Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing
Abstract
1. Introduction
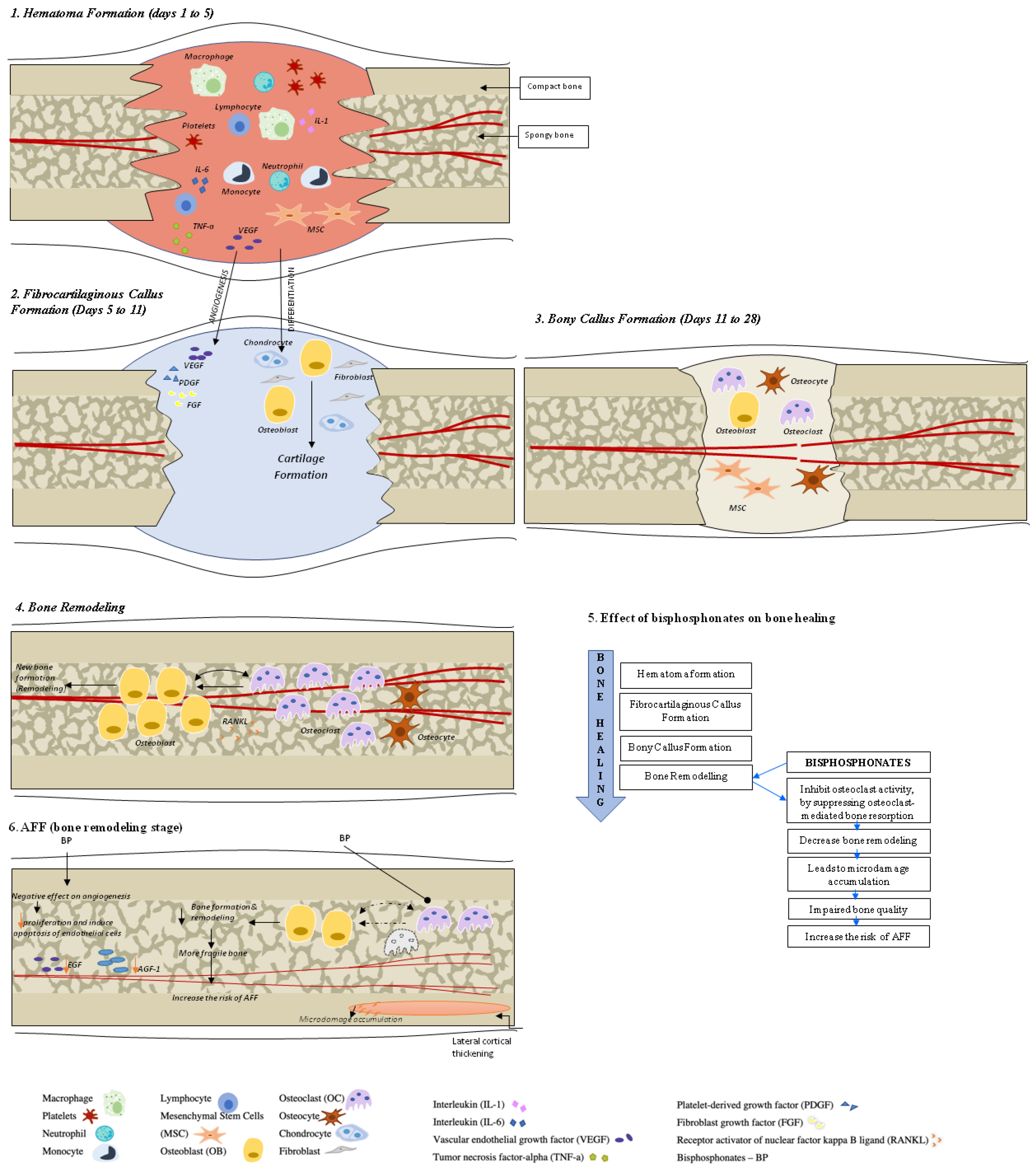
2. Fracture Healing in Healthy Bone
3. Fracture Healing in Osteoporotic Bone
4. Bone Turnover Markers (BTMs) in Fracture Healing (Table 1 and Table 2)
| Bone Turnover Marker | Origin | Expected Change in Level during the Fracture Healing | Conditions That Affect BTM Levels |
|---|---|---|---|
| P1NP | Product of the type I procollagen degradation during the procollagen-to-collagen conversion. Cells—osteoblasts [23] | Peak at 12 weeks after fracture, remains elevated at 24 weeks [11,31]. | Antiresorptive treatment (such as estrogen and BPs) lowers procollagen peptide levels [32]. Anabolic agents such as TPTD and Rmab increase procollagen peptide concentrations [32,33,34]. Renal function deviations have no influence on P1NP, so this marker can be used in patients with CKD [21]. |
| BALP | Enzyme needed in osteoid formation and mineralization [35]. Cells—osteoblasts. | The level is elevated at 4 weeks after fracture of the tibial shaft and remains increased at 1 year [11]. | BALP has several advantageous features, which include low circadian variation due to its half-life of 1 to 2 days, stability of samples, broad availability of assays, and lack of renal clearance [36]. BALP can be used in patients with CKD [37]. |
| OC | Non-collagen protein is a kind of calcium-binding protein, vitamin-K-dependent, associated with bone mineralization [23]. Cells—osteoblasts. | The level is elevated at 24 weeks after fracture of the tibial shaft and at 1 week after distal radial fracture [11]. | OC is metabolized in liver and kidneys and is influenced by renal clearance, with higher levels of OC that occur in CKD. Some anticoagulants (such as a high dose of heparin for one week) can reduce OC level by 40% [32,36]. OC is affected by renal clearance and has circadian rhythm with peak at around 4 AM [36]. |
| CTX | Degradation of mature type I collagen marker. CTX is formed in the process of bone resorption mediated by cathepsin-K [38]. | The level rises in the first week after fracture, with peak at 4 weeks after fracture, and remains elevated throughout fracture healing [11]. | Fasting morning samples are important for optimal clinical use since fasting reduces circadian variations [32]. This biomarker decreases rapidly in the course of antiresorptive therapy [38]. |
| TRACP5b | The serum enzyme activity reflects the number of active osteoclasts [35]. Cells—resorbing osteoclasts | Peak is approximately seven days after osteosynthesis and after two weeks in fractures, remaining high at 24 weeks [11]. | As this marker is not secreted in urine, it can be used in CKD patients [29]. TRAP5b levels are not affected by food intake as well, but they feature diurnal variation and increase immediately after exercise. In addition, TRAP5b samples are unstable at room temperature [38]. |
| Factor | Effect on BTM Levels |
|---|---|
| Age and gender | Highest levels are in infancy and remain high in childhood, with a nadir in women in the fourth decade and the fifth decade in men. Men <35 years old have higher BTMs vs. women due to longer lasting bone consolidation into young adulthood in men [38,39]. |
| Menopausal status | Bone formation and resorption markers are higher during a few months following the menopause onset and both of these levels remain elevated thereafter [32,36]. |
| Fractures | Elevation of bone resorption markers levels occurs within the first four weeks after a fracture, followed by increase in bone formation markers. Elevation of BTMs levels is estimated as 20–50% and may persist for up to six months [32]. |
| Pregnancy and lactation | BTMs are increasing in the course of pregnancy. They reach higher values in the third trimester and even higher levels occur postpartum [32]. Elevation of levels of both formation (BALP and P1NP) and resorption markers (cross-links and telopeptides) start from the second trimester of pregnancy. These levels reach significantly higher values than before pregnancy [32]. The serum OC concentration decreases in the first two trimesters, with normalization in the third trimester and after delivery. Lowering of bone markers levels occurs postpartum over a period of 6–12 months, with slower decline during the lactation period [32,36]. |
| Drugs intake | Glucocorticoid therapy reduces serum of formation markers (OC and P1NP by up to 40% to 50%) within a few days of therapy initiation, with little effect on bone resorption markers [36]. Intake of anticonvulsants may result in elevation of BTMs levels. It is essential to pay close attention to intake of corticosteroids, anticonvulsants, heparin, and GnRH agonists [36]. |
| Fasting status/food intake | Feeding causes suppression of BTMs, with more pronounced effect on resorption markers, which can be decreased by 20–40% in contrast to bone formation markers (10% suppression). CTX level decreases by 20% after breakfast) [29,36]. |
| Bed Rest/Immobility | 2–4 days of bed rest leads to a significant bone resorption markers elevation and, after 1 week, these levels increase by 30% to 50% vs. bone formation markers, which remain unchanged or increase only slightly [36]. |
5. Assessment of Bone Health before Bone Surgery
6. Imaging in Fracture Assessment (Table 3)
6.1. Dual-Energy X-ray Absorptiometry
| Imaging Modality and What Can Be Measured | Benefits | Limitations |
|---|---|---|
DXA
|
|
|
REMS
|
|
|
| Plain X-ray Structural bone changes |
|
|
| QCT, pQCT |
|
|
| HR-pQCT Structural bone changes, mechanical properties, microarchitecture |
|
|
| Opportunistic CT Structural changes, L1 HU |
|
|
| Opportunistic MRI Structural changes, microarchitecture Acute vs. chronic bone changes M- score |
|
|
6.2. Radiofrequency Echographic Multi-Spectrometry (REMS)
6.3. Opportunistic Computed Tomography (CT)
6.4. Opportunistic Magnetic Resonance Imaging (MRI)
6.5. Quantitative Computed Tomography (QCT)
7. Anti-Osteoporosis Medications and Bone Health before Orthopedic Surgery
7.1. Vitamin D
7.2. Anti-Osteoporosis Medication
8. Specifics of Fracture Healing and Treatment with AFF
- The fracture is associated with minimal or no trauma, such as in a fall from standing height or lower;
- The fracture line originates at the lateral cortex and is substantially transverse in its orientation, although it may become oblique as it progresses medially across the femur;
- Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex;
- The fracture is noncomminuted or minimally comminuted;
- Localized periosteal or endosteal thickening of the lateral cortex is present at the fracture site (‘‘beaking’’ or ‘‘flaring’’).
