Hypokalemia Duration in the First Year Associated with Subsequent Peritoneal Dialysis-Associated Peritonitis: A Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Data Collection and Definitions
2.3. Covariates
2.4. Study Outcome
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
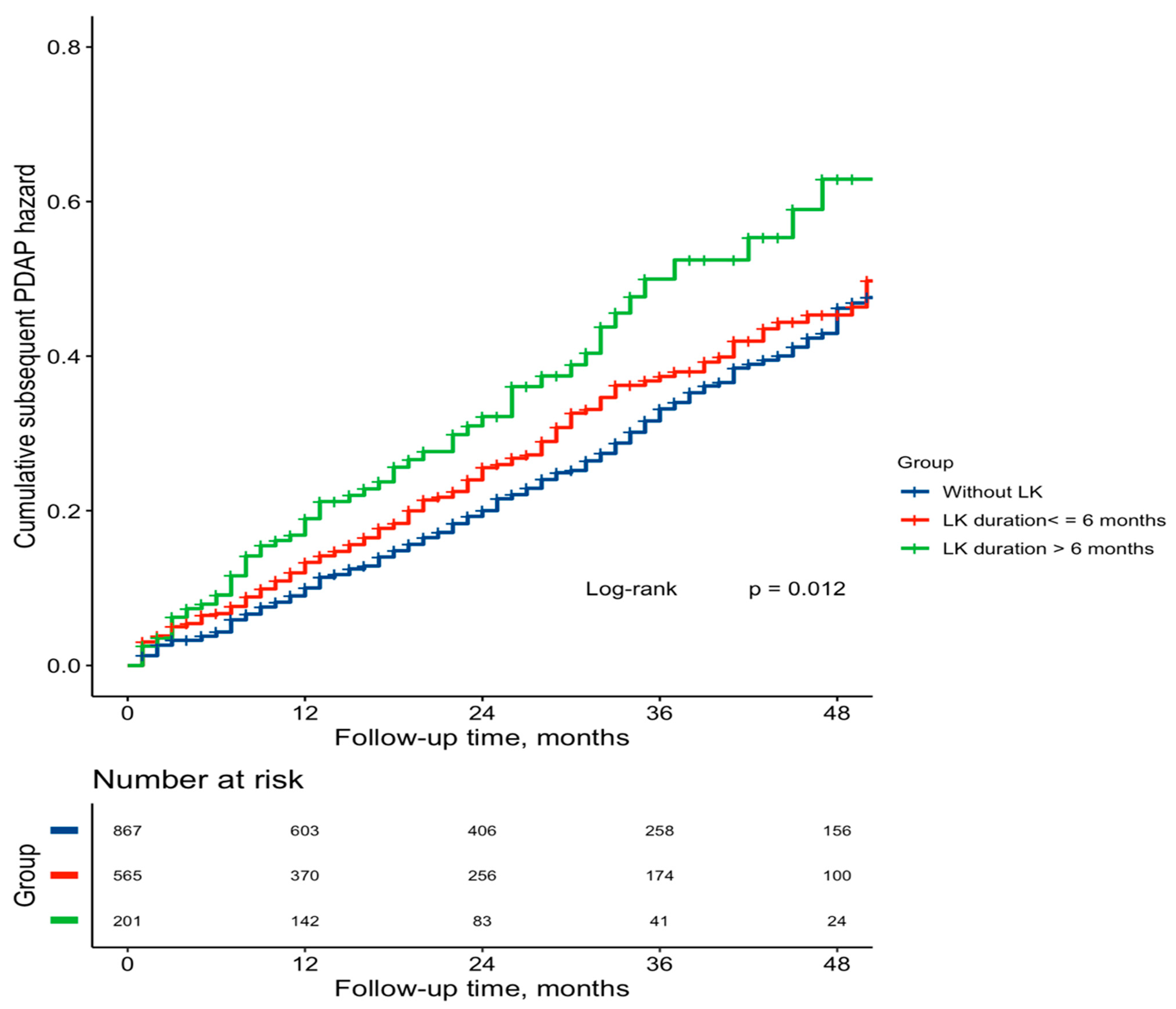
3.2. Associations of LK and SLK Duration with Subsequent PDAP Risk
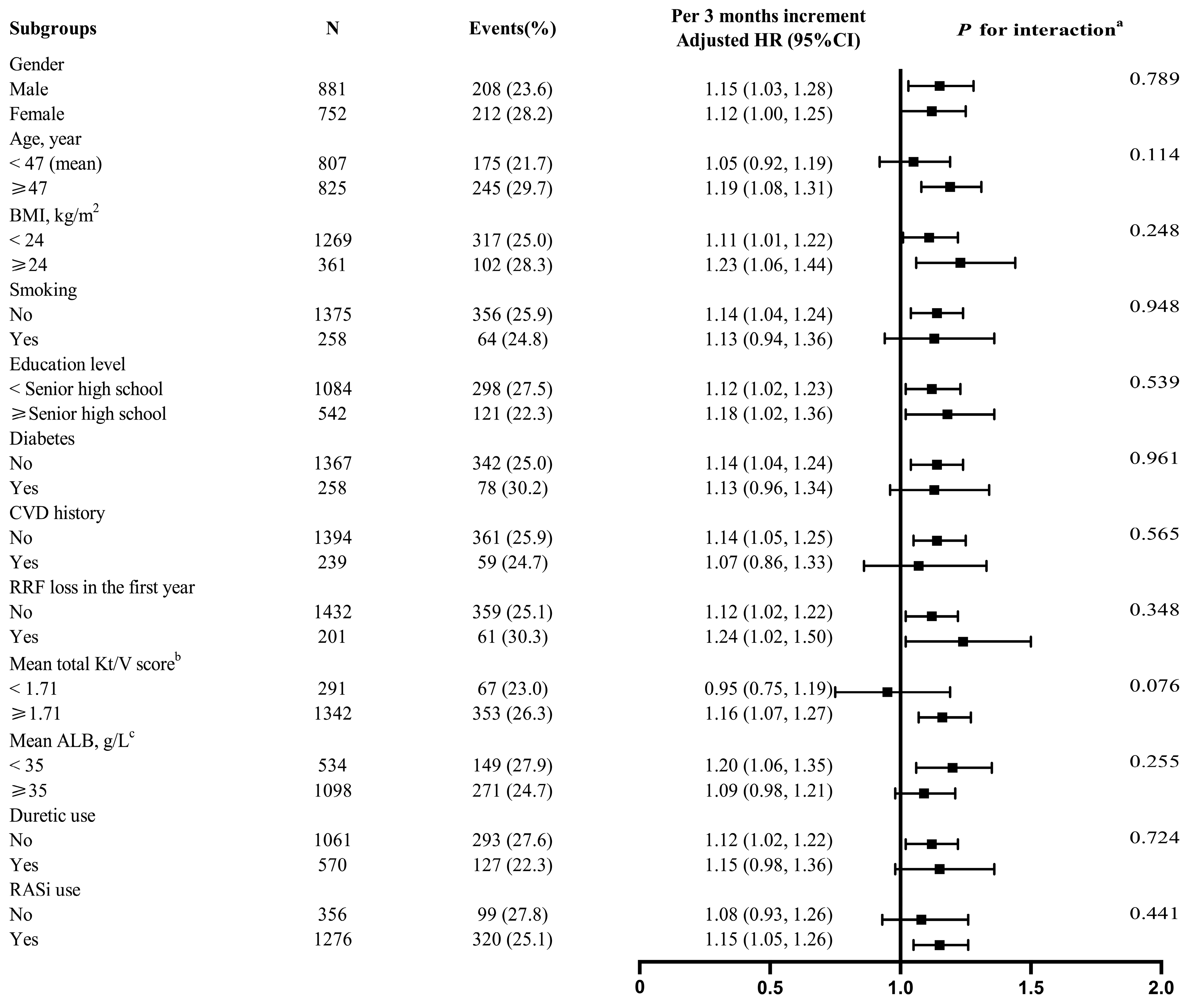
3.3. Stratified Analyses
3.4. Causative Organisms of the Subsequent PDAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perl, J.; Fuller, D.S.; Bieber, B.A.; Boudville, N.; Kanjanabuch, T.; Ito, Y.; Nessim, S.J.; Piraino, B.M.; Pisoni, R.L.; Robinson, B.M.; et al. Peritoneal Dialysis-Related Infection Rates and Outcomes: Results from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am. J. Kidney Dis. 2020, 76, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.-T.; Chow, K.M.; Cho, Y.; Fan, S.; E Figueiredo, A.; Harris, T.; Kanjanabuch, T.; Kim, Y.-L.; Madero, M.; Malyszko, J.; et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. 2022, 42, 110–153. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Badve, S.V.; Hawley, C.M.; McDonald, S.P.; Brown, F.G.; Boudville, N.; Clayton, P.; Johnson, D.W. Peritoneal dialysis outcomes after temporary haemodialysis transfer for peritonitis. Nephrol. Dial. Transplant. 2014, 29, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Boudville, N.; Kemp, A.; Clayton, P.; Lim, W.; Badve, S.V.; Hawley, C.M.; McDonald, S.P.; Wiggins, K.J.; Bannister, K.M.; Brown, F.G.; et al. Recent Peritonitis Associates with Mortality among Patients Treated with Peritoneal Dialysis. J. Am. Soc. Nephrol. 2012, 23, 1398–1405. [Google Scholar] [CrossRef]
- Hsieh, Y.-P.; Chang, C.-C.; Wen, Y.-K.; Chiu, P.-F.; Yang, Y. Predictors of Peritonitis and the Impact of Peritonitis on Clinical Outcomes of Continuous Ambulatory Peritoneal Dialysis Patients in Taiwan-10 Years’ Experience in a Single Center. Perit. Dial. Int. 2014, 34, 85–94. [Google Scholar] [CrossRef]
- Ghali, J.R.; Bannister, K.M.; Brown, F.G.; Rosman, J.B.; Wiggins, K.J.; Johnson, D.W.; McDonald, S.P. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit. Dial. Int. 2011, 31, 651–662. [Google Scholar] [CrossRef]
