Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Apparatus
2.3. Drugs
2.4. Experimental Procedure
2.4.1. Acclimation
2.4.2. Treatment
2.5. Data Analysis
3. Results
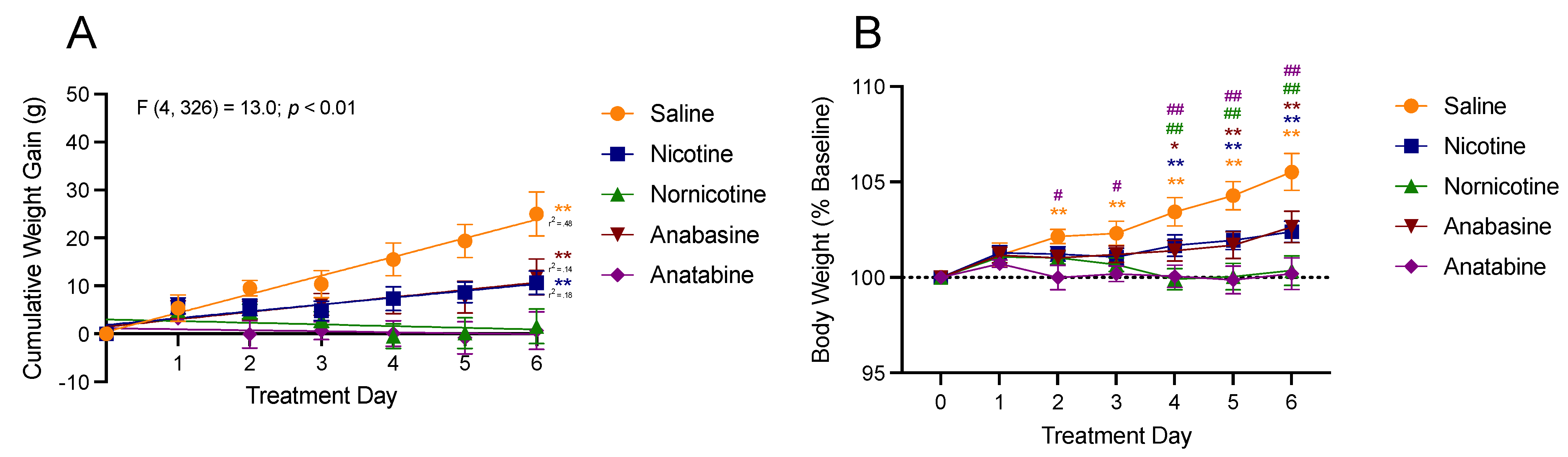
3.1. Body Weight
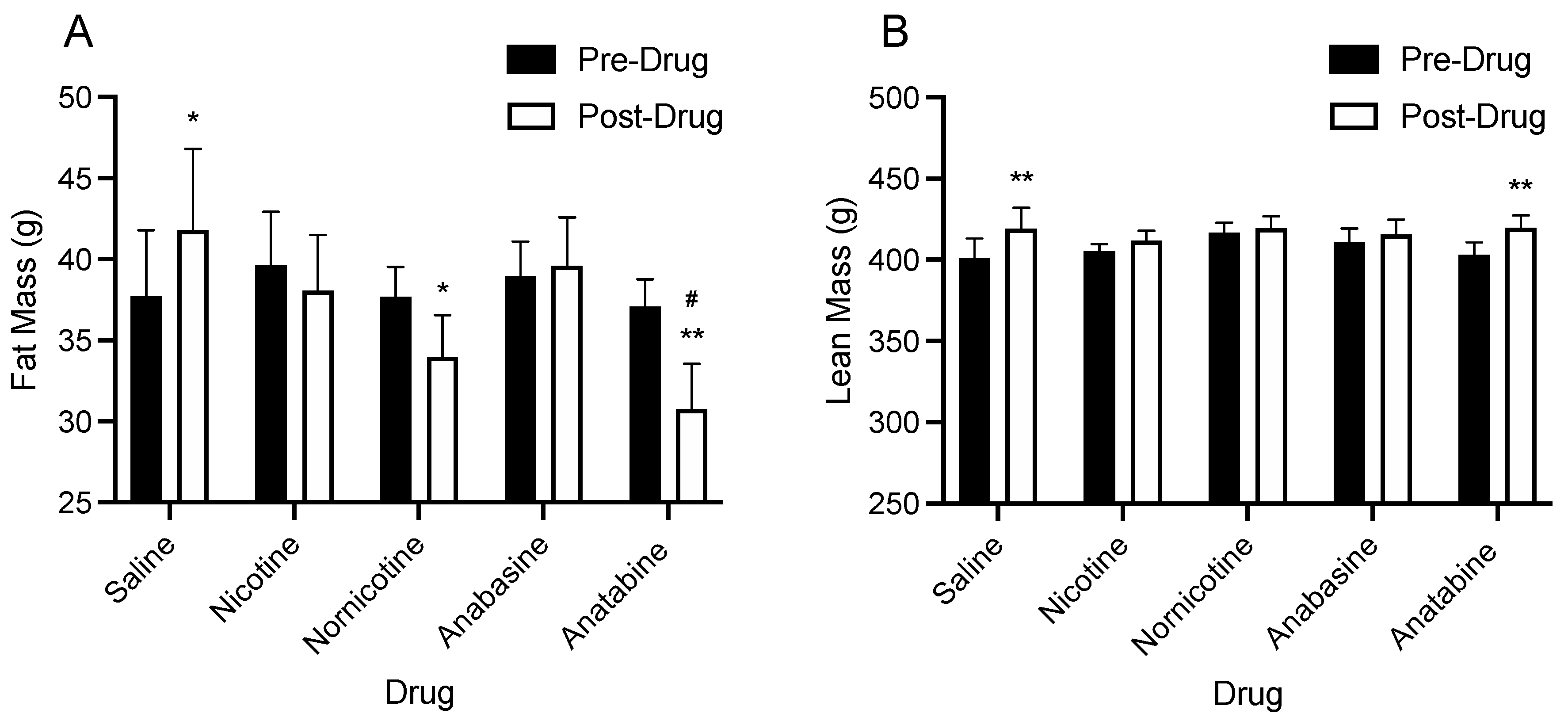
3.2. Body Composition
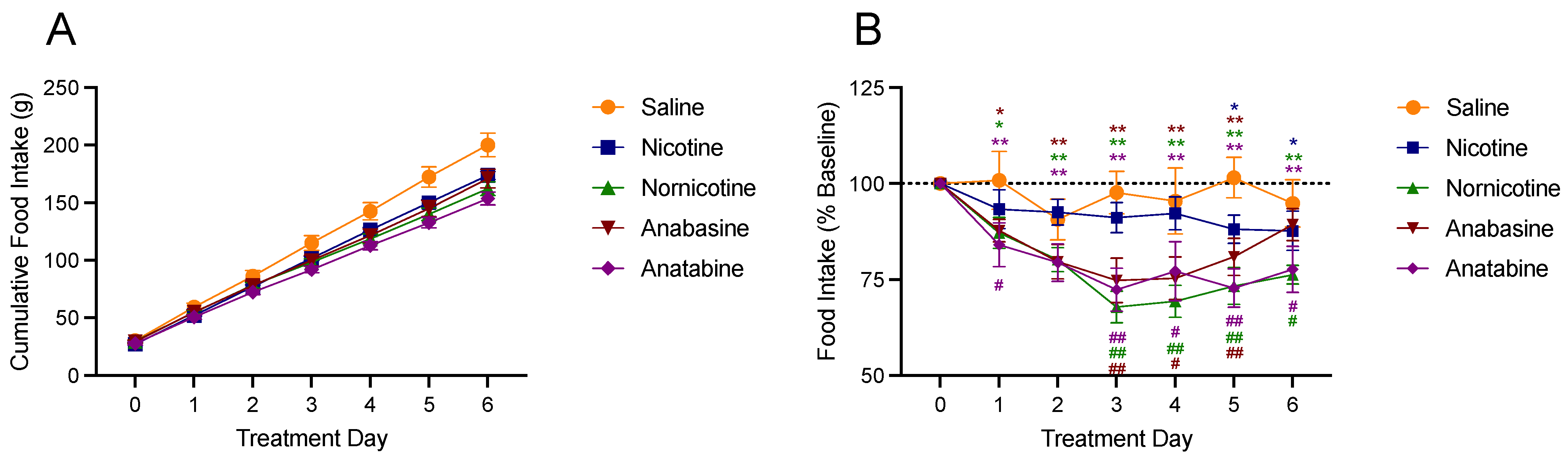
3.3. Food Intake
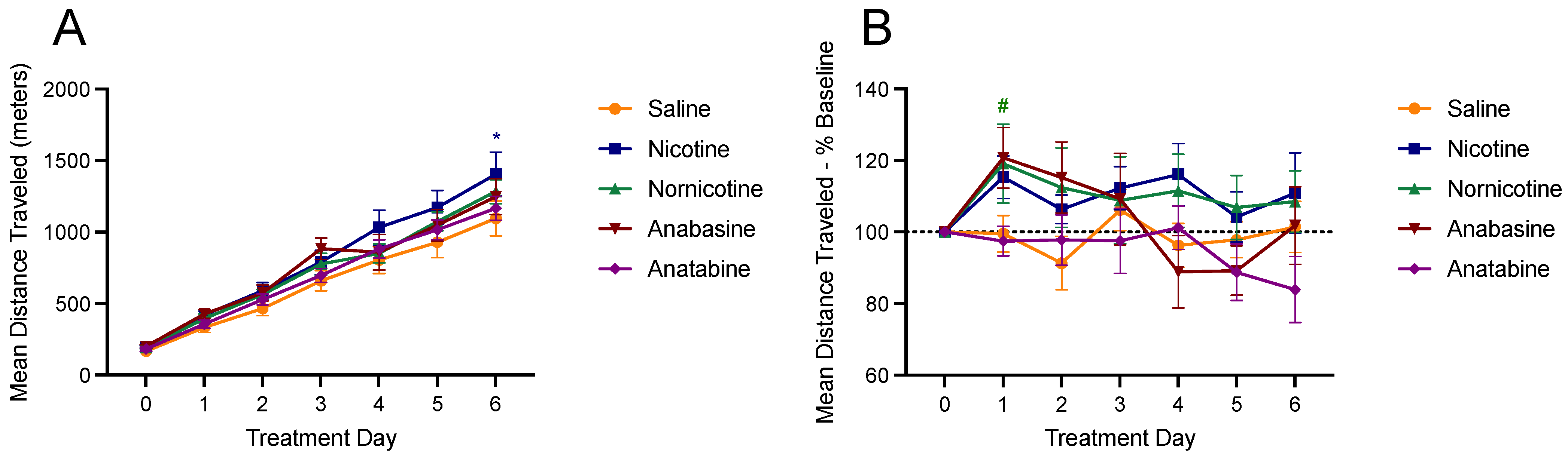
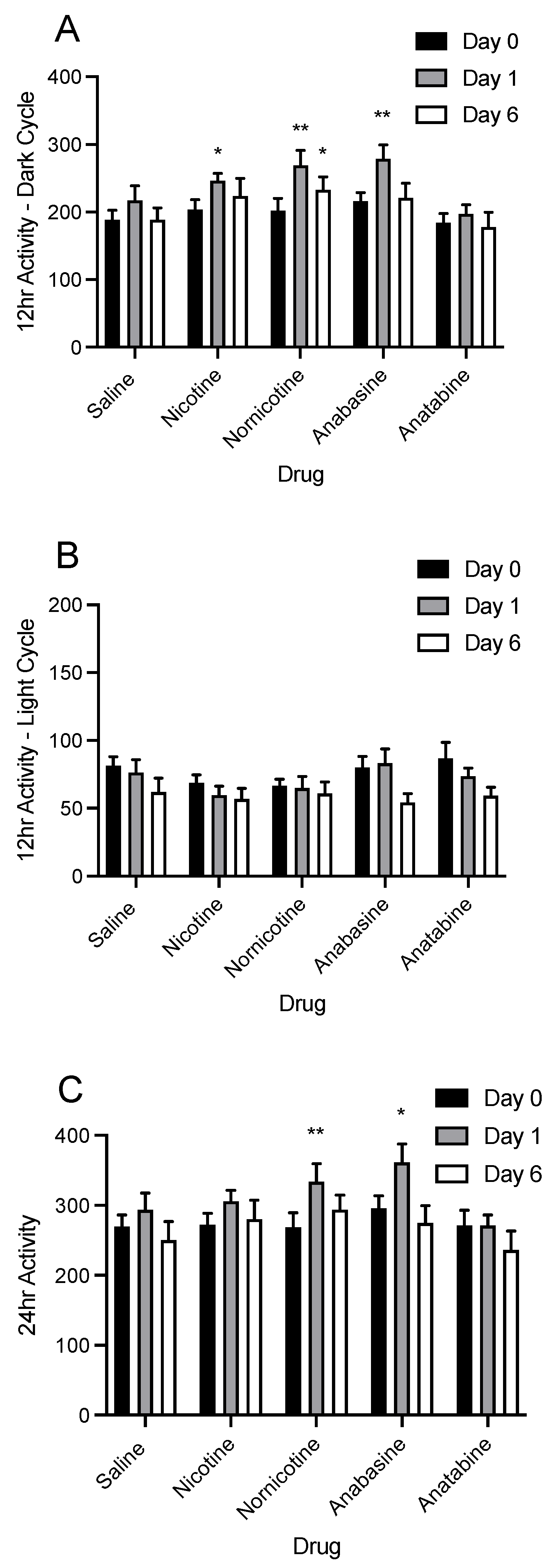
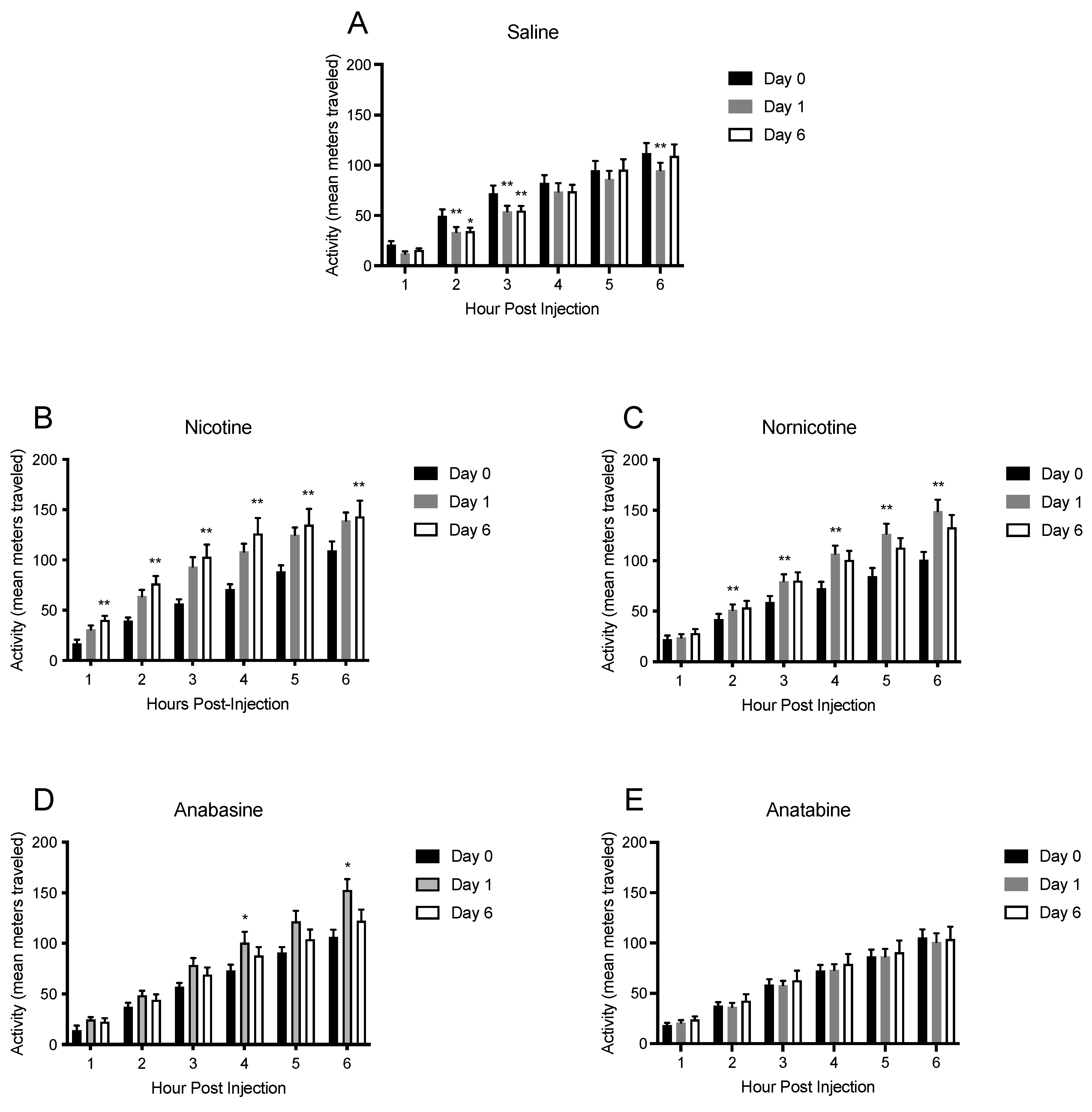
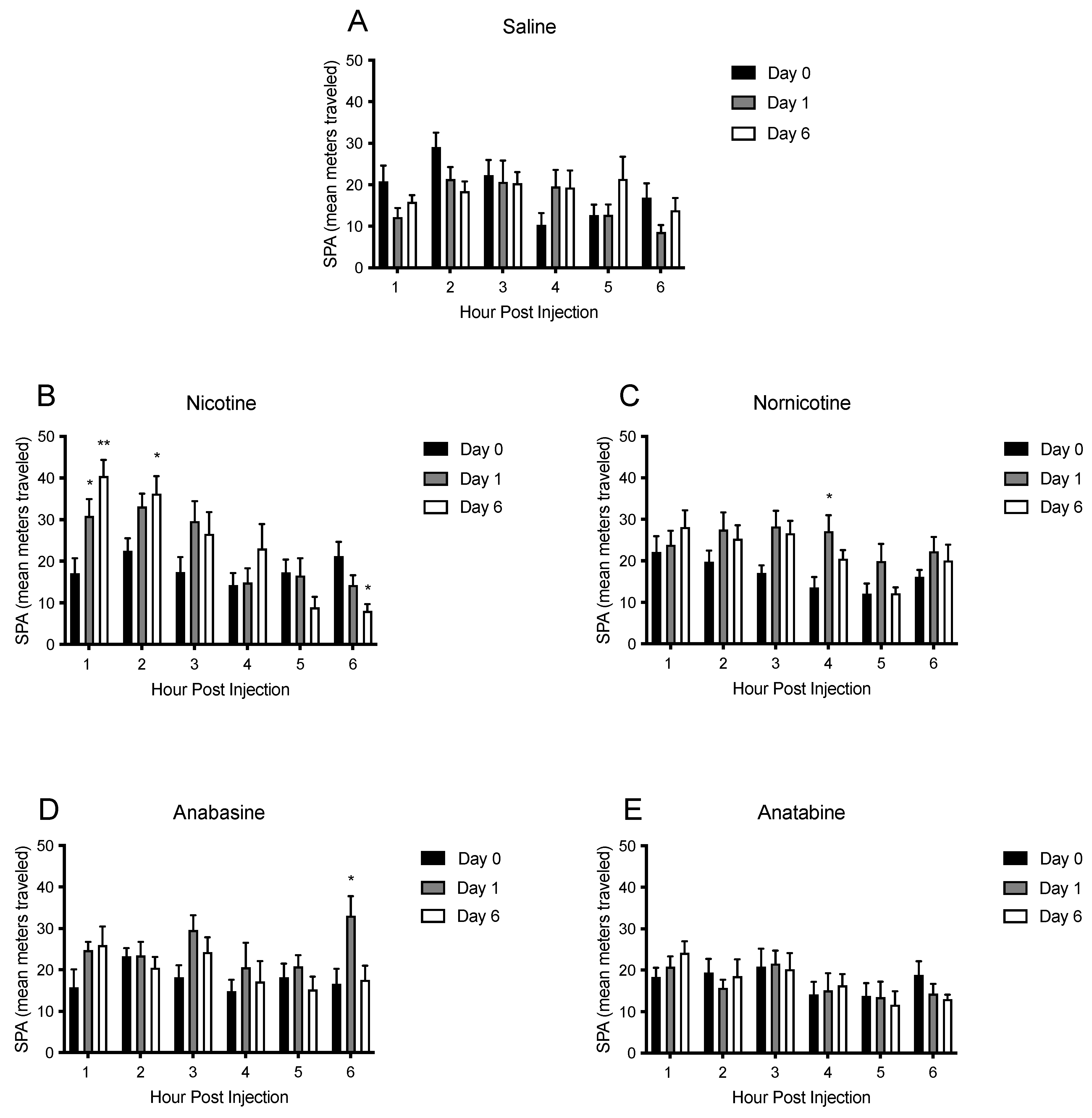
3.4. Physical Activity
4. Discussion
4.1. Body Weight, Body Composition, and Food Intake
4.2. Physical Activity and Energy Expenditure
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, Z.J.; Bleich, S.N.; Long, M.W.; Gortmaker, S.L. Association of Body Mass Index with Health Care Expenditures in the United States by Age and Sex. PLoS ONE 2021, 16, e0247307. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Hamzah, S.; Hajifaraji, M.; Radzi, C.W.J.B.W.M.; Cordell, G.A. Slimming and Appetite-Suppressing Effects of Caraway Aqueous Extract as a Natural Therapy in Physically Active Women. Phytother. Res. 2016, 30, 981–987. [Google Scholar] [CrossRef]
- Poddar, K.; Kolge, S.; Bezman, L.; Mullin, G.E.; Cheskin, L.J. Nutraceutical Supplements for Weight Loss. Nutr. Clin. Pract. 2011, 26, 539–552. [Google Scholar] [CrossRef]
- Krentz, A.J.; Fujioka, K.; Hompesch, M. Evolution of Pharmacological Obesity Treatments: Focus on Adverse Side-effect Profiles. Diabetes Obes. Metab. 2016, 18, 558–570. [Google Scholar] [CrossRef]
- Saitoh, F.; Noma, M.; Kawashima, N. The Alkaloid Contents of Sixty Nicotiana Species. Phytochemistry 1985, 24, 477–480. [Google Scholar] [CrossRef]
- Taujenis, L.; Olšauskaitė, V.; Padarauskas, A. Determination of Nicotine and Three Minor Alkaloids in Tobacco by Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry. Acta. Chromatogr. 2015, 27, 373–385. [Google Scholar] [CrossRef]
- Bardo, M.T.; Bevins, R.A.; Klebaur, J.E.; Crooks, P.A.; Dwoskin, L.P. (−)-Nornicotine Partially Substitutes for (+)-Amphetamine in a Drug Discrimination Paradigm in Rats. Pharm. Biochem. Behav. 1997, 58, 1083–1087. [Google Scholar] [CrossRef]
- Caine, S.B.; Collins, G.T.; Thomsen, M.; IV, C.W.; Lanier, R.K.; Mello, N.K. Nicotine-like Behavioral Effects of the Minor Tobacco Alkaloids Nornicotine, Anabasine, and Anatabine in Male Rodents. Exp. Clin. Psychopharm. 2014, 22, 9. [Google Scholar] [CrossRef]
- Mello, N.K.; Fivel, P.A.; Kohut, S.J.; Caine, S.B. Anatabine Significantly Decreases Nicotine Self-Administration. Exp. Clin. Psychopharm. 2014, 22, 1. [Google Scholar] [CrossRef]
- Hall, B.J.; Wells, C.; Allenby, C.; Lin, M.Y.; Hao, I.; Marshall, L.; Rose, J.E.; Levin, E.D. Differential Effects of Non-Nicotine Tobacco Constituent Compounds on Nicotine Self-Administration in Rats. Pharm. Biochem. Behav. 2014, 120, 103–108. [Google Scholar] [CrossRef]
- Levin, E.D.; Hao, I.; Burke, D.A.; Cauley, M.; Hall, B.J.; Rezvani, A.H. Effects of Tobacco Smoke Constituents, Anabasine and Anatabine, on Memory and Attention in Female Rats. J. Psychopharmacol. 2014, 28, 915–922. [Google Scholar] [CrossRef]