- A generalized increase in cortical thickness of the femoral diaphyses;
- Unilateral or bilateral prodromal symptoms, such as dull or aching pain in the groin or thigh;
- Bilateral incomplete or complete femoral diaphysis fractures;
- Delayed fracture healing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hak, D.J. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury 2018, 49, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Sardar, Z.M.; Coury, J.R.; Cerpa, M.; DeWald, C.J.; Ames, C.P.; Shuhart, C.; Watkins, C.; Polly, D.W.; Dirschl, D.R.; Klineberg, E.O.; et al. Best Practice Guidelines for Assessment and Management of Osteoporosis in Adult Patients Undergoing Elective Spinal Reconstruction. Spine 2022, 47, 128–135. [Google Scholar] [CrossRef]
- Lafuente-Gracia, L.; Borgiani, E.; Nasello, G.; Geris, L. Towards in silico Models of the Inflammatory Response in Bone Fracture Healing. Front. Bioeng Biotechnol. 2021, 9, 703725. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.R.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth factor regulation of fracture repair. J. Bone Miner. Res. 1999, 14, 1805–1815. [Google Scholar] [CrossRef]
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing: Which are the important molecules? Injury 2007, 38, 11–25. [Google Scholar] [CrossRef]
- Cox, G.; Einhorn, T.A.; Tzioupis, C.; Giannoudis, P.V. Bone-turnover markers in fracture healing. J. Bone Jt. Surg. Br. 2010, 92, 329–334. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Bone Health & Osteoporosis Foundation. What is Osteoporosis and What Causes It? Available online: https://www.nof.org/patients/what-is-osteoporosis/ (accessed on 23 June 2022).
- Pesce, V.; Speciale, D.; Sammarco, G.; Patella, S.; Spinarelli, A.; Patella, V. Surgical approach to bone healing in osteoporosis. Clin. Cases Miner Bone Metab. 2009, 6, 131–135. [Google Scholar] [PubMed]
- Doll, B.; Tegtmeier, F.; Koch, H.; Acarturk, O.; Holliger, J. Declino cellulare e molecolare nella guarigione ossea con l’avanzare dell’età. Tec. Chir. Ortop. 2003, 3, 15–20. [Google Scholar]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef]
- Maurel, D.B.; Jahn, K.; Lara-Castillo, N. Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines 2017, 5, 62. [Google Scholar] [CrossRef]
- Hamrick, M.W. The skeletal muscle secretome: An emerging player in muscle-bone crosstalk. Bonekey Rep. 2012, 1, 60. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, Y.; Wang, X.; Jin, F.; Ge, S. Short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J. Orthop. Res. 2012, 30, 574–580. [Google Scholar] [CrossRef]
- Kushchayeva, Y.; Lewiecki, E.M. Osteoporosis management with focus on spine. In Image Guided Interventions of the Spine: Principles and Clinical Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 61–92. [Google Scholar] [CrossRef]
- Sousa, C.P.; Dias, I.R.; Lopez-Peña, M.; Camassa, J.A.; Lourenço, P.J.; Judas, F.M.; Gomes, M.E.; Reis, R.L. Bone turnover markers for early detection of fracture healing disturbances: A review of the scientific literature. An. Acad. Bras Cienc. 2015, 87, 1049–1061. [Google Scholar] [CrossRef]
- Pan, C.; Liu, X.; Li, T.; Wang, G.; Sun, J. Kinetic of bone turnover markers after osteoporotic vertebral compression fractures in postmenopausal female. J. Orthop. Surg. Res. 2018, 13, 314. [Google Scholar] [CrossRef]
- Szulc, P.; Naylor, K.; Hoyle, N.R.; Eastell, R.; Leary, E.T.; for the National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos. Int. 2017, 28, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; Gerdhem, P.; Akesson, K.; Garnero, P.; Obrant, K.J. Effect of fracture on bone turnover markers: A longitudinal study comparing marker levels before and after injury in 113 elderly women. J. Bone Miner. Res. 2007, 22, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Hannon, R.; Eastell, R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos. Int. 2000, 11, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Veitch, S.; Findlay, S.; Hamer, A.; Blumsohn, A.; Eastell, R.; Ingle, B. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos. Int. 2006, 17, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Branco, J.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Cooper, C.; Cortet, B.; Diez-Perez, A.; Ferrari, S.; Gasparik, A.; et al. Algorithm for the Use of Biochemical Markers of Bone Turnover in the Diagnosis, Assessment and Follow-Up of Treatment for Osteoporosis. Adv. Ther. 2019, 36, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyere, O.; Foldes, A.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef]
- Ohishi, T.; Takahashi, M.; Kushida, K.; Hoshino, H.; Tsuchikawa, T.; Naitoh, K.; Inoue, T. Changes of biochemical markers during fracture healing. Arch. Orthop. Trauma Surg. 1998, 118, 126–130. [Google Scholar] [CrossRef]
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover - uses and limitations. Ann. Clin. Biochem. 2014, 51, 189–202. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, S.H.; Yoo, J.I.; Chung, Y.J.; Jeon, Y.K.; Yoon, B.H.; Kim, H.Y.; Lee, S.H.; Lee, J.; Hong, S. Position Statement on the Use of Bone Turnover Markers for Osteoporosis Treatment. JBM 2019, 26, 213–224. [Google Scholar] [CrossRef]
- Miyauchi, A.; Matsumoto, T.; Sugimoto, T.; Tsujimoto, M.; Warner, M.R.; Nakamura, T. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 2010, 47, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Lenora, J.; Norrgren, K.; Thorsson, O.; Wollmer, P.; Obrant, K.J.; Ivaska, K.K. Bone turnover markers are correlated with total skeletal uptake of 99mTc-methylene diphosphonate (99mTc-MDP). BMC Med. Phys. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.K.; Clarke, B.L. Biochemical Testing Relevant to Bone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Ureña-Torres, P.; Cozzolino, M.; Rodríguez-García, M.; Gómez-Alonso, C. The Non-invasive Diagnosis of Bone Disorders in CKD. Calcif. Tissue Int. 2021, 108, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Camacho, P. Use of bone turnover markers in the management of osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 366–372. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Galbusera, F.; Volkheimer, D.; Reitmaier, S.; Berger-Roscher, N.; Kienle, A.; Wilke, H.J. Pedicle screw loosening: A clinically relevant complication? Eur. Spine J. 2015, 24, 1005–1016. [Google Scholar] [CrossRef]
- Park, S.B.; Chung, C.K. Strategies of spinal fusion on osteoporotic spine. J. Korean Neurosurg. Soc. 2011, 49, 317–322. [Google Scholar] [CrossRef]
- Chin, D.K.; Park, J.Y.; Yoon, Y.S.; Kuh, S.U.; Jin, B.H.; Kim, K.S.; Cho, Y.E. Prevalence of osteoporosis in patients requiring spine surgery: Incidence and significance of osteoporosis in spine disease. Osteoporos. Int. 2007, 18, 1219–1224. [Google Scholar] [CrossRef]
- Lubelski, D.; Choma, T.J.; Steinmetz, M.P.; Harrop, J.S.; Mroz, T.E. Perioperative Medical Management of Spine Surgery Patients With Osteoporosis. Neurosurgery 2015, 77, 92–97. [Google Scholar] [CrossRef]