- Kotsanas, D.; Polkinghorne, K.R.; Korman, T.M.; Atkins, R.C.; Brown, F. Risk factors for peritoneal dialysis-related peritonitis: Can we reduce the incidence and improve patient selection? Nephrology 2007, 12, 239–245. [Google Scholar] [CrossRef]
- McDonald, S.R.; Collins, J.F.; Rumpsfeld, M.; Johnson, D.W. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit. Dial. Int. 2004, 24, 340–346. [Google Scholar] [CrossRef]
- Chow, K.M.; Szeto, C.C.; Leung, C.B.; Kwan, B.C.-H.; Law, M.C.; Li, P.K.-T. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit. Dial. Int. 2005, 25, 374–379. [Google Scholar] [CrossRef]
- Wang, Q.; Bernardini, J.; Piraino, B.; Fried, L. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am. J. Kidney Dis. 2003, 41, 664–669. [Google Scholar] [CrossRef]
- Pichitporn, W.; Kanjanabuch, T.; Phannajit, J.; Puapatanakul, P.; Chuengsaman, P.; Parapiboon, W.; Treamtrakanpon, W.; Boonyakrai, C.; Matayart, S.; Katavetin, P.; et al. Efficacy of Potassium Supplementation in Hypokalemic Patients Receiving Peritoneal Dialysis: A Randomized Controlled Trial. Am. J. Kidney Dis. 2022, 80, 580–588.e1. [Google Scholar] [CrossRef] [PubMed]
- Torlén, K.; Kalantar-Zadeh, K.; Molnar, M.Z.; Vashistha, T.; Mehrotra, R. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin. J. Am. Soc. Nephrol. 2012, 7, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.-C.; Chow, K.-M.; Kwan, B.C.-H.; Leung, C.-B.; Chung, K.-Y.; Law, M.-C.; Li, P.K.-T. Hypokalemia in Chinese peritoneal dialysis patients: Prevalence and prognostic implication. Am. J. Kidney Dis. 2005, 46, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-W.; Shu, K.-H.; Yu, T.-M.; Cheng, C.-H.; Chen, C.-H. Hypokalaemia: An independent risk factor of Enterobacteriaceae peritonitis in CAPD patients. Nephrol. Dial. Transplant. 2009, 24, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.C.; Figueiredo, A.E.; Barretti, P.; Pecoits-Filho, R.; de Moraes, T.P.; all centers that contributed to the BRAZPD II study. Low Serum Potassium Levels Increase the Infectious-Caused Mortality in Peritoneal Dialysis Patients: A Propensity-Matched Score Study. PLoS ONE 2015, 10, e0127453. [Google Scholar] [CrossRef]
