Axial Spondyloarthritis: Reshape the Future—From the “2022 GISEA International Symposium”
Abstract
:1. Introduction
2. Pathogenesis
3. Treatment
3.1. Anti-IL17
3.1.1. Secukinumab
3.1.2. Ixekizumab
3.1.3. Other IL-17 Inhibitors
3.2. IL-23 Inhibition
3.2.1. Ustekinumab
3.2.2. Guselkumab
3.2.3. Risankizumab
3.2.4. Tildrakizumab
3.3. Janus Kinases Inhibitors
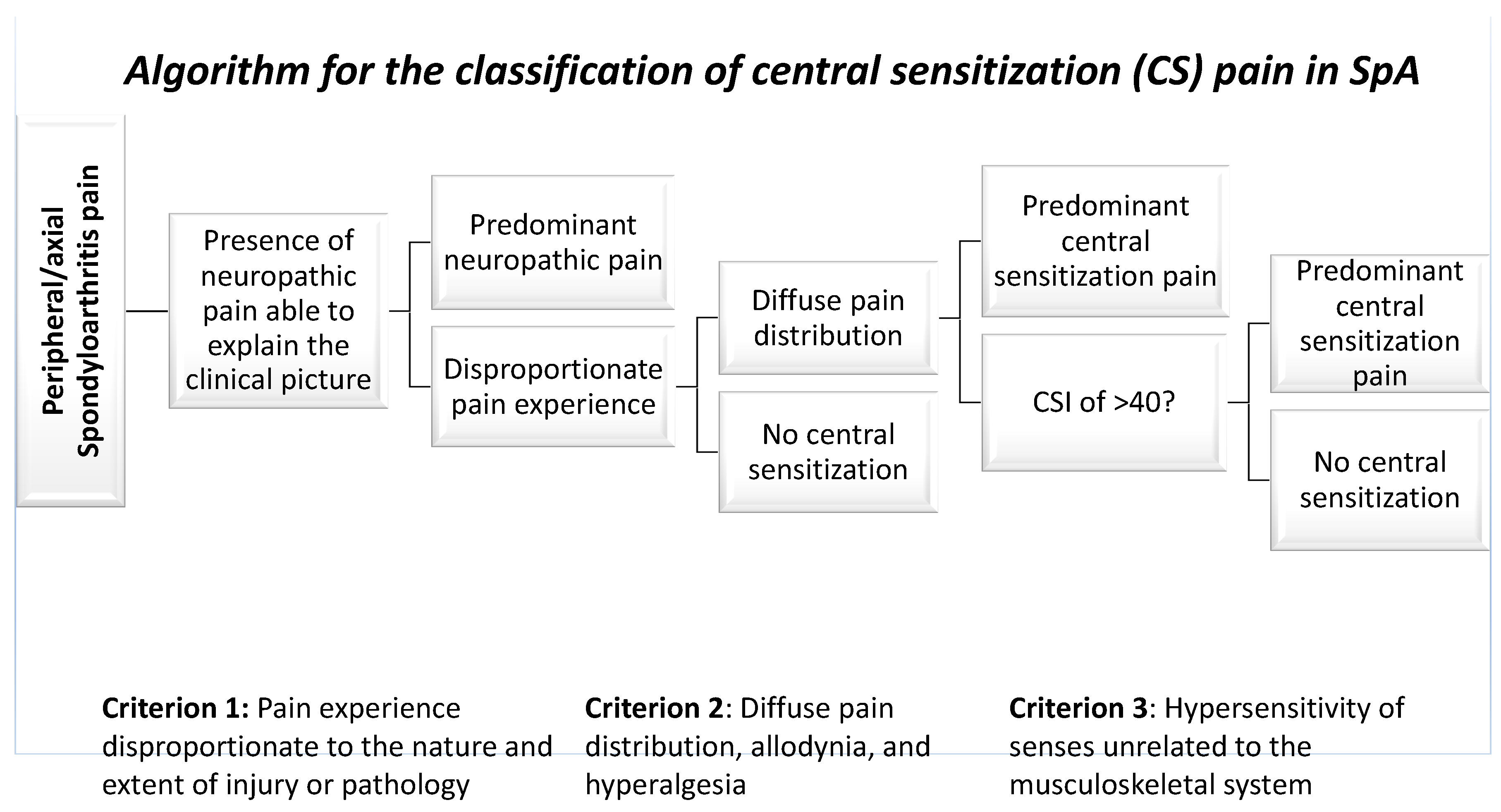
4. The Impact of Pain in SpA Management
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwartzman, S.; Ruderman, E.M. A Road Map of the Axial Spondyloarthritis Continuum. Mayo Clin. Proc. 2022, 97, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, C.; Monti, S.; Terenzi, R.; Carli, L. One Year in Review 2021: Axial Spondyloarthritis. Clin. Exp. Rheumatol. 2021, 39, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a Drug Target for Autoimmune Inflammatory Diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Kleinschek, M.A.; Muller, U.; Brodie, S.J.; Stenzel, W.; Kohler, G.; Blumenschein, W.M.; Straubinger, R.K.; McClanahan, T.; Kastelein, R.A.; Alber, G. IL-23 Enhances the Inflammatory Cell Response in Cryptococcus Neoformans Infection and Induces a Cytokine Pattern Distinct from IL-12. J. Immunol. 2006, 176, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing Spondylitis: Etiology, Pathogenesis, and Treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, J.; He, C.; Li, D.; Tong, W.; Zou, Y.; Xu, W. Role of HLA-B27 in the Pathogenesis of Ankylosing Spondylitis (Review). Mol. Med. Rep. 2017, 15, 1943–1951. [Google Scholar] [CrossRef] [Green Version]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLA-B27 Misfolding and the Unfolded Protein Response Augment Interleukin-23 Production and Are Associated with Th17 Activation in Transgenic Rats. Arthritis Rheum. 2009, 60, 2633–2643. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.T.; Kollnberger, S.D.; Wedderburn, L.R.; Bowness, P. Expansion and Enhanced Survival of Natural Killer Cells Expressing the Killer Immunoglobulin-like Receptor KIR3DL2 in Spondylarthritis. Arthritis Rheum. 2005, 52, 3586–3595. [Google Scholar] [CrossRef]
- Guiliano, D.B.; Fussell, H.; Lenart, I.; Tsao, E.; Nesbeth, D.; Fletcher, A.J.; Campbell, E.C.; Yousaf, N.; Williams, S.; Santos, S.; et al. Endoplasmic Reticulum Degradation-Enhancing α-Mannosidase-like Protein 1 Targets Misfolded HLA-B27 Dimers for Endoplasmic Reticulum-Associated Degradation. Arthritis Rheumatol. 2014, 66, 2976–2988. [Google Scholar] [CrossRef] [Green Version]
- York, I.A.; Chang, S.C.; Saric, T.; Keys, J.A.; Favreau, J.M.; Goldberg, A.L.; Rock, K.L. The ER Aminopeptidase ERAP1 Enhances or Limits Antigen Presentation by Trimming Epitopes to 8-9 Residues. Nat. Immunol. 2002, 3, 1177–1184. [Google Scholar] [CrossRef]