- Morita, A.; Kobayashi, N.; Choe, H.; Tezuka, T.; Higashihira, S.; Inaba, Y. Preoperative factors predicting the severity of BMD loss around the implant after Total hip Arthroplasty. BMC Musculoskelet. Disord. 2021, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Aro, H.T.; Alm, J.J.; Moritz, N.; Mäkinen, T.J.; Lankinen, P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: A 2-year RSA study of 39 patients. Acta Orthop. 2012, 83, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Virtama, P. Uneven distribution of bone minerals and covering effect of non-mineralized tissue as reasons for impaired detectability of bone density from roentgenograms. Ann. Med. Intern. Fenn. 1960, 49, 57–65. [Google Scholar] [PubMed]
- McCullagh, C.D.; McCoy, K.; Crawford, V.L.; Taggart, H. How reliable is a radiological report in osteoporosis in diagnosing low bone density? Ulster Med. J. 2003, 72, 34–37. [Google Scholar]
- Naylor, K.L.; McArthur, E.; Leslie, W.D.; Fraser, L.A.; Jamal, S.A.; Cadarette, S.M.; Pouget, J.G.; Lok, C.E.; Hodsman, A.B.; Adachi, J.D.; et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014, 86, 810–818. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.R.; Oh, H.J. Fracture risk in chronic kidney disease: A Korean population-based cohort study. Kidney Res. Clin. Pract. 2019, 38, 220–228. [Google Scholar] [CrossRef]
- Kim, C.W.; Kim, H.J.; Lee, C.R.; Wang, L.; Rhee, S.J. Effect of chronic kidney disease on outcomes of total joint arthroplasty: A meta-analysis. Knee Surg. Relat. Res. 2020, 32, 12. [Google Scholar] [CrossRef]
- Chou, T.A.; Ma, H.H.; Tsai, S.W.; Chen, C.F.; Wu, P.K.; Chen, W.M. Dialysis patients have comparable results to patients who have received kidney transplant after total joint arthroplasty: A systematic review and meta-analysis. EFORT Open Rev. 2021, 6, 618–628. [Google Scholar] [CrossRef]
- Popat, R.; Ali, A.M.; Holloway, I.P.; Sarraf, K.M.; Hanna, S.A. Outcomes of total hip arthroplasty in haemodialysis and renal transplant patients: Systematic review. Hip Int. 2021, 31, 207–214. [Google Scholar] [CrossRef]
- Iimori, S.; Mori, Y.; Akita, W.; Kuyama, T.; Takada, S.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—A single-center cohort study. Nephrol. Dial. Transplant. 2012, 27, 345–351. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Lok, C.E.; Langsetmo, L.; Cheung, A.M.; Szabo, E.; Pearce, D.; Fusaro, M.; Wald, R.; Weinstein, J.; Jamal, S.A. Bone mineral density predicts fractures in chronic kidney disease. J. Bone Miner. Res. 2015, 30, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Bandirali, M.; Sconfienza, L.M.; D’Alonzo, N.K.; Di Leo, G.; Papini, G.D.; Ulivieri, F.M.; Sardanelli, F. Prevalence and type of errors in dual-energy X-ray absorptiometry. Eur. Radiol. 2015, 25, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Agnollitto, P.M.; Petrini, M.; Biacca, A.; Ulivieri, F.M.; Sconfienza, L.M.; Messina, C. Operator-Related Errors and Pitfalls in Dual Energy X-Ray Absorptiometry: How to Recognize and Avoid Them. Acad. Radiol. 2021, 28, 1272–1286. [Google Scholar] [CrossRef]
- Martineau, P.; Bazarjani, S.; Zuckier, L.S. Artifacts and Incidental Findings Encountered on Dual-Energy X-Ray Absorptiometry: Atlas and Analysis. Semin. Nucl. Med. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Morgan, S.L.; Prater, G.L. Quality in dual-energy X-ray absorptiometry scans. Bone 2017, 104, 13–28. [Google Scholar] [CrossRef]
- Gupta, A.; Upadhyaya, S.; Patel, A.; Fogel, H.A.; Cha, T.; Schwab, J.; Bono, C.; Hershman, S. DEXA sensitivity analysis in patients with adult spinal deformity. Spine J. 2020, 20, 174–180. [Google Scholar] [CrossRef]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Krohn, K.; Schwartz, E.N.; Chung, Y.S.; Lewiecki, E.M. Dual-energy X-ray Absorptiometry Monitoring with Trabecular Bone Score: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 501–505. [Google Scholar] [CrossRef]
- Pothuaud, L.; Barthe, N.; Krieg, M.A.; Mehsen, N.; Carceller, P.; Hans, D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: A preliminary spine BMD-matched, case-control study. J. Clin. Densitom. 2009, 12, 170–176. [Google Scholar] [CrossRef]
- Winzenrieth, R.; Dufour, R.; Pothuaud, L.; Hans, D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: Analyzing the odds of vertebral fracture. Calcif. Tissue Int. 2010, 86, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Rabier, B.; Heraud, A.; Grand-Lenoir, C.; Winzenrieth, R.; Hans, D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone 2010, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Lamy, O.; Metzger, M.; Krieg, M.A.; Aubry-Rozier, B.; Stoll, D.; Hans, D. [OsteoLaus: Prediction of osteoporotic fractures by clinical risk factors and DXA, IVA and TBS]. Rev. Med. Suisse 2011, 7, 2132–2136. [Google Scholar]
- Hans, D.; Goertzen, A.L.; Krieg, M.A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, A.; Brandi, M.L.; Al-Daghri, N.; Branco, J.C.; Bruyère, O.; Cavalli, L.; Cooper, C.; Cortet, B.; Dawson-Hughes, B.; Dimai, H.P.; et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art-outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin. Exp. Res. 2019, 31, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S.; Peccarisi, M.; Pisani, P.; Franchini, R.; Greco, A.; De Marco, T.; Grimaldi, A.; Quarta, L.; Quarta, E.; Muratore, M.; et al. An Advanced Quantitative Echosound Methodology for Femoral Neck Densitometry. Ultrasound Med. Biol. 2016, 42, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Franchini, R.; Greco, A.; Soloperto, G.; Chiriacò, F.; Casciaro, E.; Aventaggiato, M.; Renna, M.D.; Pisani, P.; Di Paola, M.; et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med. Biol. 2015, 41, 281–300. [Google Scholar] [CrossRef]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Adami, G.; Arioli, G.; Bianchi, G.; Brandi, M.L.; Caffarelli, C.; Cianferotti, L.; Gatti, D.; Girasole, G.; Gonnelli, S.; Manfredini, M.; et al. Radiofrequency echographic multi spectrometry for the prediction of incident fragility fractures: A 5-year follow-up study. Bone 2020, 134, 115297. [Google Scholar] [CrossRef]
- Bojinca, V.C.; Popescu, C.C.; Decianu, R.D.; Dobrescu, A.; Balanescu, S.M.; Balanescu, A.R.; Bojinca, M. A novel quantitative method for estimating bone mineral density using B-mode ultrasound and radiofrequency signals-a pilot study on patients with rheumatoid arthritis. Exp. Ther. Med. 2019, 18, 1661–1668. [Google Scholar] [CrossRef]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogues, X.; Ovejero Crespo, D.; Quarta, E.; Brandi, M.L. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef] [PubMed]
- Valentina Anna, D.; Maria Luisa, B.; Greta, C.; Sergio, C.; Delia, C.; Francesco, C.; Pasquo Elvira, D.; Stefano, G.; Fiorella Anna, L.; Paola, P.; et al. First assessment of bone mineral density in healthy pregnant women by means of Radiofrequency Echographic Multi Spectrometry (REMS) technology. Eur. J Obstet. Gynecol. Reprod. Biol. 2021, 263, 44–49. [Google Scholar] [CrossRef]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic Osteoporosis Screening at Routine Abdominal and Thoracic CT: Normative L1 Trabecular Attenuation Values in More than 20 000 Adults. Radiology 2019, 291, 360–367. [Google Scholar] [CrossRef] [PubMed]
- 2019 ISCD Official Positions Adult. 2019. Available online: https://iscd.org/learn/official-positions/adult-positions/ (accessed on 23 June 2022).