- Fan, X.; Huang, R.; Wang, J.; Ye, H.; Guo, Q.; Yi, C.; Lin, J.; Zhou, Q.; Shao, F.; Yu, X.; et al. Risk factors for the first episode of peritonitis in Southern Chinese continuous ambulatory peritoneal dialysis patients. PLoS ONE 2014, 9, e107485. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.A.; de Jesus, J.S.; Cordeiro, L.; Piraciaba, M.C.T.; de Araujo, L.K.R.P.; Martins, C.S.W.; Dalboni, M.A.; Pereira, B.J.; Silva, B.C.; Moysés, R.M.A.; et al. Hypokalemia and hyperkalemia in patients on peritoneal dialysis: Incidence and associated factors. Int. Urol. Nephrol. 2020, 52, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, Y.; Gong, N.; Xiao, Z.; Zhang, F.; Zhong, X.; Yi, Z.; Yang, C.; Zhang, G.; Ai, J. Degree and duration of hypokalemia associated with peritonitis in patients undergoing peritoneal dialysis. Int. J. Clin. Pract. 2021, 75, e14188. [Google Scholar] [CrossRef]
- Jung, J.Y.; Chang, J.H.; Lee, H.H.; Chung, W.; Kim, S. De novo hypokalemia in incident peritoneal dialysis patients: A 1-year observational study. Electrolyte Blood Press. 2009, 7, 73–78. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Ming, P.; Qureshi, A.R.; Lindholm, B.; Bo, Y.; Yang, H. Peritonitis: Episode Sequence, Microbiological Variation, Risk Factors and Clinical Outcomes in a North China Peritoneal Dialysis Center. Kidney Blood Press Res. 2018, 43, 1573–1584. [Google Scholar] [CrossRef]
- Davies, S.J.; Zhao, J.; Morgenstern, H.; Zee, J.; Bieber, B.; Fuller, D.S.; Sloand, J.A.; Vychytil, A.; Kawanishi, H.; Johnson, D.W.; et al. Low Serum Potassium Levels and Clinical Outcomes in Peritoneal Dialysis-International Results from PDOPPS. Kidney Int. Rep. 2021, 6, 313–324. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am. J. Kidney Dis. 1997, 30 (3 Suppl. 2), S67–S136. [Google Scholar] [CrossRef] [PubMed]
- Tzamaloukas, A.H.; Avasthi, P.S. Temporal profile of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am. J. Nephrol. 1987, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.; Druml, W. Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Langote, A.; Hiremath, S.; Ruzicka, M.; McCormick, B.B. Spironolactone is effective in treating hypokalemia among peritoneal dialysis patients. PLoS ONE 2017, 12, e0187269. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.-C.; Leung, C.-B.; Chow, K.-M.; Kwan, B.C.-H.; Law, M.-C.; Wang, A.Y.-M.; Lui, S.-F.; Li, P.K.-T. Change in bacterial aetiology of peritoneal dialysis-related peritonitis over 10 years: Experience from a centre in South-East Asia. Clin. Microbiol. Infect. 2005, 11, 837–839. [Google Scholar] [CrossRef]
