Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Outbreak Investigation
- (1)
- Identification of infected carcasses and parts thereof containing muscle tissue;
- (2)
- Handling of infected carcasses and parts thereof;
- (3)
- Search of the sources of infection and its spread among fauna;
- (4)
- Determination of the species of detected Trichinella spp.
2.2. Epidemiolological Investigation
- Clinical criteria: any person with at least three of the following six criteria: fever, muscle pain, diarrhea, swelling of the face, eosinophilia, and subconjunctival, subungual and retinal hemorrhages.
- Laboratory criteria: at least one of the following two criteria: demonstrating the presence of Trichinella larvae in a muscle biopsy, demonstrating the presence of specific antibodies to Trichinella (IFA, ELISA, or Western blot).
- Epidemiological criteria: at least one of the following two epidemiological links: exposure to contaminated food (meat), exposure to the same source.
2.3. Animal Species Identification as Meat Origin
2.4. Larvae Detection and Identification
3. Results
3.1. Outbreak Investigation
3.2. Epidemiological Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Czarkowski, M.P.; Staszewska-Jakubik, E.; Kondej, B. Infectious Diseases and Poisonings in Poland in 2016; National Institute of Public Health—National Institute of Hygiene, Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection: Warsaw, Poland, 2017; pp. 1–166. [Google Scholar]
- Czarkowski, M.P.; Staszewska-Jakubik, E.; Kondej, B. Infectious Diseases and Poisonings in Poland in 2017; National Institute of Public Health—National Institute of Hygiene, Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection: Warsaw, Poland, 2018; pp. 1–168. [Google Scholar]
- Czarkowski, M.P.; Kondej, E.; Sadłocha, A. Infectious Diseases and Poisonings in Poland in 2018; National Institute of Public Health—National Institute of Hygiene, Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection: Warsaw, Poland, 2019; pp. 1–168. [Google Scholar]
- Czarkowski, M.N.; Szmulik-Misiurek, A.; Zbrzeźniak, J. Infectious Diseases and Poisonings in Poland in 2019; National Institute of Public Health—National Institute of Hygiene, Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection: Warsaw, Poland, 2020; pp. 1–168. [Google Scholar]
- Anon. Infectious Diseases and Poisonings in Poland in 2021. Preliminary Data State on 22.07.2021; National Institute of Public Health—National Institute of Hygiene, Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection: Warsaw, Poland, 2021; pp. 1–89. [Google Scholar]
- Teunis, P.F.M.; Koningstein, M.; Takumi, K.; van der Giessen, J.W.B. Human beings are highly susceptible to low doses of Trichinella spp. Epidemiol. Infect. 2012, 140, 210–218. [Google Scholar] [CrossRef]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Y.N.; Tong, M.W.; Sun, N.; Xuan, Y.H.; Kang, Y.J.; Vallée, I.; Boireau, P.; Cheng, S.P.; Liu, M.Y. Serological tools for detection of Trichinella infection in animals and humans. One Health 2016, 2, 25–30. [Google Scholar] [CrossRef]
- Bruschi, F.; Gómez-Morales, M.A.; Hill, D.E. International Commission on Trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and humans. Food Waterborne Parasitol. 2019, 14, e00032. [Google Scholar] [CrossRef]
- Pozio, E.; Sacchini, D.; Sacchi, L.; Tamburrini, A.; Alberici, F. Failure of Mebendazole in the Treatment of Humans with Trichinella spiralis Infection at the Stage of Encapsulating Larvae. Clin. Infect. Dis. 2001, 32, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://koscian.poznan.lasy.gov.pl/en/lesnictwa (accessed on 3 January 2022).