- Lee, S.Y.; Kwon, S.S.; Kim, H.S.; Yoo, J.H.; Kim, J.; Kim, J.Y.; Min, B.C.; Moon, S.J.; Sung, K.H. Reliability and validity of lower extremity computed tomography as a screening tool for osteoporosis. Osteoporos. Int. 2015, 26, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Buckens, C.F.; Dijkhuis, G.; de Keizer, B.; Verhaar, H.J.; de Jong, P.A. Opportunistic screening for osteoporosis on routine computed tomography? An external validation study. Eur. Radiol. 2015, 25, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Sadineni, R.T.; Pasumarthy, A.; Bellapa, N.C.; Velicheti, S. Imaging Patterns in MRI in Recent Bone Injuries Following Negative or Inconclusive Plain Radiographs. J. Clin. Diagn. Res. 2015, 9, 10–13. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Punyanitya, M.; Shapses, S.; Heshka, S.; Heymsfield, S.B. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos. Int. 2007, 18, 641–647. [Google Scholar] [CrossRef]
- Shen, W.; Scherzer, R.; Gantz, M.; Chen, J.; Punyanitya, M.; Lewis, C.E.; Grunfeld, C. Relationship between MRI-Measured Bone Marrow Adipose Tissue and Hip and Spine Bone Mineral Density in African-American and Caucasian Participants: The CARDIA Study. J. Clin. Endocrinol. Metab. 2012, 97, 1337–1346. [Google Scholar] [CrossRef]
- Yeung, D.K.; Griffith, J.F.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285. [Google Scholar] [CrossRef]
- Griffith, J.F.; Yeung, D.K.; Antonio, G.E.; Wong, S.Y.; Kwok, T.C.; Woo, J.; Leung, P.C. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology 2006, 241, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, D.; Lin, C.S.; Hatipoglu, H.G.; Fertikh, D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. Am. J. Neuroradiol. 2001, 22, 1620–1627. [Google Scholar] [PubMed]
- Bandirali, M.; Di Leo, G.; Papini, G.D.E.; Messina, C.; Sconfienza, L.M.; Ulivieri, F.M.; Sardanelli, F. A new diagnostic score to detect osteoporosis in patients undergoing lumbar spine MRI. Eur. Radiol. 2015, 25, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Krueger, D.; Vallarta-Ast, N. An overlying fat panniculus affects femur bone mass measurement. J. Clin. Densitom. 2003, 6, 199–204. [Google Scholar] [CrossRef]
- Blake, G.M.; McKeeney, D.B.; Chhaya, S.C.; Ryan, P.J.; Fogelman, I. Dual energy X-ray absorptiometry: The effects of beam hardening on bone density measurements. Med. Phys. 1992, 19, 459–465. [Google Scholar] [CrossRef]
- Weigert, J.; Cann, C. DXA in obese patients: Are normal values really normal. J. Womens Imaging 1999, 1, 11–17. [Google Scholar]
- Löffler, M.T.; Jacob, A.; Valentinitsch, A.; Rienmüller, A.; Zimmer, C.; Ryang, Y.M.; Baum, T.; Kirschke, J.S. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur. Radiol. 2019, 29, 4980–4989. [Google Scholar] [CrossRef]
- Engelke, K.; Lang, T.; Khosla, S.; Qin, L.; Zysset, P.; Leslie, W.D.; Shepherd, J.A.; Schousboe, J.T. Clinical Use of Quantitative Computed Tomography (QCT) of the Hip in the Management of Osteoporosis in Adults: The 2015 ISCD Official Positions-Part I. J. Clin. Densitom. 2015, 18, 338–358. [Google Scholar] [CrossRef]
- Cann, C.E.; Adams, J.E.; Brown, J.K.; Brett, A.D. CTXA hip—An extension of classical DXA measurements using quantitative CT. PLoS ONE 2014, 9, e91904. [Google Scholar] [CrossRef]
- American College of Radiology. ACR–SPR–SSR Practice Parameter for the Perfomance Of Musculoskeletal Quantitative Computed Tomography (QCT). 2008. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/qct.pdf/ (accessed on 30 July 2022).
- Lichtenstein, A.; Ferreira-Júnior, M.; Sales, M.M.; Aguiar, F.B.; Fonseca, L.A.; Sumita, N.M.; Duarte, A.J. Vitamin D: Non-skeletal actions and rational use. Rev. Assoc. Med. Bras. 2013, 59, 495–506. [Google Scholar] [CrossRef][Green Version]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.K.; Miao, D.; Bolivar, I.; Li, J.; Huo, R.; Hendy, G.N.; Goltzman, D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 2004, 279, 16754–16766. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Thadhani, R.; Slatopolsky, E. Vitamin D receptor and analogs. Semin Nephrol. 2004, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Ebert, K.; Rolvien, T.; Oehler, N.; Lohmann, J.; Papavero, L.; Kothe, R.; Amling, M.; Barvencik, F.; Mussawy, H. A retrospective analysis of bone mineral status in patients requiring spinal surgery. BMC Musculoskelet. Disord. 2018, 19, 53. [Google Scholar] [CrossRef]
- Kerezoudis, P.; Rinaldo, L.; Drazin, D.; Kallmes, D.; Krauss, W.; Hassoon, A.; Bydon, M. Association Between Vitamin D Deficiency and Outcomes Following Spinal Fusion Surgery: A Systematic Review. World Neurosurg 2016, 95, 71–76. [Google Scholar] [CrossRef]
- Monaco, M.D.; Vallero, F.; Monaco, R.D.; Tappero, R.; Cavanna, A. 25-Hydroxyvitamin D, parathyroid hormone, and functional recovery after hip fracture in elderly patients. J. Bone Miner. Metab. 2006, 24, 42–47. [Google Scholar] [CrossRef]
- Prentice, R.; Pettinger, M.; Jackson, R.; Wactawski-Wende, J.; Lacroix, A.; Anderson, G.; Chlebowski, R.; Manson, J.; Van Horn, L.; Vitolins, M. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos. Int. 2013, 24, 567–580. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Mowé, M.; Haug, E.; Bøhmer, T. Low serum calcidiol concentration in older adults with reduced muscular function. J. Am. Geriatr. Soc. 1999, 47, 220–226. [Google Scholar] [CrossRef]
- Dhesi, J.K.; Bearne, L.M.; Moniz, C.; Hurley, M.V.; Jackson, S.H.; Swift, C.G.; Allain, T.J. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J. Bone Miner. Res. 2002, 17, 891–897. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.; Schlotthauer, T.; Pospeschill, M.; Scholz, M.; Lazarescu, A.; Pollähne, W. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp. Clin. Endocrinol. Diabetes 2001, 109, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.S.; Wark, J.D.; Scherer, S.C.; Walton, S.L.; Chick, P.; Di Carlantonio, M.; Zajac, J.D.; Flicker, L. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J. Am. Geriatr. Soc. 1999, 47, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Giorgino, R.; Rini, G.; Bevilacqua, M.; Maugeri, D.; Adami, S. Prevalence of hypovitaminosis D in elderly women in Italy: Clinical consequences and risk factors. Osteoporos. Int. 2003, 14, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stahelin, H.B.; Urscheler, N.; Ehrsam, R.; Vonthein, R.; Perrig-Chiello, P.; Tyndall, A.; Theiler, R. Muscle strength in the elderly: Its relation to vitamin D metabolites. Arch. Phys. Med. Rehabil. 1999, 80, 54–58. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Godzik, J.; Dailey, A.T.; Schmidt, M.H.; Bisson, E.F.; Hood, R.S.; Cutler, A.; Ray, W.Z. Vitamin D Levels and 1-Year Fusion Outcomes in Elective Spine Surgery: A Prospective Observational Study. Spine 2015, 40, 1536–1541. [Google Scholar] [CrossRef]
- Formby, P.M.; Kang, D.G.; Helgeson, M.D.; Wagner, S.C. Clinical and Radiographic Outcomes of Transforaminal Lumbar Interbody Fusion in Patients with Osteoporosis. Global Spine J. 2016, 6, 660–664. [Google Scholar] [CrossRef]
- Wong, R.M.Y.; Wong, P.Y.; Liu, C.; Wong, H.W.; Chung, Y.L.; Chow, S.K.H.; Law, S.W.; Cheung, W.H. The imminent risk of a fracture-existing worldwide data: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2453–2466. [Google Scholar] [CrossRef]
- Johansson, H.; Siggeirsdottir, K.; Harvey, N.C.; Oden, A.; Gudnason, V.; McCloskey, E.; Sigurdsson, G.; Kanis, J.A. Imminent risk of fracture after fracture. Osteoporos. Int. 2017, 28, 775–780. [Google Scholar] [CrossRef]
- Wu, C.H.; Tu, S.T.; Chang, Y.F.; Chan, D.C.; Chien, J.T.; Lin, C.H.; Singh, S.; Dasari, M.; Chen, J.F.; Tsai, K.S. Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: A systematic literature review and meta-analysis. Bone 2018, 111, 92–100. [Google Scholar] [CrossRef]