- Zelenitsky, S.; Barns, L.; Findlay, I.; Alfa, M.; Ariano, R.; Fine, A.; Harding, G. Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am. J. Kidney Dis. 2000, 36, 1009–1013. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, X.; Xiang, S.; Yang, X.; Lin, J.; Zhang, X.; Shou, Z.; Chen, J. Risk Factors and Outcomes of Early-Onset Peritonitis in Chinese Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2017, 42, 1266–1276. [Google Scholar] [CrossRef]


| Characteristic | LK Duration in the First PD Year, Months | |||
|---|---|---|---|---|
| Total | 0 | ≤6 | >6 | |
| No. of participants | 1633 | 867 | 565 | 201 |
| Age, years | 46.6 ± 13.8 | 45.8 ± 13.1 | 47.2 ± 14.3 | 47.9 ± 15.2 |
| Male, No. (%) | 881 (54.0) | 498 (57.4) | 282 (49.9) | 101 (50.3) |
| BMI, kg/m2 | 21.8 ± 3.3 | 22.1 ± 3.1 | 21.5 ± 3.3 | 21.4 ± 3.5 |
| Smoking, No. (%) | 258 (15.8) | 144 (16.6) | 85 (15.0) | 29 (14.4) |
| Alcohol drinking, No. (%) | 133 (8.1) | 78 (9.0) | 41 (7.3) | 14 (7.0) |
| Education level, No. (%) | ||||
| Junior high school and below | 1084 (66.7) | 560 (65.0) | 389 (69.1) | 135 (67.2) |
| Senior high school and above | 542 (33.3) | 302 (35.0) | 174 (30.9) | 66 (32.8) |
| DKD to ESRD, No. (%) | 258 (15.9) | 138 (16.0) | 86 (15.3) | 34 (17.0) |
| CVD history, No. (%) | 239 (14.6) | 121 (14.0) | 92 (16.3) | 26 (12.9) |
| PD vintage, months | 28 (12–47) | 27 (12–48) | 29 (10–48) | 27 (13–42) |
| Baseline MAP, mmHg | 104.9 ± 14.2 | 105.9 ± 14.5 | 103.9 ± 14.1 | 103.0 ± 13.1 |
| eGFR, ml/min/1.73 m2 | 5.0 ± 2.2 | 5.1 ± 2.2 | 5.0 ± 2.1 | 5.1 ± 2.5 |
| Baseline laboratory results | ||||
| Blood HGB, g/L | 82.9 ± 19.3 | 82.9 ± 19.4 | 83.5 ± 19.9 | 81.1 ± 17.4 |
| Serum creatinine, μmol/L | 980.8 ± 365.9 | 993.7 ± 376.6 | 963.1 ± 327.9 | 974.5 ± 423.3 |
| Serum ALB, g/L | 36.5 ± 5.4 | 36.5 ± 5.4 | 36.5 ± 5.4 | 36.3 ± 5.3 |
| Serum potassium, mmol/L | 4.6 ± 0.8 | 4.7 ± 0.8 | 4.5 ± 0.8 | 4.3 ± 0.8 |
| Serum calcium, mmol/L | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 |
| Serum iPTH, pg/mL | 303.6 (157.0–488.0) | 299.3 (157.8–471.5) | 324.5 (154.5–497.5) | 331.2 (156.5–498.2) |
| Serum phosphorus, mmol/L | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.1 ± 0.7 | 2.0 ± 0.7 |
| Fasting glucose, mmol/L | 5.7 ± 2.2 | 5.8 ± 2.3 | 5.6 ± 2.1 | 5.6 ± 2.0 |
| Baseline PD characteristics | ||||
