 (0.0–0.0) | ||||
| AECOPD in the previous 12 months, n | 0.0 (0.0–1.0) | ||||
| mMRC, score | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.0 (−1.0–0.0) | 0.39 | <0.001 * |
| CAT score, points | 14.8 ± 8.0 | 12.1 ± 7.0 | −2.7 ± 5.8 | 0.46 | <0.001 * |
| SGRQ, total score | 45.1 ± 19.8 | 38.7 ± 19.1 | −6.4 ± 12.2 | 0.52 | <0.001 * |
| QVC, KgF | 30.6 ± 8.4 | 33.4 ± 10.2 | 2.7 ± 8.7 | 0.31 | <0.001 * |
| Handgrip strength, KgF | 34.3 ± 9.2 | 34.0 ± 10.1 | −0.3 ± 7.5 | 0.05 | 0.619 |
| BPAAT, score | 0.0 (0.0–3.0) | 4.0 (2.0–6.0) | 2.0 (1.0–4.0) | 0.69 | <0.001 * |
| Brief-BESTest | 19.0 (15.0–22.0) | 21.0 (18.0–24.0) | 2.0 (0.0–4.0) | 0.63 | <0.001 * |
| 1 min STS, repetitions | 23.0 (18.0–29.0) | 27.0 (21.0–33.0) | 3.0 (0.0–6.0) | 0.58 | <0.001 * |
| 1 min STS <70% predicted, n (%) | 59 (48.8) | 38 (31.4) | 21 (17.4) | <0.001 * | |
| 6MWD, m | 419.6 (331.9–508.6) | 465.0 (386.7–540.3) | 41.0 (7.0–75.3) | 0.56 | <0.001 * |
| 6MWD < 70% predicted, n (%) | 27 (22.3) | 20 (16.5) | 7 (5.8) | <0.001 * |
| 1-Min STS | 6MWT | 1-Min STS and 6MWT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders (n = 66) | Non-Responders (n = 55) | p-Value | Responders (n = 69) | Non-Responders (n = 52) | p-Value | Responders in Both Measures (n = 43) | Non-Responders in Both Measures (n = 78) | p-Value | |
| Age, years | 69.0 (64.0–75.0) | 70.0 (67.5–74.5) | 0.150 | 69.0 (65.0–71.5) | 69.0 (65.0–71.5) | 0.344 | 69.0 (61.5–73.0) | 70.0 (66.0–75.0) | 0.178 |
| Sex, n (%) | |||||||||
| Male | 50 (75.8) | 49 (89.1) | 0.058 | 57 (82.6) | 42 (80.8) | 0.795 | 34 (79.1) | 65 (83.3) | 0.561 |
| Female | 16 (24.2) | 6 (10.9) | 12 (17.4) | 10 (19.2) | 9 (20.9) | 13 (16.7) | |||
| BMI, kg/m2 | 27.0 (24.3–30.1) | 24.2 (22.1–28.3) | 0.008 | 27.0 ± 4.6 | 25.7 ± 5.0 | 0.143 | 27.5 (25.0–30.2) | 24.6 (22.4–28.6) | 0.009 |
| Smoking status, n (%) | |||||||||
| Never | 13 (19.7) | 9 (16.4) | 0.475 | 11 (15.9) | 11 (21.2) | 0.668 | 6 (14.0) | 16 (20.5) | 0.504 |
| Former | 46 (69.7) | 36 (65.5) | 49 (71.0) | 33 (63.5) | 32 (74.4) | 50 (64.1) | |||
| Current | 7 (10.6) | 10 (18.2) | 9 (13.0) | 8 (15.4) | 5 (11.6) | 12 (15.4) | |||
| Pack-years, n | 38.8 (12.3–64.0) | 30.0 (6.1–55.0) | 0.342 | 34.1 (13.1–60.0) | 28.5 (3.2–69−0) | 0.756 | 38.8 (17.0–60.0) | 30.0 (5.9–60.8) | 0.510 |
| LTOT, n (%) | 8 (12.1) | 4 (7.3) | 0.374 | 6 (8.7) | 6 (11.5) | 0.605 | 4 (9.3) | 8 (10.3) | 0.867 |
| NIV, n (%) | 11 (16.7) | 5 (9.1) | 0.221 | 11 (15.9) | 5 (9.6) | 0.309 | 9 (20.9) | 7 (9.0) | 0.063 |
| CCI, score | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.513 | 4.0 (3.0–5.0) | 4.0 (3.0–4.5) | 0.313 | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.980 |
| FEV1, % predicted | 54.3 ± 17.0 | 47.8 ± 18.4 | 0.049 | 50.4 ± 16.0 | 52.6 ± 20.3 | 0.521 | 54.0 (41.0–63.0) | 47.0 (36.0–63.7) | 0.273 |
| GOLD grade, n (%) | |||||||||
| 1 | 6 (9.1) | 3 (5.5) | 0.054 | 3 (4.3) | 6 (11.5) | 0.180 | 2 (4.7) | 7 (9.0) | 0.064 |
| 2 | 33 (50.0) | 19 (34.5) | 33 (47.8) | 19 (36.5) | 25 (58.1) | 27 (34.6) | |||
| 3 | 24 (36.4) | 23 (41.8) | 28 (40.6) | 19 (36.5) | 14 (32.6) | 33 (42.3) | |||
| 4 | 3 (4.5) | 10 (18.2) | 5 (7.2) | 8 (15.4) | 2 (4.7) | 11 (14.1) | |||
| GOLD group, n (%) | |||||||||
| A | 14 (21.2) | 18 (32.7) | 0.525 | 16 (23.2) | 16 (30.8) | 0.405 | 9 (20.9) | 23 (29.5) | 0.375 |