- Genant, H.K.; Engelke, K.; Bolognese, M.A.; Mautalen, C.; Brown, J.P.; Recknor, C.; Goemaere, S.; Fuerst, T.; Yang, Y.C.; Grauer, A.; et al. Effects of Romosozumab Compared With Teriparatide on Bone Density and Mass at the Spine and Hip in Postmenopausal Women With Low Bone Mass. J. Bone Miner. Res. 2017, 32, 181–187. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Ferrari, S.; Khan, A.; Lane, N.E.; Lippuner, K.; Matsumoto, T.; Milmont, C.E.; Libanati, C.; Grauer, A. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J. Bone Miner. Res. 2018, 33, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Engelke, K.; Keaveny, T.M.; Chines, A.; Chapurlat, R.; Foldes, A.J.; Nogues, X.; Civitelli, R.; De Villiers, T.; Massari, F.; et al. Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: Results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial. J. Bone Miner. Res. 2021, 36, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; Bolognese, M.A.; Brown, J.P.; Reginster, J.Y.; Langdahl, B.L.; Shi, Y.; Timoshanko, J.; Libanati, C.; Chines, A.; Oates, M.K. Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus 2021, 5, e10512. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lin, H.; Zhu, X.; Chen, X.; Fan, L.; Liu, C. Zoledronic acid increases bone mineral density and improves health-related quality of life over two years of treatment in Chinese women with postmenopausal osteoporosis. Endokrynol. Pol. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Augoulea, A.; Tsakonas, E.; Triantafyllopoulos, I.; Rizos, D.; Armeni, E.; Tsoltos, N.; Tournis, S.; Deligeoroglou, E.; Antoniou, A.; Lambrinoudaki, I. Comparative effects of denosumab or bisphosphonate treatment on bone mineral density and calcium metabolism in postmenopausal women. J. Musculoskelet. Neuronal. Interact. 2017, 17, 444–449. [Google Scholar]
- Kendler, D.; Chines, A.; Clark, P.; Ebeling, P.R.; McClung, M.; Rhee, Y.; Huang, S.; Stad, R.K. Bone mineral density after transitioning from denosumab to alendronate. J. Clin. Endocrinol. Metab. 2020, 105, e255–e264. [Google Scholar] [CrossRef]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.Y.; Harris, A.G.; et al. Effect of Abaloparatide vs. Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef]
- McClung, M.R.; Harvey, N.C.; Fitzpatrick, L.A.; Miller, P.D.; Hattersley, G.; Wang, Y.; Cosman, F. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause 2018, 25, 767–771. [Google Scholar] [CrossRef]
- Saag, K.G.; Williams, S.A.; Wang, Y.; Weiss, R.J.; Cauley, J.A. Effect of Abaloparatide on Bone Mineral Density and Fracture Incidence in a Subset of Younger Postmenopausal Women with Osteoporosis at High Risk for Fracture. Clin. Ther. 2020, 42, 1099–1107. [Google Scholar] [CrossRef]
- Eastell, R.; Nickelsen, T.; Marin, F.; Barker, C.; Hadji, P.; Farrerons, J.; Audran, M.; Boonen, S.; Brixen, K.; Gomes, J.M. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: Final results of the randomized, controlled European Study of Forsteo (EUROFORS). J. Bone Miner. Res. 2009, 24, 726–736. [Google Scholar] [CrossRef]
- Shane, E.; Shiau, S.; Recker, R.R.; Lappe, J.M.; Agarwal, S.; Kamanda-Kosseh, M.; Bucovsky, M.; Stubby, J.; Cohen, A. Denosumab After Teriparatide in Premenopausal Women With Idiopathic Osteoporosis. J. Clin. Endocrinol. Metab. 2021, 107, e1528–e1540. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222–6230. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Murakami, H.; Takahashi, N.; Sasaki, T.; Udagawa, N.; Tanaka, S.; Nakamura, I.; Zhang, D.; Barbier, A.; Suda, T. A possible mechanism of the specific action of bisphosphonates on osteoclasts: Tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone 1995, 17, 137–144. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Cremers, S.C.; Pillai, G.; Papapoulos, S.E. Pharmacokinetics/pharmacodynamics of bisphosphonates: Use for optimisation of intermittent therapy for osteoporosis. Clin. Pharmacokinet 2005, 44, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.M.; Pfaar, U.; Schweitzer, A.; Wiegand, H.; Skerjanec, A.; Schran, H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 2008, 36, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, V.; Dicembrino, F.; Frusciante, V.; Chiodini, I.; Minisola, S.; Scillitani, A. Different patterns of global and regional skeletal uptake of 99mTc-methylene diphosphonate with age: Relevance to the pathogenesis of bone loss. J. Nucl. Med. 2000, 41, 1478–1483. [Google Scholar]
- Israel, O.; Front, D.; Hardoff, R.; Ish-Shalom, S.; Jerushalmi, J.; Kolodny, G.M. In vivo SPECT quantitation of bone metabolism in hyperparathyroidism and thyrotoxicosis. J. Nucl. Med. 1991, 32, 1157–1161. [Google Scholar]
- Boivin, G.Y.; Chavassieux, P.M.; Santora, A.C.; Yates, J.; Meunier, P.J. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000, 27, 687–694. [Google Scholar] [CrossRef]
- Seeman, E. Bone quality. Osteoporos. Int. 2003, 14, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj Fuleihan, G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Cenzer, I.; Nguyen, B.; Lee, S.J. Time to Benefit of Bisphosphonate Therapy for the Prevention of Fractures Among Postmenopausal Women With Osteoporosis: A Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 33–41. [Google Scholar] [CrossRef]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.E.; Cauley, J.A.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA 2006, 296, 2927–2938. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Bauer, D.C.; Cummings, S.R.; Cauley, J.A.; Ensrud, K.E.; Palermo, L.; Wallace, R.B.; Hochberg, M.C.; Feldstein, A.C.; Lombardi, A.; et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX trial. J. Bone Miner. Res. 2010, 25, 976–982. [Google Scholar] [CrossRef]
- Mellstrom, D.D.; Sorensen, O.H.; Goemaere, S.; Roux, C.; Johnson, T.D.; Chines, A.A. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif. Tissue Int. 2004, 75, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Reid, I.R.; Boonen, S.; Bucci-Rechtweg, C.; Cauley, J.A.; Cosman, F.; Cummings, S.R.; Hue, T.F.; Lippuner, K.; Lakatos, P.; et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Hosking, D.; Devogelaer, J.P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Chines, A.; Olszynski, W.P.; McKeever, C.D.; McClung, M.R.; Zhou, X.; Grauer, A. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos. Int. 2008, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.; Brown, K.; Cheung, A.; Kim, S.; Juurlink, D.; Cadarette, S.M. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study. Ann. Intern. Med. 2022, 175, 335–343. [Google Scholar] [CrossRef]
- Diez-Perez, A.; Naylor, K.E.; Abrahamsen, B.; Agnusdei, D.; Brandi, M.L.; Cooper, C.; Dennison, E.; Eriksen, E.F.; Gold, D.T.; Guañabens, N.; et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos. Int. 2017, 28, 767–774. [Google Scholar] [CrossRef]
- Fretes, N.; Vellios, E.; Sharma, A.; Ajiboye, R.M. Radiographic and functional outcomes of bisphosphonate use in lumbar fusion: A systematic review and meta-analysis of comparative studies. Eur. Spine J. 2020, 29, 272–281. [Google Scholar] [CrossRef]
- Tu, C.W.; Huang, K.F.; Hsu, H.T.; Li, H.Y.; Yang, S.S.; Chen, Y.C. Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J. Surg. Res. 2014, 192, 112–116. [Google Scholar] [CrossRef]
- Xue, D.; Li, F.; Chen, G.; Yan, S.; Pan, Z. Do bisphosphonates affect bone healing? A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2014, 9, 45. [Google Scholar] [CrossRef]