| Dialysate GLUC, % | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 |
| UF volume, ml/24 h | 320 (200–500) | 300 (200–500) | 350 (200–500) | 325 (200–500) |
| Urine volume, ml/24 h | 1000 (600–1200) | 1000 (650–1300) | 900 (600–1200) | 1000 (550–1200) |
| Mean values of total Kt/V score a | 2.2 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 0.6 |
| Medication use in the first year, No. (%) | ||||
| Diuretic | 570 (35.0) | 329 (38.0) | 197 (34.9) | 44 (21.9) |
| RASi | 1276 (78.2) | 708 (81.8) | 424 (75.0) | 144 (71.6) |
| Subsequent PDAP | N | Events, N (%) | Crude Model a | Adjusted Model b | ||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |||
| Cox model | ||||||
| LK duration | ||||||
| Continuous, per 3 months | 1633 | 420 (25.7) | 1.13 (1.05, 1.21) | 0.002 | 1.13 (1.05, 1.23) | 0.002 |
| Categories | ||||||
| 0 month | 867 | 205 (23.6) | Ref. | Ref. | ||
| ≤6 months | 565 | 150 (26.5) | 1.16 (0.94, 1.43) | 0.165 | 1.18 (0.94, 1.48) | 0.146 |
| >6 months | 201 | 65 (32.3) | 1.51 (1.14, 2.00) | 0.004 | 1.53 (1.14, 2.06) | 0.005 |
| SLK duration | ||||||
| Continuous, per 3 months | 1633 | 420 (25.7) | 1.23 (1.06, 1.42) | 0.006 | 1.19 (1.02, 1.38) | 0.027 |
| Categories | ||||||
| 0 month | 1378 | 345 (25.0) | Ref. | Ref. | ||
| ≤6 months | 234 | 65 (27.8) | 1.18 (0.91, 1.54) | 0.220 | 1.12 (0.85, 1.49) | 0.414 |
| >6 months | 21 | 10 (47.6) | 2.54 (1.35, 4.76) | 0.004 | 2.17 (1.14, 4.14) | 0.019 |
| Competing risk model | ||||||
| LK duration | ||||||
| Continuous, per 3 months | 1633 | 420 (25.7) | 1.12 (1.06, 1.19) | 0.002 | 1.13 (1.06, 1.21) | 0.003 |
| Categories | ||||||
| 0 month | 867 | 205 (23.6) | Ref. | Ref. | ||
| ≤6 months | 565 | 150 (26.5) | 1.22 (1.03, 1.46) | 0.056 | 1.21 (1.00, 1.47) | 0.095 |
| >6 months | 201 | 65 (32.3) | 1.47 (1.16, 1.86) | 0.007 | 1.51 (1.18, 1.94) | 0.007 |
| SLK duration | ||||||
| Continuous, per 3 months | 1633 | 420 (25.7) | 1.22 (1.09, 1.37) | 0.004 | 1.18 (1.03, 1.34) | 0.038 |
| Categories | ||||||
| 0 month | 1378 | 345 (25.0) | Ref. | Ref. | ||
| ≤6 months | 234 | 65 (27.8) | 1.16 (0.93, 1.44) | 0.270 | 1.06 (0.84, 1.35) | 0.680 |
| >6 months | 21 | 10 (47.6) | 2.64 (1.61, 4.32) | 0.001 | 2.28 (1.33, 3.89) | 0.011 |
| Organisms | Overall | Without LK | With LK | p-Value |
|---|---|---|---|---|
| No. of PDAP | 420 | 205 | 215 | |