- Paris, D.; Beaulieu-Abdelahad, D.; Abdullah, L.; Bachmeier, C.; Ait-Ghezala, G.; Reed, J.; Verma, M.; Crawford, F.; Mullan, M. Anti-Inflammatory Activity of Anatabine via Inhibition of STAT3 Phosphorylation. Eur. J. Pharm. 2013, 698, 145–153. [Google Scholar] [CrossRef]
- Verma, M.; Beaulieu-Abdelahad, D.; Ait-Ghezala, G.; Li, R.; Crawford, F.; Mullan, M.; Paris, D. Chronic Anatabine Treatment Reduces Alzheimer’s Disease (AD)-Like Pathology and Improves Socio-Behavioral Deficits in a Transgenic Mouse Model of AD. PLoS ONE 2015, 10, e0128224. [Google Scholar] [CrossRef][Green Version]
- Barreto, G.E.; Iarkov, A.; Moran, V.E. Beneficial Effects of Nicotine, Cotinine and Its Metabolites as Potential Agents for Parkinson’s Disease. Front. Aging. Neurosci. 2015, 6, 340. [Google Scholar] [CrossRef]
- Morin, A.; Mouzon, B.; Ferguson, S.; Paris, D.; Saltiel, N.; Browning, M.; Mullan, M.; Crawford, F. A 3-Month-Delayed Treatment with Anatabine Improves Chronic Outcomes in Two Different Models of Repetitive Mild Traumatic Brain Injury in HTau Mice. Sci. Rep. 2021, 11, 7900. [Google Scholar] [CrossRef]
- Castro, P.A.R.; Kogel, U.; Sasso, G.L.; Phillips, B.W.; Sewer, A.; Titz, B.; Garcia, L.; Kondylis, A.; Guedj, E.; Peric, D.; et al. Anatabine Ameliorates Intestinal Inflammation and Reduces the Production of Pro-Inflammatory Factors in a Dextran Sulfate Sodium Mouse Model of Colitis. J. Inflamm. 2020, 17, 29. [Google Scholar] [CrossRef]
- Holtman, J.R.; Crooks, P.A.; Johnson-Hardy, J.K.; Wala, E.P. The Analgesic and Toxic Effects of Nornicotine Enantiomers Alone and in Interaction with Morphine in Rodent Models of Acute and Persistent Pain. Pharm. Biochem. Behav. 2010, 94, 352–362. [Google Scholar] [CrossRef]
- Audrain-McGovern, J.; Benowitz, N. Cigarette Smoking, Nicotine, and Body Weight. Clin. Pharm. 2017, 90, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Bunney, P.E.; Burroughs, D.; Hernandez, C.; LeSage, M.G. The Effects of Nicotine Self-Administration and Withdrawal on Concurrently Available Chow and Sucrose Intake in Adult Male Rats. Physiol. Behav. 2016, 154, 49–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of Smoking for Body Weight, Body Fat Distribution, and Insulin Resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Filozof, C.; Pinilla, M.C.F.; Fernández-Cruz, A. Smoking Cessation and Weight Gain. Obes. Rev. 2004, 5, 95–103. [Google Scholar] [CrossRef]
- Stamford, B.A.; Matter, S.; Fell, R.D.; Papanek, P. Effects of Smoking Cessation on Weight Gain, Metabolic Rate, Caloric Consumption, and Blood Lipids. Am. J. Clin. Nutr. 1986, 43, 486–494. [Google Scholar] [CrossRef]
- Lisko, J.G.; Stanfill, S.B.; Duncan, B.W.; Watson, C.H. Application of GC-MS/MS for the Analysis of Tobacco Alkaloids in Cigarette Filler and Various Tobacco Species. Anal. Chem. 2013, 85, 3380–3384. [Google Scholar] [CrossRef]
- Alijevic, O.; McHugh, D.; Rufener, L.; Mazurov, A.; Hoeng, J.; Peitsch, M. An Electrophysiological Characterization of Naturally Occurring Tobacco Alkaloids and Their Action on Human A4b2 and A7 Nicotinic Acetylcholine Receptors. Phytochemistry 2020, 170, 112187. [Google Scholar] [CrossRef]