- Pozio, E.; Hoberg, E.; La Rosa, G.; Zarlenga, D.S. Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus. Infect. Genet. Evol. 2009, 9, 606–616. [Google Scholar] [CrossRef]
- Czarkowski, M.P.; Cielebąk, E.; Kondej, B.; Staszewska, E. Infectious Diseases and Poisonings in Poland in 2011; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Disease and Infection Prevention and Control: Warsaw, Poland, 2012; pp. 1–146. [Google Scholar]
- Czarkowski, M.P.; Cielebąk, E.; Kondej, B.; Staszewska, E. Infectious Diseases and Poisonings in Poland in 2012; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Disease and Infection Prevention and Control: Warsaw, Poland, 2013; pp. 1–146. [Google Scholar]
- Czarkowski, M.P.; Cielebąk, E.; Kondej, B.; Staszewska, B. Infectious Diseases and Poisonings in Poland in 2013; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Disease and Infection Prevention and Control: Warsaw, Poland, 2014; pp. 1–156. [Google Scholar]
- Czarkowski, M.P.; Cielebąk, E.; Staszewska-Jakubik, E.; Kondej, B. Infectious Diseases and Food Poisonings in Poland in 2014; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Diseases and Infection Prevention and Control: Warsaw, Poland, 2015; pp. 1–165. [Google Scholar]
- Czarkowski, M.P.; Cielebąk, E.; Staszewska-Jakubik, E.; Kondej, B. Infectious Diseases and Food Poisonings in Poland in 2015; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Disease and Infection Prevention and Control: Warsaw, Poland, 2016; pp. 1–166. [Google Scholar]
- Czarkowski, M.P.; Cielebąk, E.; Staszewska-Jakubik, E.; Kondej, B. Infectious Diseases and Food Poisonings in Poland in 2016; National Institute of Public Health—National Institute of Hygiene, Department of Epidemiology, Chief Sanitary Inspectorate—Department of Communicable Disease and Infection Prevention and Control: Warsaw, Poland, 2017; pp. 1–166. [Google Scholar]
- Flis, M.; Grela, E.R.; Gugała, D. Epizootic and epidemiological situation of Trichinella sp. Infection in Poland in 2006–2015 in view of wild boar population dynamics. J. Vet. Res. 2017, 61, 181–187. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official control for Trichinella in meat. OJEU 2015, 212, 7–34. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R1375 (accessed on 6 December 2021).
- Konopka, B. Guideline of the Chief Veterinary Officer on Interventional Action to Be Undertaken in Case of Detection of Trichinella in Wild or Farmed Animals. Nr GIW pr 0200.1.1.2021, 02nd March 2021. 2021, pp. 1–11. Available online: https://www.e-bip.org.pl/upload/00471/16395/0367877-33167819.pdf (accessed on 6 December 2021).
- European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues to be Covered by Epidemiological Surveillance as well as Relevant Case Definitions. OJEU 2018, 170, 1–74. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN (accessed on 6 January 2022).
- Vutova, K.; Velev, V.; Chipeva, R.; Yancheva, N.; Petkova, S.; Tomov, T.; Pozio, E.; Robertson, L.J. Clinical and epidemiological descriptions from trichinellosis outbreaks in Bulgaria. Exp. Parasitol. 2020, 212, 107874. [Google Scholar] [CrossRef] [PubMed]
- Różycki, M.; Chmurzyńska, E.; Bilska-Zając, E.; Karamon, J.; Cencek, T. Isoelectric focusing of proteins in the pH gradient as a tool for identification of species origin of raw meat. J. Vet. Res. 2018, 62, 151–159. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Pozio, E.; Gayda, J.; Thaben, N.; Bahn, P.; Nöckler, K. Magnetic stirrer method for the detection of Trichinella larvae in muscle samples. J. Vis. Exp. 2017, 121, e55354. [Google Scholar] [CrossRef]
- Pozio, E.; Zarlenga, D. International Commission on Trichinellosis: Recommendations for genotyping Trichinella muscle stage larvae. Food Waterborne Parasitol. 2019, 15, e00033. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; La Rossa, G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. In PCR Detection of Microbial Pathogens; Sachse, K., Frey, J., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 216, pp. 299–309. [Google Scholar] [CrossRef]
- Bilska-Zając, E.; Różycki, M.; Korpysa-Dzirba, W.; Bełcik, A.; Ziętek-Barszcz, A.; Włodarczyk-Ramus, M.; Gontarczyk, A.; Cencek, T. Trichinella outbreaks on pig farms in Poland in 2012–2020. Pathogens 2021, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Antunović, B.; Blagojević, B.; Johler, S.; Guldimann, C.; Pinto, M.V.; Vågsholm, I.; Meemken, D.; Alvseike, O.; Georgiev, M.; Alban, L. Challenges and opportunities in the implementation of new meat inspection systems in Europe. Trends Food Sci. Technol. 2021, 116, 460–467. [Google Scholar] [CrossRef]
- Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; Ducrot, C.; Hope, J.; Johnston, M.; Klein, G.; Kruse, H.; Lücker, E.; et al. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on the “Risk assessment of a revised inspection of slaughter animals in areas with low prevalence of Trichinella”. EFSA J. 2005, 200, 1–411. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/200 (accessed on 6 December 2021).
- Anon, Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31992L0043 (accessed on 6 December 2021).