- Edwards, B.J.; Bunta, A.D.; Lane, J.; Odvina, C.; Rao, D.S.; Raisch, D.W.; McKoy, J.M.; Omar, I.; Belknap, S.M.; Garg, V.; et al. Bisphosphonates and nonhealing femoral fractures: Analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: A systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. J. Bone Joint Surg. Am. 2013, 95, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Nieves, J.W.; Dempster, D.W. Treatment Sequence Matters: Anabolic and Antiresorptive Therapy for Osteoporosis. J. Bone Miner. Res. 2017, 32, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.; Yang, Y.C.; Vittinghoff, E.; Adami, S.; Boonen, S.; Bauer, D.C.; Bianchi, G.; Bolognese, M.A.; Christiansen, C.; Eastell, R.; et al. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J. Bone Miner. Res. 2012, 27, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guanabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Makras, P.; Yavropoulou, M.P.; Tabacco, G.; Naciu, A.M.; Palermo, A. Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review. J. Clin. Med. 2021, 10, 152. [Google Scholar] [CrossRef]
- Aubry-Rozier, B.; Liebich, G.S.D. Can we avoid the loss of bone mineral density one year after denosumab discontinuation? The Reolaus bone project. In Proceedings of the European Congress of Rheumatology, EULAR 2019, Madrid, Spain, 12–15 June 2019. [Google Scholar]
- Kendler, D.L.; Roux, C.; Benhamou, C.L. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 2010, 25, 72–81. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwinski, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Makras, P.; Appelman-Dijkstra, N.M.; Papapoulos, S.E.; van Wissen, S.; Winter, E.M.; Polyzos, S.A.; Yavropoulou, M.P.; Anastasilakis, A.D. The Duration of Denosumab Treatment and the Efficacy of Zoledronate to Preserve Bone Mineral Density After Its Discontinuation. J. Clin. Endocrinol. Metab. 2021, 106, e4155–e4162. [Google Scholar] [CrossRef]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1940. [Google Scholar] [CrossRef]
- Adami, S.; Libanati, C.; Boonen, S.; Cummings, S.R.; Ho, P.R.; Wang, A.; Siris, E.; Lane, J. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: Results from the FREEDOM trial. JBJS 2012, 94, 2113–2119. [Google Scholar] [CrossRef]
- Rolighed, L.; Rejnmark, L.; Christiansen, P. Bone Involvement in Primary Hyperparathyroidism and Changes After Parathyroidectomy. Eur. Endocrinol. 2014, 10, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Dobnig, H.; Sipos, A.; Jiang, Y.; Fahrleitner-Pammer, A.; Ste-Marie, L.G.; Gallagher, J.C.; Pavo, I.; Wang, J.; Eriksen, E.F. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3970–3977. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsson, M.; Aspenberg, P. Abaloparatide versus teriparatide: A head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018, 89, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, J.J.; Mitlak, B.H.; Wang, O.; Genant, H.K.; Eriksen, E.F. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J. Bone Miner. Res. 2003, 18, 1932–1941. [Google Scholar] [CrossRef]
- Boyce, E.G.; Mai, Y.; Pham, C. Abaloparatide: Review of a Next-Generation Parathyroid Hormone Agonist. Ann. Pharmacother. 2018, 52, 462–472. [Google Scholar] [CrossRef]
- Choksi, P.; Jepsen, K.J.; Clines, G.A. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol. 2018, 4, 12. [Google Scholar] [CrossRef]
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 2016, 157, 141–149. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Pal, S.; Chattopadhyay, N. Abaloparatide, the second generation osteoanabolic drug: Molecular mechanisms underlying its advantages over the first-in-class teriparatide. Biochem. Pharmacol. 2019, 166, 185–191. [Google Scholar] [CrossRef]
- Carlson, B.C.; Robinson, W.A.; Wanderman, N.R.; Sebastian, A.S.; Nassr, A.; Freedman, B.A.; Anderson, P.A. A Review and Clinical Perspective of the Impact of Osteoporosis on the Spine. Geriatr. Orthop. Surg. Rehabil. 2019, 10. [Google Scholar] [CrossRef]
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Miyagi, M.; Suzuki, M.; et al. More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery. Asian Spine J. 2015, 9, 573–580. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Aizawa, T.; Sasaki, S.; Ando, S.; Maekawa, S.; Aonuma, H.; Tsuchie, H.; Sasaki, H.; Kasukawa, Y.; Shimada, Y. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: A comparison between treatment with and without teriparatide. J. Bone Miner. Metab. 2015, 33, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Inoue, G.; Ueno, M.; Nakazawa, T.; Imura, T.; Saito, W.; Uchida, K.; Ohtori, S.; Toyone, T.; Takahira, N.; Takaso, M. Teriparatide increases the insertional torque of pedicle screws during fusion surgery in patients with postmenopausal osteoporosis. J. Neurosurg. Spine 2014, 21, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; O’Dea, L.S.L.; Zanchetta, J.R.; Kumar, P.; Banks, K.; McKay, K.; Lyttle, C.R.; Hattersley, G. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 2015, 100, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.; Hattersley, G.; Fitzpatrick, L.; Harris, A.; Shevroja, E.; Banks, K.; Leder, B.; Zanchetta, J.; Hans, D. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): A 24-week randomized clinical trial. Osteoporos. Int. 2018, 29, 323–328. [Google Scholar] [CrossRef]
- Watts, N.; Hattersley, G.; Fitzpatrick, L.; Wang, Y.; Williams, G.; Miller, P.; Cosman, F. Abaloparatide effect on forearm bone mineral density and wrist fracture risk in postmenopausal women with osteoporosis. Osteoporos. Int. 2019, 30, 1187–1194. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Silverman, S.; Fujiwara, S.; Saag, K.; Napoli, N.; Soen, S.; Enomoto, H.; Melby, T.E.; Disch, D.P.; Marin, F.; et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone 2018, 116, 58–66. [Google Scholar] [CrossRef]
- Cosman, F.; Hattersley, G.; Hu, M.y.; Williams, G.C.; Fitzpatrick, L.A.; Black, D.M. Effects of Abaloparatide-SC on Fractures and Bone Mineral Density in Subgroups of Postmenopausal Women With Osteoporosis and Varying Baseline Risk Factors. J. Bone Miner. Res. 2017, 32, 17–23. [Google Scholar] [CrossRef]
- Miller, P.D.; Lewiecki, E.M.; Krohn, K.; Schwartz, E. Teriparatide: Label changes and identifying patients for long-term use. Cleve Clin. J. Med. 2021, 88, 489–493. [Google Scholar] [CrossRef]
- BioSpace. Radius Announces Update on TYMLOS® (Abaloparatide) Label. 2021. Available online: https://www.biospace.com/article/releases/radius-announces-update-on-tymlos-abaloparatide-label (accessed on 25 September 2022).
- Cosman, F.; Nieves, J.; Woelfert, L.; Formica, C.; Gordon, S.; Shen, V.; Lindsay, R. Parathyroid hormone added to established hormone therapy: Effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J. Bone Miner. Res. 2001, 16, 925–931. [Google Scholar] [CrossRef]
- Cohen, A.; Kamanda-Kosseh, M.; Recker, R.R.; Lappe, J.M.; Dempster, D.W.; Zhou, H.; Cremers, S.; Bucovsky, M.; Stubby, J.; Shane, E. Bone Density After Teriparatide Discontinuation in Premenopausal Idiopathic Osteoporosis. J. Clin. Endocrinol. Metab. 2015, 100, 4208–4214. [Google Scholar] [CrossRef]
- Shah, A.D.; Shoback, D.; Lewiecki, E.M. Sclerostin inhibition: A novel therapeutic approach in the treatment of osteoporosis. Int. J. Womens Health 2015, 7, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Bolster, M.B. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des. Devel. Ther. 2017, 11, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Chapurlat, R.; Portero-Muzy, N.; Roux, J.P.; Garcia, P.; Brown, J.P.; Libanati, C.; Boyce, R.W.; Wang, A.; Grauer, A. Bone-Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women With Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment. J. Bone Miner. Res. 2019, 34, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Ferrari, S.; Lewiecki, E.M.; Jaller-Raad, J.; Zerbini, C.; Milmont, C.E.; Meisner, P.D.; Libanati, C.; Grauer, A. Romosozumab FRAME study: A post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J. Bone Miner. Res. 2018, 33, 1407–1416. [Google Scholar] [CrossRef]