- O’Leary, K.; Parameswaran, N.; McIntosh, J.M.; Quik, M. Cotinine Selectively Activates a Subpopulation of A3/A6β2 Nicotinic Receptors in Monkey Striatum. J. Pharm. Exp. 2008, 325, 646–654. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Abizaid, A.; Rao, Y.; Salas, R.; DiLeone, R.J.; Gündisch, D.; Diano, S.; Biasi, M.D.; Horvath, T.L.; Gao, X.-B.; et al. Nicotine Decreases Food Intake Through Activation of POMC Neurons. Science 2011, 332, 1330–1332. [Google Scholar] [CrossRef]
- Papke, R.L.; Dwoskin, L.P.; Crooks, P.A. The Pharmacological Activity of Nicotine and Nornicotine on NAChRs Subtypes: Relevance to Nicotine Dependence and Drug Discovery. J. Neurochem. 2007, 101, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic Acetylcholine Receptors and the Regulation of Neuronal Signalling. Trends Pharm. Sci. 2004, 25, 317–324. [Google Scholar] [CrossRef]
- Gotti, C.; Zoli, M.; Clementi, F. Brain Nicotinic Acetylcholine Receptors: Native Subtypes and Their Relevance. Trends Pharm. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Kenny, P.J. Molecular Mechanisms Underlying Behaviors Related to Nicotine Addiction. Cold Spring Harb. Perspect. Med. 2013, 3, a012112. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar] [CrossRef]
- de Wit, H.; Zacny, J. Abuse Potential of Nicotine Replacement Therapies. Cns Drugs 1995, 4, 456–468. [Google Scholar] [CrossRef]
- Houezec, J.L. Role of Nicotine Pharmacokinetics in Nicotine Addiction and Nicotine Replacement Therapy: A Review. Int. J. Tuberc. Lung Dis. 2003, 7, 811–819. [Google Scholar]
- Avena, N.M.; Rada, P.V. Cholinergic Modulation of Food and Drug Satiety and Withdrawal. Physiol. Behav. 2012, 106, 332–336. [Google Scholar] [CrossRef]
- Bäckberg, M.; Meister, B. Abnormal Cholinergic and GABAergic Vascular Innervation in the Hypothalamic Arcuate Nucleus of Obese Tub/Tub Mice. Synapse 2004, 52, 245–257. [Google Scholar] [CrossRef]
- García, A.P.; Aitta-aho, T.; Schaaf, L.; Heeley, N.; Heuschmid, L.; Bai, Y.; Barrantes, F.J.; Apergis-Schoute, J. Nicotinic A4 Receptor-Mediated Cholinergic Influences on Food Intake and Activity Patterns in Hypothalamic Circuits. PLoS ONE 2015, 10, e0133327. [Google Scholar] [CrossRef][Green Version]
- Herman, A.M.; Ortiz-Guzman, J.; Kochukov, M.; Herman, I.; Quast, K.B.; Patel, J.M.; Tepe, B.; Carlson, J.C.; Ung, K.; Selever, J.; et al. A Cholinergic Basal Forebrain Feeding Circuit Modulates Appetite Suppression. Nature 2016, 538, 253. [Google Scholar] [CrossRef]
- Marrero, M.B.; Lucas, R.; Salet, C.; Hauser, T.A.; Mazurov, A.; Lippiello, P.M.; Bencherif, M. An A7 Nicotinic Acetylcholine Receptor-Selective Agonist Reduces Weight Gain and Metabolic Changes in a Mouse Model of Diabetes. J. Pharm. Exp. 2010, 332, 173–180. [Google Scholar] [CrossRef]
- Jo, Y.; Talmage, D.A.; Role, L.W. Nicotinic Receptor-mediated Effects on Appetite and Food Intake. J. Neurobiol. 2002, 53, 618–632. [Google Scholar] [CrossRef]
- McFadden, K.L.; Cornier, M.-A.; Tregellas, J.R. The Role of Alpha-7 Nicotinic Receptors in Food Intake Behaviors. Front. Psychol. 2014, 5, 553. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Picciotto, M.R. Nicotinic Regulation of Energy Homeostasis. Nicotine Tob. Res. 2012, 14, 1270–1290. [Google Scholar] [CrossRef]