- De Bruyne, A.; Ancelle, T.; Vallée, I.; Boireau, P.; Dupouy-Camet, J. Human trichinellosis acquired from wild boar meat: A continuing parasitic risk in France. Euro Surveill. 2006, 11, 3048. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. Trichinella spp. imported with live animals and meat. Vet. Parasitol. 2015, 213, 46–55. [Google Scholar] [CrossRef]
- Sadkowska-Todys, M.; Baumann-Popczyk, A.; Wnukowska, N.; Popczyk, B.; Kucharczyk, B.; Gołąb, E. Occurrence and prevalence of selected zoonotic agents: Echinococcus multilocularis, Trichinella spiralis and hepatitis E virus (HEV) in the population of Polish hunters--results of the study conducted in 2010–2012. Przegl. Epidemiol. 2015, 69, 673–678. [Google Scholar]
- Franssen, F.; Swart, A.; van der Giessen, J.; Havelaar, A.; Takumi, K. Parasite to patient: A quantitative risk model for Trichinella spp. in pork and wild boar meat. Int. J. Food Microbiol. 2017, 241, 262–275. [Google Scholar] [CrossRef][Green Version]
- Moskwa, B.; Cybulska, A.; Kornacka, A.; Cabaj, W.; Bień, J. Wild boars meat as a potential source of human trichinellosis in Poland: Current data. Acta Parasitol. 2015, 60, 530–535. [Google Scholar] [CrossRef]
- Bilska-Zajac, E.; Różycki, M.; Chmurzyńska, E.; Antolak, E.; Próchniak, M.; Grądziel-Krukowska, K.; Karamon, J.; Sroka, J.; Zdybel, J.; Cencek, T. First case of Trichinella nativa infection in wild boar in Central Europe-molecular characterization of the parasite. Parasitol. Res. 2017, 116, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Zając, E.; Franssen, F.; Różycki, M.; Swart, A.; Karamon, J.; Sroka, J.; Zdybel, J.; Ziętek-Barszcz, A.; Cencek, T. Intraspecific genetic variation in Trichinella spiralis and Trichinella britovi populations circulating in different geographical regions of Poland. Int. J. Parasitol. Parasites Wildl. 2019, 10, 101–112. [Google Scholar] [CrossRef]