- Cortes, A.; Pulit, S.L.; Leo, P.J.; Pointon, J.J.; Robinson, P.C.; Weisman, M.H.; Ward, M.; Gensler, L.S.; Zhou, X.; Garchon, H.J.; et al. Major Histocompatibility Complex Associations of Ankylosing Spondylitis Are Complex and Involve Further Epistasis with ERAP1. Nat. Commun. 2015, 6, 7146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccia, F.; Bombardieri, M.; Principato, A.; Giardina, A.; Tripodo, C.; Porcasi, R.; Peralta, S.; Franco, V.; Giardina, E.; Craxi, A.; et al. Overexpression of Interleukin-23, but Not Interleukin-17, as an Immunologic Signature of Subclinical Intestinal Inflammation in Ankylosing Spondylitis. Arthritis Rheum. 2009, 60, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, M.E.; Ciccia, F.; Willner, D.; Warrington, N.; Robinson, P.C.; Gardiner, B.; Marshall, M.; Kenna, T.J.; Triolo, G.; Brown, M.A. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015, 67, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Alessandro, R.; Luchetti, M.M.; Milling, S.; Saieva, L.; Cypers, H.; Stampone, T.; Di Benedetto, P.; et al. Dysbiosis and Zonulin Upregulation Alter Gut Epithelial and Vascular Barriers in Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 2017, 76, 1123–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, P.; Lambrecht, S.; Verheugen, E.; Pauwels, E.; Kollias, G.; Armaka, M.; Verhoye, M.; Van Der Linden, A.; Achten, R.; Lories, R.J.; et al. Proof of Concept: Enthesitis and New Bone Formation in Spondyloarthritis Are Driven by Mechanical Strain and Stromal Cells. Ann. Rheum. Dis. 2014, 73, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wang, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Baeten, D.; Østergaard, M.; Wei, J.C.C.; Sieper, J.; Järvinen, P.; Tam, L.S.; Salvarani, C.; Kim, T.H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 Inhibitor, for Ankylosing Spondylitis: Results of a Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept, Dose-Finding Phase 2 Study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A Cellular and Molecular View of T Helper 17 Cell Plasticity in Autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Yeremenko, N.; Noordenbos, T.; Cantaert, T.; Van Tok, M.; Van De Sande, M.; Cañete, J.D.; Tak, P.P.; Baeten, D. Disease-Specific and Inflammation-Independent Stromal Alterations in Spondylarthritis Synovitis. Arthritis Rheum. 2013, 65, 174–185. [Google Scholar] [CrossRef]
- Noack, M.; Ndongo-Thiam, N.; Miossec, P. Interaction among Activated Lymphocytes and Mesenchymal Cells through Podoplanin Is Critical for a High IL-17 Secretion. Arthritis Res. Ther. 2016, 18, 148. [Google Scholar] [CrossRef]
- Noack, M.; Ndongo-Thiam, N.D.; Miossec, P. Role of Podoplanin in the High Interleukin-17A Secretion Resulting from Interactions between Activated Lymphocytes and Psoriatic Skin-Derived Mesenchymal Cells. Clin. Exp. Immunol. 2016, 186, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of Autoantibody Activity by the IL-23-TH17 Axis Determines the Onset of Autoimmune Disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Bloch, Y.; Składanowska, K.; Savvides, S.N.; Adamopoulos, I.E. Pathophysiology and Inhibition of IL-23 Signaling in Psoriatic Arthritis: A Molecular Insight. Clin. Immunol. 2019, 206, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wendling, D.; Cedoz, J.P.; Racadot, E.; Dumoulin, G. Serum IL-17, BMP-7, and Bone Turnover Markers in Patients with Ankylosing Spondylitis. Jt. Bone Spine 2007, 74, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sanchez, C.; Jaimes, D.A.; Londoño, J.; De Avila, J.; Castellanos, J.E.; Bello, J.M.; Bautista, W.; Valle-Oñate, R. Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin. Exp. Rheumatol. 2011, 29, 828–834. [Google Scholar] [PubMed]