- Dezfuli, G.; Kellar, K.J.; Dretchen, K.L.; Tizabi, Y.; Sahibzada, N.; Gillis, R.A. Evidence for the Role of B2 Nachr Desensitization in Regulating Body Weight in Obese Mice. Neuropharmacology 2016, 110, 165–174. [Google Scholar] [CrossRef]
- Grebenstein, P.E.; Harp, J.L.; Rowland, N.E. The Effects of Noncontingent and Self-Administered Cytisine on Body Weight and Meal Patterns in Male Sprague–Dawley Rats. Pharm. Biochem. Behav. 2013, 110, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bunney, P.E.; Hansen, M.; LeSage, M. Effects of Isolated Tobacco Alkaloids and Tobacco Products on Deprivation-Induced Food Intake and Meal Patterns in Rats. Pharm. Biochem. Behav. 2018, 165, 45–55. [Google Scholar] [CrossRef]
- Bishop, C.; Parker, G.C.; Coscina, D.V. Systemic Nicotine Alters Whole-Body Fat Utilization in Female Rats. Physiol. Behav. 2004, 80, 563–567. [Google Scholar] [CrossRef]
- Chen, H.; Vlahos, R.; Bozinovski, S.; Jones, J.; Anderson, G.P.; Morris, M.J. Effect of Short-Term Cigarette Smoke Exposure on Body Weight, Appetite and Brain Neuropeptide Y in Mice. Neuropsychopharmacology 2005, 30, 1300597. [Google Scholar] [CrossRef]
- Grebenstein, P.E.; Thompson, I.E.; Rowland, N.E. The Effects of Extended Intravenous Nicotine Administration on Body Weight and Meal Patterns in Male Sprague Dawley Rats. Psychopharmacology 2013, 228, 359–366. [Google Scholar] [CrossRef]
- Grunberg, N.E.; Bowen, D.J. The Role of Physical Activity in Nicotine’s Effects on Body Weight. Pharm. Biochem. Behav. 1985, 23, 851–854. [Google Scholar] [CrossRef]
- Grunberg, N.E.; Bowen, D.J.; Maycock, V.A.; Nespor, S.M. The Importance of Sweet Taste and Caloric Content in the Effects of Nicotine on Specific Food Consumption. Psychopharmacology 1985, 87, 198–203. [Google Scholar] [CrossRef]
- Bowen, D.J.; Eury, S.E.; Grunberg, N.E. Nicotine’s Effects on Female Rats’ Body Weight: Caloric Intake and Physical Activity. Pharm. Biochem. Behav. 1986, 25, 1131–1136. [Google Scholar] [CrossRef]
- Mangubat, M.; Lutfy, K.; Lee, M.L.; Pulido, L.; Stout, D.; Davis, R.; Shin, C.-S.; Shahbazian, M.; Seasholtz, S.; Sinha-Hikim, A.; et al. Effect of Nicotine on Body Composition in Mice. J. Endocrinol. 2012, 212, 317–326. [Google Scholar] [CrossRef]
- Rupprecht, L.E.; Smith, T.T.; Donny, E.C.; Sved, A.F. Self-Administered Nicotine Suppresses Body Weight Gain Independent of Food Intake in Male Rats. Nicotine Tob. Res. 2016, 18, 1869–1876. [Google Scholar] [CrossRef]
- Sztalryd, C.; Hamilton, J.; Horwitz, B.A.; Johnson, P.; Kraemer, F.B. Alterations of Lipolysis and Lipoprotein Lipase in Chronically Nicotine-Treated Rats. Am. J. Physiol. -Endoc. Metab. 1996, 270, E215–E223. [Google Scholar] [CrossRef]
- Yoshida, T.; Sakane, N.; Umekawa, T.; Kogure, A.; Kondo, M.; Kumamoto, K.; Kawada, T.; Nagase, I.; Saito, M. Nicotine Induces Uncoupling Protein 1 in White Adipose Tissue of Obese Mice. Int. J. Obes. 1999, 23, 0800870. [Google Scholar] [CrossRef][Green Version]
- Rupprecht, L.E.; Kreisler, A.D.; Spierling, S.R.; de Guglielmo, G.; Kallupi, M.; George, O.; Donny, E.C.; Zorrilla, E.P.; Sved, A.F. Self-Administered Nicotine Increases Fat Metabolism and Suppresses Weight Gain in Male Rats. Psychopharmacology 2018, 235, 1131–1140. [Google Scholar] [CrossRef]