- Sadkowska-Todys, M.; Gołab, E. Trichinellosis in Poland in 2011. Przegl. Epidemiol. 2013, 67, 259–261, 363–364. [Google Scholar] [PubMed]
- Golab, E.; Szulc, M.; Sadkowska-Todys, M. Outbreak of trichinellosis in North-Western Poland—Update and exported cases, June–July 2007. Euro Surveill. 2007, 12, 3234. [Google Scholar] [CrossRef]
- Nöckler, K.; Wichmann-Schauer, H.; Hiller, P.; Müller, A.; Bogner, K. Trichinellosis outbreak in Bavaria caused by cured sausage from Romania, January 2007. Euro Surveill. 2007, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Bartuliene, A.; Liausediene, R.; Motiejuniene, V. Trichinellosis outbreak in Lithuania, Ukmerge region, June 2009. Euro Surveill. 2009, 14, 19336. [Google Scholar] [CrossRef] [PubMed]
- Barruet, R.; Devez, A.; Dupony-Camet, J.; Karadjian, G.; Plausa, D.; Chydériotis, G.; Vallée, J.; Sofronic-Milosavljevic, L.; Year, H. A common source for a trichinellosis outbreak reported in France and Serbia in 2017. Euro Surveill. 2020, 25, 1900527. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Schink, S.; Mayer-Scholl, A.; Ziesch, C.; Schönfelder, R.; Wichmann-Schauer, H.; Stark, K.; Nöckler, K. Outbreak of trichinellosis due to wild boar meat and evaluation of the effectiveness of post exposure prophylaxis, Germany, 2013. Clin. Infect. Dis. 2015, 60, e98–e104. [Google Scholar] [CrossRef] [PubMed]