| Gram-positive | 168 (40.0) | 78 (38.1) | 90 (41.9) | 0.425 |
| Staphylococcus aureus | 25 (6.0) | 17 (8.3) | 8 (3.7) | 0.048 |
| CNS a | 70 (16.7) | 30 (14.6) | 40 (18.6) | 0.275 |
| Streptococcus spp. | 47 (11.2) | 19 (9.3) | 28 (13.0) | 0.222 |
| Enterococcus spp. | 7 (1.7) | 3 (1.5) | 4 (1.9) | 0.949 |
| Others | 19 (4.4) | 9 (4.4) | 10 (4.7) | 0.898 |
| Gram-negative | 109 (25.9) | 57 (27.7) | 52 (24.2) | 0.398 |
| Escherichia coli | 59 (14.0) | 26 (12.7) | 33 (15.3) | 0.432 |
| Klebsiella spp. | 21 (5.0) | 17 (8.3) | 4 (1.9) | 0.003 |
| Acinetobacter spp. | 5 (1.2) | 3 (1.4) | 2 (1.0) | 0.957 |
| Others | 24 (5.7) | 11 (5.3) | 13 (6.0) | 0.764 |
| Enterobacteriaceae | 80 (19.0) | 43 (21.0) | 37 (17.2) | 0.326 |
| Multiple organisms | 16 (3.8) | 11 (5.4) | 5 (2.3) | 0.104 |
| Fungi | 10 (2.4) | 7 (3.4) | 3 (1.4) | 0.300 |
| Culture-negative | 117 (27.9) | 52 (25.4) | 65 (30.2) | 0.266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Z.; Zhuo, Q.; Zhong, S.; Wang, F.; Xie, C.; Gong, N.; Zhong, X.; Yi, Z.; Kong, Y.; Liu, D.; et al. Hypokalemia Duration in the First Year Associated with Subsequent Peritoneal Dialysis-Associated Peritonitis: A Multicenter Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7518. https://doi.org/10.3390/jcm11247518
Huo Z, Zhuo Q, Zhong S, Wang F, Xie C, Gong N, Zhong X, Yi Z, Kong Y, Liu D, et al. Hypokalemia Duration in the First Year Associated with Subsequent Peritoneal Dialysis-Associated Peritonitis: A Multicenter Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(24):7518. https://doi.org/10.3390/jcm11247518
Chicago/Turabian StyleHuo, Zhihao, Qianqian Zhuo, Shaoxin Zhong, Fang Wang, Chao Xie, Nirong Gong, Xiaohong Zhong, Zhixiu Yi, Yaozhong Kong, Dehui Liu, and et al. 2022. "Hypokalemia Duration in the First Year Associated with Subsequent Peritoneal Dialysis-Associated Peritonitis: A Multicenter Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 24: 7518. https://doi.org/10.3390/jcm11247518
APA StyleHuo, Z., Zhuo, Q., Zhong, S., Wang, F., Xie, C., Gong, N., Zhong, X., Yi, Z., Kong, Y., Liu, D., Dou, X., Wang, G., & Ai, J. (2022). Hypokalemia Duration in the First Year Associated with Subsequent Peritoneal Dialysis-Associated Peritonitis: A Multicenter Retrospective Cohort Study. Journal of Clinical Medicine, 11(24), 7518. https://doi.org/10.3390/jcm11247518