| B | 39 (59.1) | 27 (49.1) | 42 (60.9) | 24 (46.2) | 28 (65.1) | 38 (48.7) | |||
| C | 2 (3.0) | 1 (1.8) | 1 (1.4) | 2 (3.8) | 1 (2.3) | 2 (2.6) | |||
| D | 11 (16.7) | 9 (16.4) | 10 (14.5) | 10 (19.2) | 5 (11.6) | 15 (19.2) | |||
| Respiratory-related hospital admissions in the previous 12 months, n | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.775 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.298 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.455 |
| AECOPD in the previous 12 months, n | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.083 | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.983 | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.399 |
| mMRC, score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.441 | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.679 | 2.0 (1.0–2.5) | 2.0 (1.0–3.0) | 0.420 |
| CAT, total score | 15.9 ± 8.2 | 13.4 ± 7.5 | 0.078 | 15.5 ± 7.9 | 13.8 ± 8.0 | 0.228 | 13.2 ± 7.8 | 14.4 ± 8.0 | 0.425 |
| SGRQ, total score | 53.7 (30.2–62.8) | 43.1 (30.1–53.5) | 0.060 | 52.6 (30.1–61.8) | 45.1 (30.2–56.9) | 0.321 | 53.7 (29.6–61.1) | 45.1 (30.2–57.4) | 0.399 |
| QVC, KgF | 30.2 ± 8.6 | 31.0 ± 8.2 | 0.561 | 30.7 ± 8.9 | 30.4 ± 7.7 | 0.875 | 30.1 ± 9.1 | 30.8 ± 8.0 | 0.679 |
| Handgrip strength, KgF | 34.3 ± 10.4 | 34.4 ± 7.7 | 0.924 | 34.3 ± 9.9 | 34.3 ± 8.4 | 0.987 | 34.6 ± 10.5 | 34.2 ± 8.5 | 0.803 |
| BPAAT, score | 0.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.513 | 0.0 (0.0–2.0) | 1.0 (0.0–4.0) | 0.038 | 0.0 (0.0–1.0) | 0.5 (0.0–3.0) | 0.156 |
| Brief-BESTest, score | 19.0 (14.0–22.0) | 18.0 (16.0–22.0) | 0.876 | 18.0 (15.0–22.0) | 19.0 (16.0–22.0) | 0.373 | 19.0 (13.5–22.0) | 18.0 (16.0–22.0) | 0.580 |
| 1 min STS, repetitions | 22.5 (18.0–27.0) | 25.0 (20.5–31.0) | 0.035 | 22.0 (18.0–28.0) | 25.5 (20.0–31.5) | 0.113 | 22.0 (17.5–26.5) | 24.5 (20.0–30.0) | 0.041 |
| 1 min STS <70% predicted, n (%) | 37 (56.1) | 22 (40.0) | 0.078 | 37 (53.6) | 22 (42.3) | 0.218 | 28 (65.1) | 31 (39.7) | 0.008 |
| 6MWD, m | 404.4 ± 135.2 | 419.2 ± 115.9 | 0.524 | 390.0 (295.0–480.0) | 489.2 (363.2–534.5) | <0.001 | 386.4 (287.4–478.1) | 441.3 (356.2–523.5) | 0.042 |
| 6MWD <70% predicted, n (%) | 17 (25.8) | 10 (18.2) | 0.319 | 21 (30.4) | 6 (11.5) | 0.013 | 15 (34.9) | 12 (15.4) | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto-Miranda, S.; Mendes, M.A.; Cravo, J.; Andrade, L.; Spruit, M.A.; Marques, A. Functional Status Following Pulmonary Rehabilitation: Responders and Non-Responders. J. Clin. Med. 2022, 11, 518. https://doi.org/10.3390/jcm11030518
Souto-Miranda S, Mendes MA, Cravo J, Andrade L, Spruit MA, Marques A. Functional Status Following Pulmonary Rehabilitation: Responders and Non-Responders. Journal of Clinical Medicine. 2022; 11(3):518. https://doi.org/10.3390/jcm11030518
Chicago/Turabian StyleSouto-Miranda, Sara, Maria A. Mendes, João Cravo, Lília Andrade, Martijn A. Spruit, and Alda Marques. 2022. "Functional Status Following Pulmonary Rehabilitation: Responders and Non-Responders" Journal of Clinical Medicine 11, no. 3: 518. https://doi.org/10.3390/jcm11030518
APA StyleSouto-Miranda, S., Mendes, M. A., Cravo, J., Andrade, L., Spruit, M. A., & Marques, A. (2022). Functional Status Following Pulmonary Rehabilitation: Responders and Non-Responders. Journal of Clinical Medicine, 11(3), 518. https://doi.org/10.3390/jcm11030518