- Miyata, G.; Meguid, M.M.; Fetissov, S.O.; Torelli, G.F.F.; Kim, H.-J. Nicotine’s Effect on Hypothalamic Neurotransmitters and Appetite Regulation. Surgery 1999, 126, 255–263. [Google Scholar] [CrossRef]
- Miyata, G.; Meguid, M.M.; Varma, M.; Fetissov, S.O.; Kim, H.-J. Nicotine Alters the Usual Reciprocity between Meal Size and Meal Number in Female Rat. Physiol. Behav. 2001, 74, 169–176. [Google Scholar] [CrossRef]
- Bellinger, L.; Cepeda-Benito, A.; Wellman, P.J. Meal Patterns in Male Rats during and after Intermittent Nicotine Administration. Pharm. Biochem. Behav. 2003, 74, 495–504. [Google Scholar] [CrossRef]
- Bellinger, L.; Cepeda-Benito, A.; Bullard, R.L.; Wellman, P.J. Effect of i.c.v. Infusion of the α-MSH Agonist MTII on Meal Patterns in Male Rats Following Nicotine Withdrawal. Life Sci. 2003, 73, 1861–1872. [Google Scholar] [CrossRef]
- Bagnol, D.; Lu, X.-Y.; Kaelin, C.B.; Day, H.E.W.; Ollmann, M.; Gantz, I.; Akil, H.; Barsh, G.S.; Watson, S.J. Anatomy of an Endogenous Antagonist: Relationship between Agouti-Related Protein and Proopiomelanocortin in Brain. J. Neurosci. 1999, 19, RC26. [Google Scholar] [CrossRef]
- Corrigall, W.A. Hypocretin Mechanisms in Nicotine Addiction: Evidence and Speculation. Psychopharmacology 2009, 206, 23. [Google Scholar] [CrossRef]
- Fornari, A.; Pedrazzi, P.; Lippi, G.; Picciotto, M.R.; Zoli, M.; Zini, I. Nicotine Withdrawal Increases Body Weight, Neuropeptide Y and Agouti-Related Protein Expression in the Hypothalamus and Decreases Uncoupling Protein-3 Expression in the Brown Adipose Tissue in High-Fat Fed Mice. Neurosci. Lett. 2007, 411, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.K.; Parker, S.L.; Matta, S.G.; Fu, Y.; Sharp, B.M.; Li, M.D. Nicotine Up-Regulates Expression of Orexin and Its Receptors in Rat Brain. Endocrinology 2000, 141, 3623–3629. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Dandekar, M.P.; Kokare, D.M.; Subhedar, N.K. Involvement of Neuropeptide Y Y1 Receptors in the Acute, Chronic and Withdrawal Effects of Nicotine on Feeding and Body Weight in Rats. Eur. J. Pharm. 2009, 609, 78–87. [Google Scholar] [CrossRef]
- Rasmussen, D.D. Effects of Chronic Nicotine Treatment and Withdrawal on Hypothalamic Proopiomelanocortin Gene expression and Neuroendodrine Regulation. Psychoneuroendocrinology 1998, 23, 245–259. [Google Scholar] [CrossRef]
- Meister, B.; Gömüç, B.; Suarez, E.; Ishii, Y.; Dürr, K.; Gillberg, L. Hypothalamic Proopiomelanocortin (POMC) Neurons Have a Cholinergic Phenotype. Eur. J. Neurosci. 2006, 24, 2731–2740. [Google Scholar] [CrossRef]
- Huang, L.Z.; Winzer-Serhan, U.H. Nicotine Regulates MRNA Expression of Feeding Peptides in the Arcuate Nucleus in Neonatal Rat Pups. Dev. Neurobiol. 2007, 67, 363–377. [Google Scholar] [CrossRef]
- Withey, S.L.; Doyle, M.R.; Bergman, J.; Desai, R.I. Involvement of Nicotinic Receptor Subtypes in Behavioral Effects of Nicotinic Drugs in Squirrel Monkeys. J. Pharmacol. Exp. Ther. 2018, 366, 8070. [Google Scholar] [CrossRef]
- Harris, A.C.; Tally, L.; Muelken, P.; Banal, A.; Schmidt, C.E.; Cao, Q.; LeSage, M.G. Effects of Nicotine and Minor Tobacco Alkaloids on Intracranial-Self-Stimulation in Rats. Drug Alcohol. Depen. 2015, 153, 330–334. [Google Scholar] [CrossRef]