- Chen, W.S.; Chang, Y.S.; Lin, K.C.; Lai, C.C.; Wang, S.H.; Hsiao, K.H.; Lee, H.T.; Chen, M.H.; Tsai, C.Y.; Chou, C.T. Association of Serum Interleukin-17 and Interleukin-23 Levels with Disease Activity in Chinese Patients with Ankylosing Spondylitis. J. Chin. Med. Assoc. 2012, 75, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xueyi, L.; Lina, C.; Zhenbiao, W.; Qing, H.; Qiang, L.; Zhu, P. Levels of Circulating Th17 Cells and Regulatory T Cells in Ankylosing Spondylitis Patients with an Inadequate Response to Anti-TNF-α Therapy. J. Clin. Immunol. 2013, 33, 151–161. [Google Scholar] [CrossRef]
- Van Baarsen, L.G.M.; Lebre, M.C.; van der Coelen, D.; Aarrass, S.; Tang, M.W.; Ramwadhdoebe, T.H.; Gerlag, D.M.; Tak, P.P. Heterogeneous Expression Pattern of Interleukin 17A (IL-17A), IL-17F and Their Receptors in Synovium of Rheumatoid Arthritis, Psoriatic Arthritis and Osteoarthritis: Possible Explanation for Nonresponse to Anti-IL-17 Therapy? Arthritis Res. Ther. 2014, 16, 426. [Google Scholar] [CrossRef] [Green Version]
- Glatt, S.; Baeten, D.; Baker, T.; Griffiths, M.; Ionescu, L.; Lawson, A.D.G.; Maroof, A.; Oliver, R.; Popa, S.; Strimenopoulou, F.; et al. Dual IL-17A and IL-17F Neutralisation by Bimekizumab in Psoriatic Arthritis: Evidence from Preclinical Experiments and a Randomised Placebo-Controlled Clinical Trial That IL-17F Contributes to Human Chronic Tissue Inflammation. Ann. Rheum. Dis. 2018, 77, 523–532. [Google Scholar] [CrossRef]
- Schmidt, E.G.W.; Larsen, H.L.; Kristensen, N.N.; Poulsen, S.S.; Pedersen, A.M.L.; Claesson, M.H.; Pedersen, A.E. TH17 Cell Induction and Effects of IL-17A and IL-17F Blockade in Experimental Colitis. Inflamm. Bowel Dis. 2013, 19, 1567–1576. [Google Scholar] [CrossRef]
- Henness, S.; Van Thoor, E.; Ge, Q.; Armour, C.L.; Hughes, J.M.; Ammit, A.J. IL-17A Acts via P38 MAPK to Increase Stability of TNF-Alpha-Induced IL-8 MRNA in Human ASM. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1283–L1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taams, L.S.; Steel, K.J.A.; Srenathan, U.; Burns, L.A.; Kirkham, B.W. IL-17 in the Immunopathogenesis of Spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srenathan, U.; Steel, K.; Taams, L.S. IL-17+ CD8+ T Cells: Differentiation, Phenotype and Role in Inflammatory Disease. Immunol. Lett. 2016, 178, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papotto, P.H.; Ribot, J.C.; Silva-Santos, B. IL-17+ Γδ T Cells as Kick-Starters of Inflammation. Nat. Immunol. 2017, 18, 604–611. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Spits, H. Human Innate Lymphoid Cells. Blood 2014, 124, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.M.; Deodhar, A.; Gensler, L.S.; Dubreuil, M.; Yu, D.; Khan, M.A.; Haroon, N.; Borenstein, D.; Wang, R.; Biehl, A.; et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 1599–1613. [Google Scholar] [CrossRef]