- Tian, A.; Jia, H.; Zhu, S.; Lu, B.; Li, Y.; Ma, J.; Ma, X. Romosozumab versus Teriparatide for the Treatment of Postmenopausal Osteoporosis: A Systematic Review and Meta-analysis through a Grade Analysis of Evidence. Orthop. Surg. 2021, 13, 1941–1950. [Google Scholar] [CrossRef]
- Schemitsch, E.H.; Miclau, T.; Karachalios, T.; Nowak, L.L.; Sancheti, P.; Poolman, R.W.; Caminis, J.; Daizadeh, N.; Dent-Acosta, R.E.; Egbuna, O.; et al. A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures. J. Bone Joint Surg. Am. 2020, 102, 693–702. [Google Scholar] [CrossRef]
- Yoshiki, F.; Nishikawa, A.; Taketsuna, M.; Kajimoto, K.; Enomoto, H. Efficacy and safety of teriparatide in bisphosphonate-pretreated and treatment-naive patients with osteoporosis at high risk of fracture: Post hoc analysis of a prospective observational study. J. Orthop. Sci. 2017, 22, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Wallace, P.M.; Lee, H.; Neer, R.M.; Burnett-Bowie, S.A. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): Extension of a randomised controlled trial. Lancet 2015, 386, 1147–1155. [Google Scholar] [CrossRef]
- Kendler, D.; Bone, H.; Massari, F.; Gielen, E.; Palacios, S.; Maddox, J.; Yan, C.; Yue, S.; Dinavahi, R.; Libanati, C. Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos. Int. 2019, 30, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Papapoulos, S.E.; Polyzos, S.A.; Appelman-Dijkstra, N.M.; Makras, P. Zoledronate for the Prevention of Bone Loss in Women Discontinuing Denosumab Treatment. A Prospective 2-Year Clinical Trial. J. Bone Miner. Res. 2019, 34, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Dinavahi, R.V.; Lazaretti-Castro, M.; Ebeling, P.R.; Adachi, J.D.; Miyauchi, A.; Gielen, E.; Milmont, C.E.; Libanati, C.; Grauer, A. One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: Results of the FRAME Extension Study. J. Bone Miner. Res. 2019, 34, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Niimi, R.; Kono, T.; Nishihara, A.; Hasegawa, M.; Kono, T.; Sudo, A. Efficacy of Switching From Teriparatide to Bisphosphonate or Denosumab: A Prospective, Randomized, Open-Label Trial. JBMR Plus 2018, 2, 289–294. [Google Scholar] [CrossRef]
- Markiewicz, M.R.; Margarone, J.E.R.; Campbell, J.H.; Aguirre, A. Bisphosphonate-associated osteonecrosis of the jaws: A review of current knowledge. J. Am. Dent. Assoc. 2005, 136, 1669–1674. [Google Scholar] [CrossRef][Green Version]
- Bagan, J.V.; Jimenez, Y.; Murillo, J.; Hernandez, S.; Poveda, R.; Sanchis, J.M.; Diaz, J.M.; Scully, C. Jaw osteonecrosis associated with bisphosphonates: Multiple exposed areas and its relationship to teeth extractions. Study of 20 cases. Oral Oncol. 2006, 42, 327–329. [Google Scholar] [CrossRef]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J. Oral Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Boquete-Castro, A.; Gomez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants Res. 2016, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T. Antiresorptive Agent-Related Osteonecrosis of the Jaw (ARONJ): A Twist of Fate in the Bone. Tohoku J. Exp. Med. 2019, 247, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Grbic, J.T.; Black, D.M.; Lyles, K.W.; Reid, D.M.; Orwoll, E.; McClung, M.; Bucci-Rechtweg, C.; Su, G. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: Data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J. Am. Dent. Assoc. 2010, 141, 1365–1370. [Google Scholar] [CrossRef]
- Everts-Graber, J.; Lehmann, D.; Burkard, J.P.; Schaller, B.; Gahl, B.; Hauselmann, H.; Studer, U.; Ziswiler, H.R.; Reichenbach, S.; Lehmann, T. Risk of Osteonecrosis of the Jaw Under Denosumab Compared to Bisphosphonates in Patients With Osteoporosis. J. Bone Miner. Res. 2022, 37, 340–348. [Google Scholar] [CrossRef]
- Sim, I.W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Papatziamos Hjelle, C.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef]
- Schilcher, J.; Aspenberg, P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop. 2009, 80, 413–415. [Google Scholar] [CrossRef]
- Bauer, D.C. Atypical Femoral Fracture Risk in Patients Treated With Bisphosphonates: Comment on “Increasing Occurrence of Atypical Femoral Fractures Associated With Bisphosphonate Use”. Arch. Intern. Med. 2012, 172, 936–937. [Google Scholar] [CrossRef]
- Dell, R.M.; Adams, A.L.; Greene, D.F.; Funahashi, T.T.; Silverman, S.L.; Eisemon, E.O.; Zhou, H.; Burchette, R.J.; Ott, S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012, 27, 2544–2550. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, J.; Sandberg, O.; Isaksson, H.; Aspenberg, P. Histology of 8 atypical femoral fractures: Remodeling but no healing. Acta Orthop. 2014, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Xia, Z.; Dunford, J.E.; Oppermann, U.; Kwaasi, A.; Hulley, P.A.; Kavanagh, K.L.; Triffitt, J.T.; Lundy, M.W.; Phipps, R.J. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 2007, 1117, 209–257. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Spevak, L.; Weinstein, R. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos. Int. 2009, 20, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, A.; Rajapakse, C.S.; Ural, A. Assessment of the multifactorial causes of atypical femoral fractures using a novel multiscale finite element approach. Bone 2020, 135, 115318. [Google Scholar] [CrossRef]
- Donnelly, E.; Saleh, A.; Unnanuntana, A.; Lane, J.M. Atypical femoral fractures: Epidemiology, etiology, and patient management. Curr. Opin. Support. Palliat. Care 2012, 6, 348–354. [Google Scholar] [CrossRef]
- Oh, Y.; Yamamoto, K.; Hashimoto, J.; Fujita, K.; Yoshii, T.; Fukushima, K.; Kurosa, Y.; Wakabayashi, Y.; Kitagawa, M.; Okawa, A. Biological activity is not suppressed in mid-shaft stress fracture of the bowed femoral shaft unlike in “typical” atypical subtrochanteric femoral fracture: A proposed theory of atypical femoral fracture subtypes. Bone 2020, 137, 115453. [Google Scholar] [CrossRef]
- Hirano, F.; Okuma, K.F.; Zenke, Y.; Menuki, K.; Ohnishi, H.; Fukuda, F.; Sakai, A.; Yamamoto, N.; Shimakura, T.; Sano, H.; et al. Disturbance of osteonal bone remodeling and high tensile stresses on the lateral cortex in atypical femoral fracture after long-term treatment with Risedronate and Alfacalcidol for osteoporosis. Bone Rep. 2021, 14, 101091. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.P.; Delaisse, J.M. Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 2021, 145, 115850. [Google Scholar] [CrossRef]
- Giusti, A.; Hamdy, N.A.; Papapoulos, S.E. Atypical fractures of the femur and bisphosphonate therapy: A systematic review of case/case series studies. Bone 2010, 47, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- Van de Laarschot, D.M.; McKenna, M.J.; Abrahamsen, B.; Langdahl, B.; Cohen-Solal, M.; Guañabens, N.; Eastell, R.; Ralston, S.H.; Zillikens, M.C. Medical management of patients after atypical femur fractures: A systematic review and recommendations from the European Calcified Tissue Society. J. Clin. Endocrinol. Metab. 2020, 105, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
| Therapy | ROI | Change vs. Baseline, % | Treatment, Months | Patient Population | Ref. |
|---|---|---|---|---|---|
| Teriparatide | LS | 10.04 ± 5.23% | 12 | Japanese W and men | [34] |
| Teriparatide | LS | 6.9% | 12 | Postmenopausal W, 55–85 years | [115] |
| Teriparatide | FN | 2.01 ± 4.63% | 12 | Japanese W and men | [34] |
| Teriparatide | TH | 2.72 ± 4.04% | 12 | Japanese W and men | [34] |
| Teriparatide | TH | 0.8% | 12 | Postmenopausal W, 55–85 years | [115] |
| Romosozumab (FRAME) | LS | BMD increase: 96% of patients ≥ 3% 89% > 6%, 68% ≥ 10%, | 12 | Postmenopausal W | [116] |
| Romosozumab (ARCH) | LS | BMD increase: 14.7% | 12 | Postmenopausal W | [117] |