- Dwoskin, L.P.; Teng, L.H.; Crooks, P.A. Nornicotine, a Nicotine Metabolite and Tobacco Alkaloid: Desensitization of Nicotinic Receptor-Stimulated Dopamine Release from Rat Striatum. Eur. J. Pharm. 2001, 428, 69–79. [Google Scholar] [CrossRef]
- Jacob, P.; Yu, L.; Shulgin, A.T.; Benowitz, N.L. Minor Tobacco Alkaloids as Biomarkers for Tobacco Use: Comparison of Users of Cigarettes, Smokeless Tobacco, Cigars, and Pipes. Am. J. Public Health 1999, 89, 731–736. [Google Scholar] [CrossRef]
- Pescatore, K.A.; Glowa, J.R.; Riley, A.L. Strain Differences in the Acquisition of Nicotine-Induced Conditioned Taste Aversion. Pharm. Biochem. Behav. 2005, 82, 751–757. [Google Scholar] [CrossRef]
- Mao, D.; McGehee, D.S. Nicotine and Behavioral Sensitization. J. Mol. Neurosci. 2010, 40, 154–163. [Google Scholar] [CrossRef]
- Dwoskin, L.P.; Crooks, P.A.; Teng, L.; Green, T.A.; Bardo, M.T. Acute and Chronic Effects of Nornicotine on Locomotor Activity in Rats: Altered Response to Nicotine. Psychopharmacology 1999, 145, 442–451. [Google Scholar] [CrossRef]
- Kiianmaa, K.; Tuomainen, P.; Makova, N.; Seppä, T.; Mikkola, J.A.; Petteri Piepponen, T.; Ahtee, L.; Hyytiä, P. The effects of nicotine on locomotor activity and dopamine overflow in the alcohol-preferring AA and alcohol-avoiding ANA rats. Eur. J. Pharmacol. 2000, 407, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sudakov, S.K.; Nazarova, G.A.; Alekseeva, E.V.; Kolppaakov, A.A. Effect of Activated Peripheral κ-Opioid Receptors on the Action of Nicotine and Its Withdrawal in Nicotine-Dependent Rats. Bull. Exp. Biol. Med. 2014, 156, 609–611. [Google Scholar] [CrossRef] [PubMed]
- de Morentin, P.B.M.; Whittle, A.J.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Vidal-Puig, A.; López, M. Nicotine Induces Negative Energy Balance Through Hypothalamic AMP-Activated Protein Kinase. Diabetes 2012, 61, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Howe, C.; Bullen, C.; McRobbie, H.; Glover, M.; Parag, V.; Williman, J.; Veale, R.; Nosa, V.; Barnes, J. Study Protocol for a Non-Inferiority Trial of Cytisine versus Nicotine Replacement Therapy in People Motivated to Stop Smoking. BMC Public Health 2011, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, A.S.; Hawrylak, M.; Mardekian, S.K. Bupropion Differentially Alters the Aversive, Locomotor and Rewarding Properties of Nicotine in CD-1 Mice. Pharm. Biochem. Behav. 2008, 90, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Collins, L.; Rowell, P.; Goldsmith, L.J.; Moffatt, R.; Stamford, B. The Effect of Smoking on Energy Expenditure and Plasma Catecholamine and Nicotine Levels during Light Physical Activity. Nicotine Tob. Res. 1999, 1, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.-N.; Hong, G.-H.; Choi, S.-H.; Shin, K.-H.; Chun, B.-G. High Fat Diet Altered the Mechanism of Energy Homeostasis Induced by Nicotine and Withdrawal in C57BL/6 Mice. Mol. Cells 2010, 30, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A.; Epstein, L.H.; Stiller, R.L.; Marks, B.L.; Jacob, R.G. Acute Effects of Nicotine on Resting Metabolic Rate in Cigarette Smokers. Am. J. Clin. Nutr. 1989, 50, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A. Metabolic Effects of Cigarette Smoking. J. Appl. Physiol. 1992, 72, 401–409. [Google Scholar] [CrossRef]