- Atzeni, F.; Carriero, A.; Boccassini, L.; D’Angelo, S. Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis. ImmunoTargets Ther. 2021, 10, 141–153. [Google Scholar] [CrossRef]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; Van Der Heijde, D.; McInnes, I.; Van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-Interleukin-17A Monoclonal Antibody Secukinumab in Treatment of Ankylosing Spondylitis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef] [Green Version]
- Sieper, J.; Deodhar, A.; Marzo-Ortega, H.; Aelion, J.A.; Blanco, R.; Jui-Cheng, T.; Andersson, M.; Porter, B.; Richards, H.B. Secukinumab Efficacy in Anti-TNF-Naive and Anti-TNF-Experienced Subjects with Active Ankylosing Spondylitis: Results from the MEASURE 2 Study. Ann. Rheum. Dis. 2017, 76, 571–575. [Google Scholar] [CrossRef]
- Kishimoto, M.; Taniguchi, A.; Fujishige, A.; Kaneko, S.; Haemmerle, S.; Porter, B.O.; Kobayashi, S. Efficacy and Safety of Secukinumab in Japanese Patients with Active Ankylosing Spondylitis: 24-Week Results from an Open-Label Phase 3 Study (MEASURE 2-J). Mod. Rheumatol. 2020, 30, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavelka, K.; Kivitz, A.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Pricop, L.; Andersson, M.; Readie, A.; Porter, B. Efficacy, Safety, and Tolerability of Secukinumab in Patients with Active Ankylosing Spondylitis: A Randomized, Double-Blind Phase 3 Study, MEASURE 3. Arthritis Res. Ther. 2017, 19, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivitz, A.J.; Wagner, U.; Dokoupilova, E.; Supronik, J.; Martin, R.; Talloczy, Z.; Richards, H.B.; Porter, B. Efficacy and Safety of Secukinumab 150 Mg with and without Loading Regimen in Ankylosing Spondylitis: 104-Week Results from MEASURE 4 Study. Rheumatol. Ther. 2018, 5, 447–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Baraliakos, X.; Kiltz, U. Secukinumab (AIN457) in the Treatment of Ankylosing Spondylitis. Expert Opin. Biol. Ther. 2016, 16, 711–722. [Google Scholar] [CrossRef]
- Deodhar, A.; Blanco, R.; Dokoupilová, E.; Hall, S.; Kameda, H.; Kivitz, A.J.; Poddubnyy, D.; van de Sande, M.; Wiksten, A.S.; Porter, B.O.; et al. Improvement of Signs and Symptoms of Nonradiographic Axial Spondyloarthritis in Patients Treated with Secukinumab: Primary Results of a Randomized, Placebo-Controlled Phase III Study. Arthritis Rheumatol. 2021, 73, 110–120. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Fonti, G.L.; Conigliaro, P.; Sunzini, F.; Scrivo, R.; Navarini, L.; Triggianese, P.; Peluso, G.; Scolieri, P.; Caccavale, R.; et al. One-Year Effectiveness, Retention Rate, and Safety of Secukinumab in Ankylosing Spondylitis and Psoriatic Arthritis: A Real-Life Multicenter Study. Expert Opin. Biol. Ther. 2020, 20, 813–821. [Google Scholar] [CrossRef]
- Michelsen, B.; Lindström, U.; Codreanu, C.; Ciurea, A.; Zavada, J.; Loft, A.G.; Pombo-Suarez, M.; Onen, F.; Kvien, T.K.; Rotar, Z.; et al. Drug Retention, Inactive Disease and Response Rates in 1860 Patients with Axial Spondyloarthritis Initiating Secukinumab Treatment: Routine Care Data from 13 Registries in the EuroSpA Collaboration. RMD Open 2020, 6, e001280. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, C.H.; Chi, C.C. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-Analysis of Real-World Evidence. BioDrugs 2020, 34, 669–679. [Google Scholar] [CrossRef]