| Romosozumab | LS | 9.1% | 12 | Postmenopausal W, 55–85 years | [118] |
| Romosozumab | LS | 12.3% | 12 | Postmenopausal W, 55–85 years | [115] |
| Romosozumab (FRAME) | TH/FN | BMD increase: 78% of patients ≥ 3%, 47% > 6%, 16% ≥10% | 12 | Postmenopausal W | [116] |
| Romosozumab | FN | 3.9% | 12 | Postmenopausal W, 55–85 yo | [118] |
| Romosozumab | TH | 4.6% | 12 | Postmenopausal W, 55–85 yo | [118] |
| Romosozumab | TH | 3.9% | 12 | Postmenopausal W, 55–85 yo | [115] |
| Zoledronic acid | LS | 3.93 ± 0.34% | 12 | Chinese Postmenopausal W | [119] |
| Zoledronic acid | FN | 2.69 ± 0.46% | 12 | Chinese Postmenopausal W | [119] |
| Zoledronic acid | TH | 2.81 ± 0.32% | 12 | Chinese Postmenopausal W | [119] |
| Alendronate (ARCH) | LS | 4.4% | 12 | Postmenopausal W | [117] |
| Alendronate or Zoledronic acid | LS | 4.5% ± 11.6 | At least 12 | Postmenopausal W, 53–66 years | [120] |
| Alendronate or Zoledronic acid | FN | 3.8% ± 7.3 | At least 12 | Postmenopausal W, 53–66 years | [120] |
| Denosumab | LS | 5.4% | 12 | Postmenopausal W, >55 yeras | [121] |
| Denosumab | LS | 9.03% ± 11.3 | At least 12 | Postmenopausal W, 53–66 years | [120] |
| Denosumab | TH | 3.1% | 12 | Postmenopausal W, >55 years | [121] |
| Denosumab | FN | 2.7% | 12 | Postmenopausal W, >55 years | [121] |
| Denosumab | FN | 8.7% ± 8.5 | At least 12 | Postmenopausal W, 53–66 years | [120] |
| Abaloparatide (ACTIVE) | LS | 11.2% | 18 | Postmenopausal W, 49–86 years | [122] |
| Abaloparatide (ACTIVE) | LS | 12.1% | 18 | Postmenopausal W, >80 years | [123] |
| Abaloparatide (ACTIVE) | LS | 7.81% | 18 | Postmenopausal W, <65 years | [124] |
| Abaloparatide (ACTIVE) | FN | 3.6% | 18 | Postmenopausal W, 49–86 years | [122] |
| Abaloparatide (ACTIVE) | FN | 3.6% | 18 | Postmenopausal W, >80 years | [123] |
| Abaloparatide (ACTIVE) | FN | 2.71% | 18 | Postmenopausal W, <65 years | [124] |
| Abaloparatide (ACTIVE) | TH | 4.18% | 18 | Postmenopausal W, 49–86 years | [122] |
| Abaloparatide (ACTIVE) | TH | 3.9% | 18 | Postmenopausal W, >80 years | [123] |
| Abaloparatide (ACTIVE) | TH | 3.2% | 18 | Postmenopausal W, <65 years | [124] |
| Therapy | ROI | Change vs. Baseline, % | Treatment, Months | Patient Population | Ref. |
|---|---|---|---|---|---|
| Teriparatide | LS | 13.42 ± 6.12% | 24 | Japanese W and men | [34] |
| Teriparatide | LS | 10.70% | 24 | Postmenopausal W | [125] |
| Teriparatide | LS | 14.2 ± 8.1 | 24; 2 patients −18 | Premenopausal W | [126] |
| Teriparatide | FN | 3.26 ± 4.25% | 24 | Japanese W and men | [34] |
| Teriparatide | FN | 3.50% | 24 | Postmenopausal W | [125] |
| Teriparatide | FN | 5.1 ± 5.2% | 24; 2 patients −18 | Premenopausal W | [126] |
| Teriparatide | TH | 3.67 ± 3.98% | 24 | Japanese W and men | [34] |
| Teriparatide | TH | 2.50% | 24 | Postmenopausal W | [125] |
| Teriparatide | TH | 5.3 ± 4.3% | 24; 2 patients −18 | Premenopausal W | [126] |
| Zoledronic acid | LS | 5.71 ± 0.35% | 24 | Chinese Postmenopausal W | [119] |
| Zoledronic acid | FN | 3.36 ± 0.60% | 24 | Chinese Postmenopausal W | [119] |
| Zoledronic acid | TH | 3.7 ± 0.46% | 24 | Chinese Postmenopausal W | [119] |
| Alendronate (ARCH) | LS | 7.40% | 24 | Postmenopausal W | [117] |
| Denosumab | LS | 9.20% | 36 | Postmenopausal W, 60–90 years | [127] |
| Denosumab | TH | 6.00% | 36 | Postmenopausal W, 60–90 years | [127] |
| Initial Drug | Second Drug | Effect on BMD | Reference |
|---|---|---|---|
| BPs | TPTD | BP-pretreated vs. BP-naïve patients started on teriparatide: The greatest mean increase in BMD: LS: BP-naïve: 15.46% (11.60–19.31%) at 18 mo BP-pretreated 11.20% (8.56–13.85%) at 24 mo FN: BP-naive, 5.16% [2.32–8.00%] at 24 mo BP-pretreated: 2.22% [0.72–3.72%] at 24 mo TH: BP-naive group: BMD decreased at 6 mo (NS), then increased significantly at 12, 18, and 24 mo; BP-pretreated group: BMD decreased slightly from baseline at 6, 12, and 18 mo, then increased from baseline at 24 mo (NS). The greatest increase observed in the BP-naive group: 4.46% [0.98–7.94%] at 24 mo | [198] |
| ALN for at least 6 mo | Dmab 12 mo | Switch to Dmab for 12 mo: vs. continued ALN: LS: 3.03% vs. 1.85% (p < 0.05) TH: 1.9% vs. 1.05% (NS) FN and 1/3 radius: significantly higher BMD for Dmab | [162] |
| Oral BP at least 3 yr and ALN 1 yr | Rmab 12 mo TPTD 12 mo | Effect of Rmab vs. TPTD for 12 mo after BPs: LS: 9.8% vs. 5.4% (p < 0.05) TH: 2.6% vs. −0.6% (p < 0.05) FN: 3.2% vs. −0.2% (p < 0.05) | [199] |
| Dmab 24 mo DATA-Switch study | TPTD 24 mo | Postmenopausal women 24 months of teriparatide + 24 months of Dmab: LS: decreased over first 6 mo followed by mean net 48-month increase of 14.0 ± 6.7% Increase in Dmab only: 4.8 ± 5.6% TH: progressively decreased between 24–36 mo Change after transitioning: −0.7 ± 3.1, FN: transient bone loss occurring between 24–36 mo, net 48-month increase of 4.9 ± 6.0% Change after transitioning: 1.2 ± 4.9% 1/3 forearm: net 48 mo decrease of −1.8 ± 5.9% | [200] |
| Dmab 12 mo | Rmab 12 mo | LS +11.5% TH +3.8% FN +3.2% | [118] |
| Dmab 12 mo | Rmab 12 mo (Second course) | LS +2.3% (95% CI 0.3, 4.4) TH −0.1% (95% CI −1.2, 0.9) FN +0.8% (95% CI −0.3, 2.0) | [201] |
| Dmab 12 mo DAPS study | BPs (ALN) 12 mo | 24 mo BMD change (Dmab 12 mo + ALN 12 mo): LS +5.9% TH +3.6% FN +2.5% BMD gain in ALN only LS +0.5% TH + 0.5% FN −0.2% | [121] |
| Dmab 12 mo | ZOL 1 dose 12 and 24 mo | LS 1.7% ± 1.1% at 12 mo LS 0.1% ± 1.2% at 24 mo | [202] |
| Rmab 12 mo ARCH study | BPs (ALN) 12 mo | LS: net 24-month increase of 17% | [117] |
| Rmab 12 mo FRAME Extension study | Dmab 12 mo and 24 mo | Differences in BMD increases from baseline Rmab-to-Dmab vs. placebo-to-Dmab 12 and 24 mo LS: 11.8% and 10.5% TH: 5.3% and 5.2% FN: 4.9% and 4.8% BMD after Rmab → 12 and 24 mo on Dmab LS: 13.1% → 16.6% → 18.1% TH:6% → 8.5% → 9.4% FN: 5.5% → 7.3% → 8.2% | [203] |
| TPTD 24 mo DATA-Switch study | Dmab 24 mo | Postmenopausal women 24 months of teriparatide + 24-month of Dmab: LS: net 48-month increase of 18.3 ± 8.5%, Change after transitioning: +8.6 ± 5.0% TH: net 48-month increase of 6.6 ± 3.3% Change after transitioning: +4.7 ± 2.6% FN: net 48-month increase of 8.3 ± 5.6% Change after transitioning: 5.6 ± 4.5% 1/3 forearm: net 48 mo decrease of 0.0 ± 2.9% | [200] |
| TPTD 24 mo | Dmab 12 and 24 mo | Premenopausal women with IOP 24 months of teriparatide + 24 months of Dmab: BMD increased by: LS: 21.9 ± 7.8% TH: 9.8 ± 4.6% FN: 9.5 ± 4.7% BMD increase after 12 months and 24 mo of Dmab after Teriparatide for 24 mo: LS: 5.2 ± 2.6% and 6.9 ± 2.6%, TH: 2.9 ± 2.4% and 4.6 ± 2.8% FN: 3.0 ± 3.8% and 4.7 ± 4.9% | [126] |
| TPTD 12 mo EUROFORS | Raloxifene 12 mo | BMD change after 24 mo of Raloxifene after Teriparatide for 12 mo: LS: no change TH: 2.3% FN: 3.1% | [125] |
| TPTD 24 mo | ALN or Dmab | ALN, 12 mo: LS: +1.3 ± 5.1% FN: +0.7 ± 4.6% Dmab, 12 mo: LS: +4.3 ± 3.5% FN: +1.4 ± 3.4% | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushchayeva, Y.; Pestun, I.; Kushchayev, S.; Radzikhovska, N.; Lewiecki, E.M. Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing. J. Clin. Med. 2022, 11, 7477. https://doi.org/10.3390/jcm11247477
Kushchayeva Y, Pestun I, Kushchayev S, Radzikhovska N, Lewiecki EM. Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing. Journal of Clinical Medicine. 2022; 11(24):7477. https://doi.org/10.3390/jcm11247477
Chicago/Turabian StyleKushchayeva, Yevgeniya, Iryna Pestun, Sergiy Kushchayev, Nataliia Radzikhovska, and E. Michael Lewiecki. 2022. "Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing" Journal of Clinical Medicine 11, no. 24: 7477. https://doi.org/10.3390/jcm11247477
APA StyleKushchayeva, Y., Pestun, I., Kushchayev, S., Radzikhovska, N., & Lewiecki, E. M. (2022). Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing. Journal of Clinical Medicine, 11(24), 7477. https://doi.org/10.3390/jcm11247477