- Perkins, K.A.; Sexton, J.E.; Epstein, L.H.; DiMarco, A.; Fonte, C.; Stiller, R.L.; Scierka, A.; Jacob, R.G. Acute Thermogenic Effects of Nicotine Combined with Caffeine during Light Physical Activity in Male and Female Smokers. Am. J. Clin. Nutr. 1994, 60, 312–319. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Benowitz, N.L.; Neese, R.A.; Schwartz, J.M.; Hoh, R.; Jacob, P.; Hsieh, J.; Faix, D. Effects of Cigarette Smoking and Its Cessation on Lipid Metabolism and Energy Expenditure in Heavy Smokers. J. Clin. Investig. 1994, 93, 265–272. [Google Scholar] [CrossRef]
- Moffatt, R.J.; Owens, S.G. Cessation from Cigarette Smoking: Changes in Body Weight, Body Composition, Resting Metabolism, and Energy Consumption. Metabolis 1991, 40, 465–470. [Google Scholar] [CrossRef]
- Bellinger, L.L.; Wellman, P.J.; Harris, R.B.S.; Kelso, E.W.; Kramer, P.R. The Effects of Chronic Nicotine on Meal Patterns, Food Intake, Metabolism and Body Weight of Male Rats. Pharm. Biochem. Behav. 2010, 95, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, B.; Huang, J.; Clarke, P.B.S. Effects of Nicotine, Nornicotine and Cotinine, Alone or in Combination, on Locomotor Activity and Ultrasonic Vocalization Emission in Adult Rats. Psychopharmacology 2020, 237, 2809–2822. [Google Scholar] [CrossRef]
- Green, T.A.; Brown, R.W.; Phillips, S.B.; Dwoskin, L.P.; Bardo, M.T. Locomotor Stimulant Effects of Nornicotine: Role of Dopamine. Pharm. Biochem. Behav. 2002, 74, 87–94. [Google Scholar] [CrossRef]
- Wiley, J.L.; Marusich, J.A.; Thomas, B.F.; Jackson, K.J. Determination of Behaviorally Effective Tobacco Constituent Doses in Rats. Nicotine Tob. Res. 2014, 17, 368–371. [Google Scholar] [CrossRef]
- Marusich, J.A.; Darna, M.; Wilson, A.G.; Denehy, E.D.; Ebben, A.; Deaciuc, A.G.; Dwoskin, L.P.; Bardo, M.T.; Lefever, T.W.; Wiley, J.L.; et al. Tobacco’s Minor Alkaloids: Effects on Place Conditioning and Nucleus Accumbens Dopamine Release in Adult and Adolescent Rats. Eur. J. Pharm. 2017, 814, 196–206. [Google Scholar] [CrossRef]
- Stolerman, I.P.; Garcha, H.S.; Mirza, N.R. Dissociations between the Locomotor Stimulant and Depressant Effects of Nicotinic Agonists in Rats. Psychopharmacology 1995, 117, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gahring, L.C.; Rogers, S.W. Neuronal Nicotinic Acetylcholine Receptor Expression and Function on Nonneuronal Cells. AAPS J. 2005, 7, E885–E894. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Vijayaraghavan, S. Nicotinic Receptor Signaling in Nonexcitable Cells. J. Neurobiol. 2002, 53, 524–534. [Google Scholar] [CrossRef]
- Somm, E. Nicotinic Cholinergic Signaling in Adipose Tissue and Pancreatic Islet Biology. Arch. Immunol. Exp. 2014, 62, 87–101. [Google Scholar] [CrossRef]