- van der Heijde, D.; Cheng-Chung Wei, J.; Dougados, M.; Mease, P.; Deodhar, A.; Maksymowych, W.P.; Van den Bosch, F.; Sieper, J.; Tomita, T.; Landewé, R.; et al. Ixekizumab, an Interleukin-17A Antagonist in the Treatment of Ankylosing Spondylitis or Radiographic Axial Spondyloarthritis in Patients Previously Untreated with Biological Disease-Modifying Anti-Rheumatic Drugs (COAST-V): 16 Week Results of a Phase 3 Randomised, Double-Blind, Active-Controlled and Placebo-Controlled Trial. Lancet 2018, 392, 2441–2451. [Google Scholar] [CrossRef] [Green Version]
- Deodhar, A.; Poddubnyy, D.; Pacheco-Tena, C.; Salvarani, C.; Lespessailles, E.; Rahman, P.; Järvinen, P.; Sanchez-Burson, J.; Gaffney, K.; Lee, E.B.; et al. Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results from a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients with Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol. 2019, 71, 599–611. [Google Scholar] [CrossRef]
- Papp, K.A.; Leonardi, C.; Menter, A.; Ortonne, J.-P.; Krueger, J.G.; Kricorian, G.; Aras, G.; Li, J.; Russell, C.B.; Thompson, E.H.Z.; et al. Brodalumab, an Anti-Interleukin-17-Receptor Antibody for Psoriasis. N. Engl. J. Med. 2012, 366, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Papp, K.A.; Marangell, L.B.; Koo, J.; Blauvelt, A.; Gooderham, M.; Wu, J.J.; Rastogi, S.; Harris, S.; Pillai, R.; et al. Psychiatric Adverse Events during Treatment with Brodalumab: Analysis of Psoriasis Clinical Trials. J. Am. Acad. Dermatol. 2018, 78, 81–89.e5. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.C.; Kim, T.H.; Kishimoto, M.; Ogusu, N.; Jeong, H.; Kobayashi, S. Efficacy and Safety of Brodalumab, an Anti-IL17RA Monoclonal Antibody, in Patients with Axial Spondyloarthritis: 16-Week Results from a Randomised, Placebo-Controlled, Phase 3 Trial. Ann. Rheum. Dis. 2021, 80, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijde, D.; Gensler, L.S.; Deodhar, A.; Baraliakos, X.; Poddubnyy, D.; Kivitz, A.; Farmer, M.K.; Baeten, D.; Goldammer, N.; Coarse, J.; et al. Dual Neutralisation of Interleukin-17A and Interleukin-17F with Bimekizumab in Patients with Active Ankylosing Spondylitis: Results from a 48-Week Phase IIb, Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Study. Ann. Rheum. Dis. 2020, 79, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Erdes, S.; Nasonov, E.; Kunder, E.; Pristrom, A.; Soroka, N.; Shesternya, P.; Dubinina, T.; Smakotina, S.; Raskina, T.; Krechikova, D.; et al. Primary Efficacy of Netakimab, a Novel Interleukin-17 Inhibitor, in the Treatment of Active Ankylosing Spondylitis in Adults. Clin. Exp. Rheumatol. 2020, 38, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanaugh, A.; Puig, L.; Gottlieb, A.B.; Ritchlin, C.; You, Y.; Li, S.; Song, M.; Randazzo, B.; Rahman, P.; McInnes, I.B. Efficacy and Safety of Ustekinumab in Psoriatic Arthritis Patients with Peripheral Arthritis and Physician-Reported Spondylitis: Post-Hoc Analyses from Two Phase III, Multicentre, Double-Blind, Placebo-Controlled Studies (PSUMMIT-1/PSUMMIT-2). Ann. Rheum. Dis. 2016, 75, 1984–1988. [Google Scholar] [CrossRef]