- Żukiewicz-Sobczak, W.; Zwoliński, J.; Chmielewska-Badora, J.; Galińska, E.M.; Cholewa, G.; Krasowska, E.; Zagórski, J.; Wojtyła, A.; Tomasiewicz, K.; Kłapeć, T. Prevalence of antibodies against selected zoonotic agents in forestry workers from eastern and southern Poland. Ann. Agric. Environ. Med. 2014, 21, 767–770. [Google Scholar] [CrossRef]
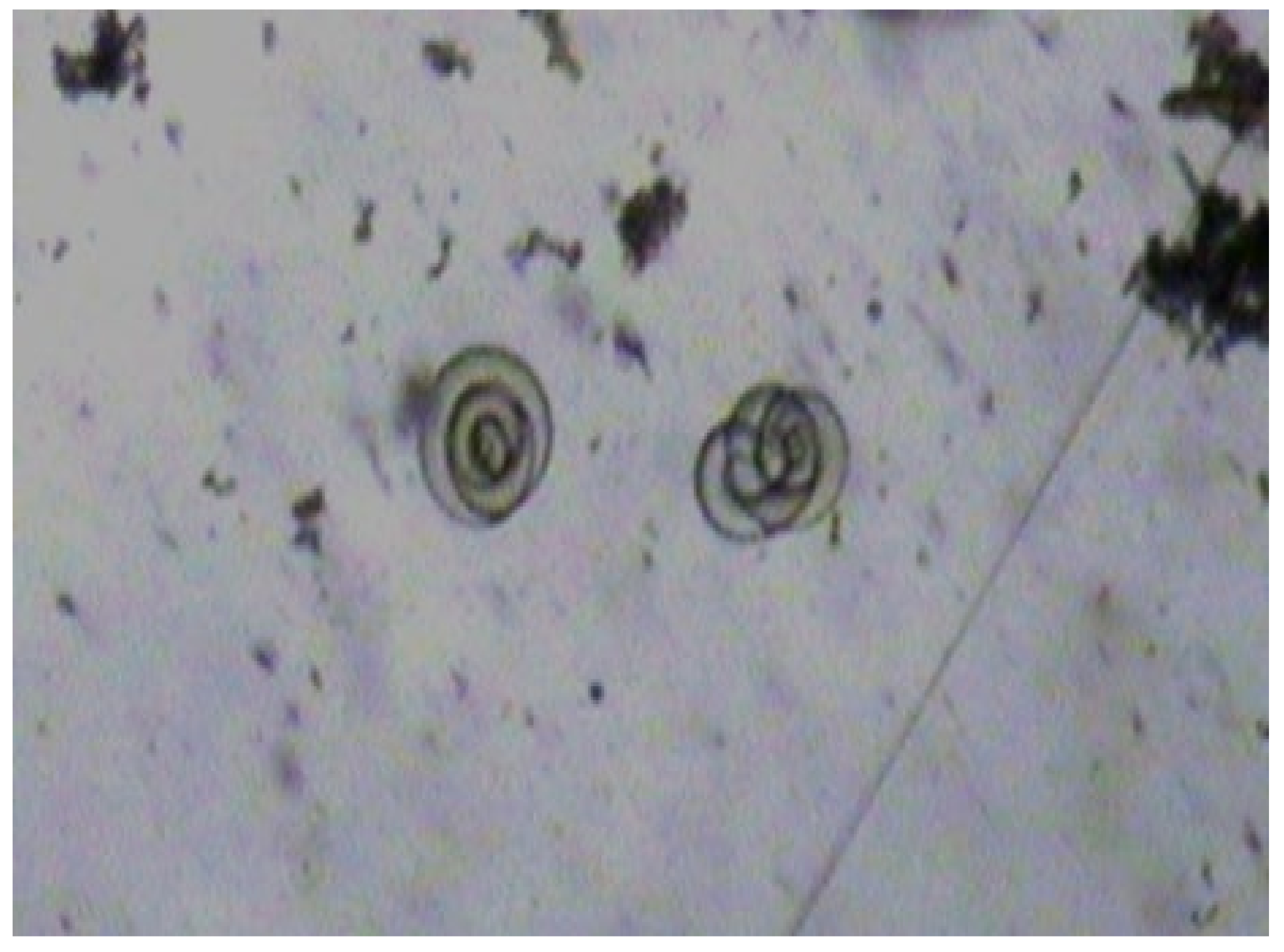
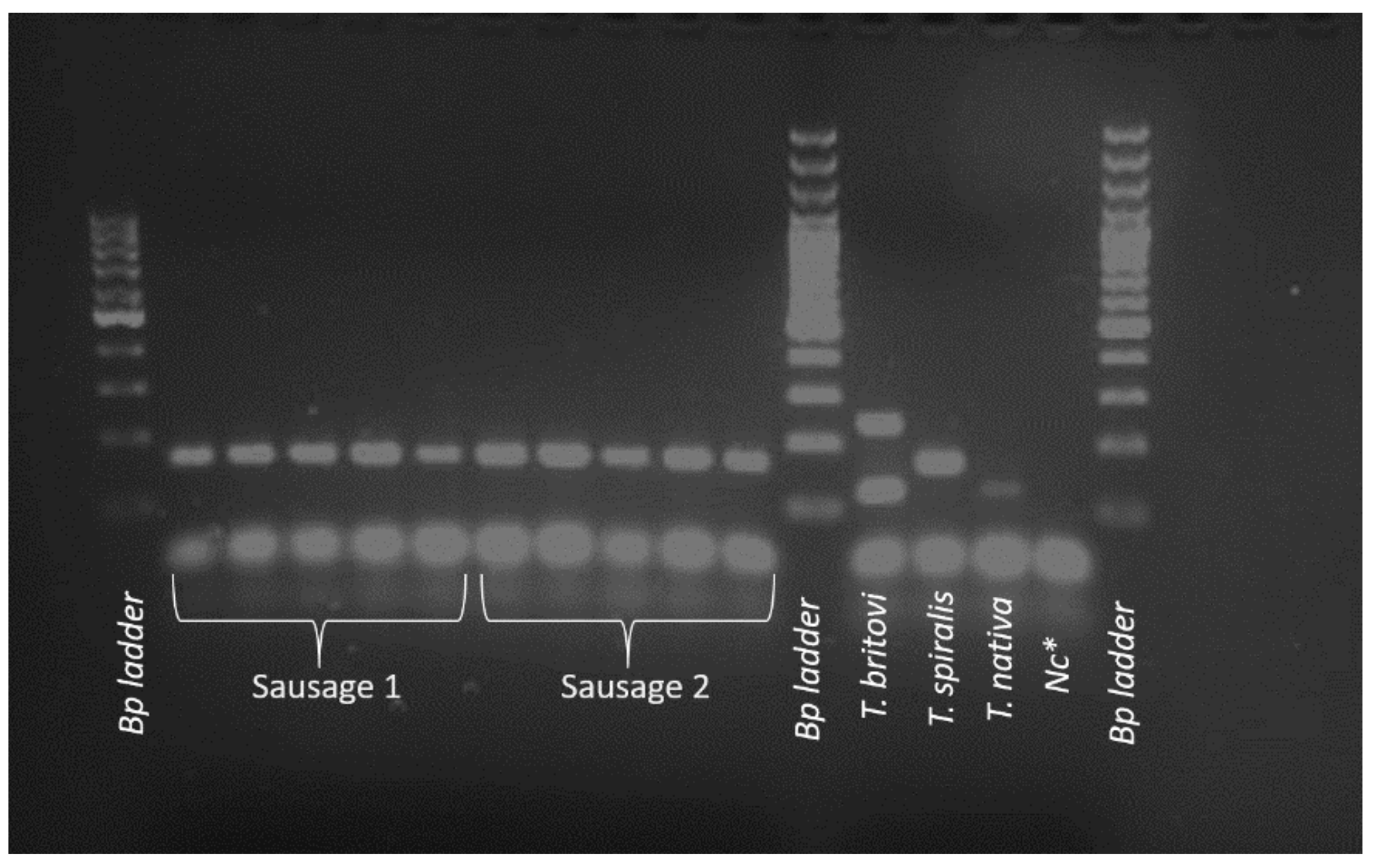
| Age | Sex * | Symptoms | ||||
|---|---|---|---|---|---|---|
| Fever | Muscle Pain | Swelling of the Eyelids | Abdominal Pain | Diarrhea | ||
| 19 | M | + | + | + | ||
| 19 | M | + | + | + | ||
| 32 | M | + | + | + | + | |
| 33 | M | + | + | + | ||
| 36 | F | + | + | + | ||
| 39 | F | + | + | + | + | |
| 47 | M | + | + | + | + | |
| 58 | M | + | + | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różycki, M.; Korpysa-Dzirba, W.; Bełcik, A.; Pelec, T.; Mazurek, J.; Cencek, T. Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat. J. Clin. Med. 2022, 11, 485. https://doi.org/10.3390/jcm11030485
Różycki M, Korpysa-Dzirba W, Bełcik A, Pelec T, Mazurek J, Cencek T. Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat. Journal of Clinical Medicine. 2022; 11(3):485. https://doi.org/10.3390/jcm11030485
Chicago/Turabian StyleRóżycki, Mirosław, Weronika Korpysa-Dzirba, Aneta Bełcik, Tomasz Pelec, Justyna Mazurek, and Tomasz Cencek. 2022. "Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat" Journal of Clinical Medicine 11, no. 3: 485. https://doi.org/10.3390/jcm11030485
APA StyleRóżycki, M., Korpysa-Dzirba, W., Bełcik, A., Pelec, T., Mazurek, J., & Cencek, T. (2022). Analysis of a Trichinellosis Outbreak in Poland after Consumption of Sausage Made of Wild Boar Meat. Journal of Clinical Medicine, 11(3), 485. https://doi.org/10.3390/jcm11030485