- Gochberg-Sarver, A.; Kedmi, M.; Gana-Weisz, M.; Bar-Shira, A.; Orr-Urtreger, A. Tnfα, Cox2 and AdipoQ Adipokine Gene Expression Levels Are Modulated in Murine Adipose Tissues by Both Nicotine and NACh Receptors Containing the Β2 Subunit. Mol. Genet. Metab. 2012, 107, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Zulian, A.; Maestrini, S.; Mencarelli, M.; Barba, A.D.; Invitti, C.; Liuzzi, A.; Blasio, A.M.D. The Nicotinic Acetylcholine Receptor A7 in Subcutaneous Mature Adipocytes: Downregulation in Human Obesity and Modulation by Diet-Induced Weight Loss. Int. J. Obes. 2012, 36, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Orr-Urtreger, A.; Korczyn, A.D. The Role of Neuronal Nicotinic Acetylcholine Receptor Subunits in Autonomic Ganglia: Lessons from Knockout Mice. Prog. Neurobiol. 2002, 68, 341–360. [Google Scholar] [CrossRef]
- Arai, K.; Kim, K.; Kaneko, K.; Iketani, M.; Otagiri, A.; Yamauchi, N.; Shibasaki, T. Nicotine Infusion Alters Leptin and Uncoupling Protein 1 MRNA Expression in Adipose Tissues of Rats. Am. J. Physiol. -Endoc. Metab. 2001, 280, E867–E876. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and Immunology of the Cholinergic Antiinflammatory Pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Xue, B.; Shi, H. Activation of the Cholinergic Antiinflammatory Pathway Ameliorates Obesity-Induced Inflammation and Insulin Resistance. Endocrinology 2011, 152, 836–846. [Google Scholar] [CrossRef]
- Mabley, J.G.; Pacher, P.; Southan, G.J.; Salzman, A.L.; Szabó, C. Nicotine Reduces the Incidence of Type I Diabetes in Mice. J. Pharm. Exp. 2002, 300, 876–881. [Google Scholar] [CrossRef]
- Schmeltz, L.R.; Blevins, T.C.; Aronoff, S.L.; Ozer, K.; Leffert, J.D.; Goldberg, M.A.; Horowitz, B.S.; Bertenshaw, R.H.; Troya, P.; Cohen, A.E.; et al. Anatabine Supplementation Decreases Thyroglobulin Antibodies in Patients with Chronic Lymphocytic Autoimmune (Hashimoto’s) Thyroiditis: A Randomized Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2014, 99, E137–E142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenkins, N.D.; Housh, T.J.; Johnson, G.O.; Traylor, D.A.; Bergstrom, H.C.; Cochrane, K.C.; Lewis, R.W.; Schmidt, R.J.; Cramer, J.T. The effects of anatabine on non-invasive indicators of muscle damage: A randomized, double-blind, placebo-controlled, crossover study. J. Int. Soc. Sports Nutr. 2013, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.V.; Weigel, C.A. Comparative Toxicity of Nicotine, Nornicotine and Anabasine to Green Peach Aphid. J. Econ. Entomol. 1948, 41, 117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebenstein, P.E.; Erickson, P.; Grace, M.; Kotz, C.M. Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity. J. Clin. Med. 2022, 11, 481. https://doi.org/10.3390/jcm11030481
Grebenstein PE, Erickson P, Grace M, Kotz CM. Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity. Journal of Clinical Medicine. 2022; 11(3):481. https://doi.org/10.3390/jcm11030481
Chicago/Turabian StyleGrebenstein, Patricia E., Paige Erickson, Martha Grace, and Catherine M. Kotz. 2022. "Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity" Journal of Clinical Medicine 11, no. 3: 481. https://doi.org/10.3390/jcm11030481
APA StyleGrebenstein, P. E., Erickson, P., Grace, M., & Kotz, C. M. (2022). Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity. Journal of Clinical Medicine, 11(3), 481. https://doi.org/10.3390/jcm11030481