- Helliwell, P.S.; Gladman, D.D.; Chakravarty, S.D.; Kafka, S.; Karyekar, C.S.; You, Y.; Campbell, K.; Sweet, K.; Kavanaugh, A.; Gensler, L.S. Effects of Ustekinumab on Spondylitis-Associated Endpoints in TNFi-Naïve Active Psoriatic Arthritis Patients with Physician-Reported Spondylitis: Pooled Results from Two Phase 3, Randomised, Controlled Trials. RMD Open 2020, 6, e001149. [Google Scholar] [CrossRef] [Green Version]
- Helliwell, P.; Gladman, D.D.; Poddubnyy, D.; Mease, P.J.; Baraliakos, X.; Kollmeier, A.; Hsia, E.C.; Xu, X.L.; Sheng, S.; Agarwal, P.; et al. Op0054 Efficacy of Guselkumab, a Monoclonal Antibody that Specifically Binds to the P19-Subunit of Il-23, on Endpoints Related to Axial Involvement in Patients with Active Psa with Imaging-Confirmed Sacroiliitis: Week-24 Results from Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Studies. Ann. Rheum. Dis. 2020, 79, 36–37. [Google Scholar] [CrossRef]
- Braun, J.; Landewé, R.B. No Efficacy of Anti-IL-23 Therapy for Axial Spondyloarthritis in Randomised Controlled Trials but in Post-Hoc Analyses of Psoriatic Arthritis-Related “Physician-Reported Spondylitis”? Ann. Rheum. Dis. 2022, 81, 466–468. [Google Scholar] [CrossRef]
- Baeten, D.; Adamopoulos, I.E. IL-23 Inhibition in Ankylosing Spondylitis: Where Did It Go Wrong? Front. Immunol. 2021, 11, 623874. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Hermann, K.G.A.; Callhoff, J.; Listing, J.; Sieper, J. Ustekinumab for the Treatment of Patients with Active Ankylosing Spondylitis: Results of a 28-Week, Prospective, Open-Label, Proof-of-Concept Study (TOPAS). Ann. Rheum. Dis. 2014, 73, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Siragusano, C.; Masala, I.F.; Antonio, C.; Valentina, P.; D’Angelo, S. IL-23 in Axial Spondyloarthritis and Psoriatic Arthritis: A Good Fit for Biological Treatment? Expert Opin. Biol. Ther. 2022, 22, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kiltz, U.; Baraliakos, X.; Regel, A.; Bühring, B.; Braun, J. Causes of Pain in Patients with Axial Spondyloarthritis. Clin. Exp. Rheumatol. 2017, 35, S102–S107. [Google Scholar]
- Essers, I.; Boonen, A.; Busch, M.; van der Heijde, D.; Keszei, A.P.; Landewé, R.; Ramiro, S.; van Tubergen, A. Fluctuations in Patient Reported Disease Activity, Pain and Global Being in Patients with Ankylosing Spondylitis. Rheumatology 2016, 55, 2014–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The Biopsychosocial Approach to Chronic Pain: Scientific Advances and Future Directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Koh, I.J.; Lee, S.Y.; In, Y. Central Sensitization Is a Risk Factor for Wound Complications after Primary Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3419–3428. [Google Scholar] [CrossRef]
- Loeser, J.D.; Treede, R.D. The Kyoto Protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Wu, Q.; Inman, R.D.; Davis, K.D. Neuropathic Pain in Ankylosing Spondylitis: A Psychophysics and Brain Imaging Study. Arthritis Rheum. 2013, 65, 1494–1503. [Google Scholar] [CrossRef]
- Koca, T.T.; Göğebakan, H.; Çetin, G.Y. Should Central Sensitization and Neuropathic Pain Be Considered in Disease Activity and Treatment Decision in Axial Ankylosing Spondilitis? Cukurova Med. J. 2019, 44, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Atzeni, F.; Boccassini, L.; Di Franco, M.; Alciati, A.; Marsico, A.; Cazzola, M.; Cassisi, G.; Sarzi-Puttini, P. Chronic Widespread Pain in Spondyloarthritis. Reumatismo 2014, 66, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Rifbjerg-Madsen, S.; Christensen, A.W.; Christensen, R.; Hetland, M.L.; Bliddal, H.; Kristensen, L.E.; Danneskiold-Samsøe, B.; Amris, K. Pain and Pain Mechanisms in Patients with Inflammatory Arthritis: A Danish Nationwide Cross-Sectional DANBIO Registry Survey. PLoS ONE 2017, 12, e0180014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A New Definition of Neuropathic Pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.H.; Kim, H.R.; Lee, K.A. Association of Neuropathic-like Pain Characteristics with Clinical and Radiographic Features in Patients with Ankylosing Spondylitis. Clin. Rheumatol. 2018, 37, 3077–3086. [Google Scholar] [CrossRef]
- Gok, K.; Cengiz, G.; Erol, K.; Ozgocmen, S. Neuropathic Pain Component in Axial Spondyloarthritis and the Influence on Disease Burden. J. Clin. Rheumatol. 2018, 24, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.A.; Celik, O.F.; Ayhan, F.F. The Important Role of Central Sensitization in Chronic Musculoskeletal Pain Seen in Different Rheumatic Diseases. Clin. Rheumatol. 2020, 39, 269–274. [Google Scholar] [CrossRef]
- Larice, S.; Ghiggia, A.; Di Tella, M.; Romeo, A.; Gasparetto, E.; Fusaro, E.; Castelli, L.; Tesio, V. Pain Appraisal and Quality of Life in 108 Outpatients with Rheumatoid Arthritis. Scand. J. Psychol. 2020, 61, 271–280. [Google Scholar] [CrossRef]
- Bennett, E.E.; Walsh, K.M.; Thompson, N.R.; Krishnaney, A.A. Central Sensitization Inventory as a Predictor of Worse Quality of Life Measures and Increased Length of Stay Following Spinal Fusion. World Neurosurg. 2017, 104, 594–600. [Google Scholar] [CrossRef]
- Salaffi, F.; Farah, S.; Mariani, C.; Sarzi-Puttini, P.; Di Carlo, M. Validity of the Central Sensitization Inventory compared with traditional measures of disease severity in fibromyalgia. Pain Pract. 2022, 22, 702–710. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Rotariu, O.; Jones, G.T.; Pathan, E.; Dean, L.E. Determining Factors Related to Poor Quality of Life in Patients with Axial Spondyloarthritis: Results from the British Society for Rheumatology Biologics Register (BSRBR-AS). Ann. Rheum. Dis. 2020, 79, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Eller-Smith, O.C.; Nicol, A.L.; Christianson, J.A. Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions. Front. Cell. Neurosci. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kieskamp, S.C.; Paap, D.; Carbo, M.J.G.; Wink, F.; Bos, R.; Bootsma, H.; Arends, S.; Spoorenberg, A. Central Sensitization, Illness Perception and Obesity Should Be Considered When Interpreting Disease Activity in Axial Spondyloarthritis. Rheumatology 2021, 60, 4476–4485. [Google Scholar] [CrossRef]
- Baraliakos, X.; Regel, A.; Kiltz, U.; Menne, H.J.; Dybowski, F.; Igelmann, M.; Kalthoff, L.; Krause, D.; Saracbasi-Zender, E.; Schmitz-Bortz, E.; et al. Patients with Fibromyalgia Rarely Fulfil Classification Criteria for Axial Spondyloarthritis. Rheumatology 2018, 57, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaffi, F.; De Angelis, R.; Carotti, M.; Gutierrez, M.; Sarzi-Puttini, P.; Atzeni, F. Fibromyalgia in Patients with Axial Spondyloarthritis: Epidemiological Profile and Effect on Measures of Disease Activity. Rheumatol. Int. 2014, 34, 1103–1110. [Google Scholar] [CrossRef]
- Nijs, J.; Torres-Cueco, R.; Paul Van Wilgen, C.; Girbés, E.L.; Struyf, F.; Roussel, N.; Van Oosterwijck, J.; Daenen, L.; Kuppens, K.; Vanderweeën, L.; et al. Applying Modern Pain Neuroscience in Clinical Practice: Criteria for the Classification of Central Sensitization Pain. Pain Physician 2014, 17, 447–457. [Google Scholar] [CrossRef]
| 1 | I feel tired and unrefreshed when I wake from sleeping. |
| 2 | My muscles feel stiff and achy. |
| 3 | I have anxiety attacks. |
| 4 | I grind or clench my teeth. |
| 5 | I have problems with diarrhea and/or constipation. |
| 6 | I need help in performing my daily activities. |
| 7 | I am sensitive to bright lights. |
| 8 | I get tired very easily when I am physically active. |
| 9 | I feel pain all over my body. |
| 10 | I have headaches. |
| 11 | I feel discomfort in my bladder and/or burning when I urinate. |
| 12 | I do not sleep well. |
| 13 | I have difficulty concentrating. |
| 14 | I have skin problems such as dryness, itchiness, or rashes. |
| 15 | Stress makes my physical symptoms get worse. |
| 16 | I feel sad or depressed. |
| 17 | I have low energy. |
| 18 | I have muscle tension in my neck and shoulders. |
| 19 | I have pain in my jaw. |
| 20 | Certain smells, such as perfumes, make me feel dizzy and nauseated. |
| 21 | I have to urinate frequently. |
| 22 | My legs feel uncomfortable and restless when I am trying to go to sleep at night. |
| 23 | I have difficulty remembering things. |
| 24 | I suffered trauma as a child. |
| 25 | I have pain in my pelvic area. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaffi, F.; Siragusano, C.; Alciati, A.; Cassone, G.; D’Angelo, S.; Guiducci, S.; Favalli, E.G.; Conti, F.; Gremese, E.; Iannone, F.; et al. Axial Spondyloarthritis: Reshape the Future—From the “2022 GISEA International Symposium”. J. Clin. Med. 2022, 11, 7537. https://doi.org/10.3390/jcm11247537
Salaffi F, Siragusano C, Alciati A, Cassone G, D’Angelo S, Guiducci S, Favalli EG, Conti F, Gremese E, Iannone F, et al. Axial Spondyloarthritis: Reshape the Future—From the “2022 GISEA International Symposium”. Journal of Clinical Medicine. 2022; 11(24):7537. https://doi.org/10.3390/jcm11247537
Chicago/Turabian StyleSalaffi, Fausto, Cesare Siragusano, Alessandra Alciati, Giulia Cassone, Salvatore D’Angelo, Serena Guiducci, Ennio Giulio Favalli, Fabrizio Conti, Elisa Gremese, Florenzo Iannone, and et al. 2022. "Axial Spondyloarthritis: Reshape the Future—From the “2022 GISEA International Symposium”" Journal of Clinical Medicine 11, no. 24: 7537. https://doi.org/10.3390/jcm11247537
APA StyleSalaffi, F., Siragusano, C., Alciati, A., Cassone, G., D’Angelo, S., Guiducci, S., Favalli, E. G., Conti, F., Gremese, E., Iannone, F., Caporali, R., Sebastiani, M., Ferraccioli, G. F., Lapadula, G., & Atzeni, F. (2022). Axial Spondyloarthritis: Reshape the Future—From the “2022 GISEA International Symposium”. Journal of Clinical Medicine, 11(24), 7537. https://doi.org/10.3390/jcm11247537









