Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review
Abstract
1. Introduction
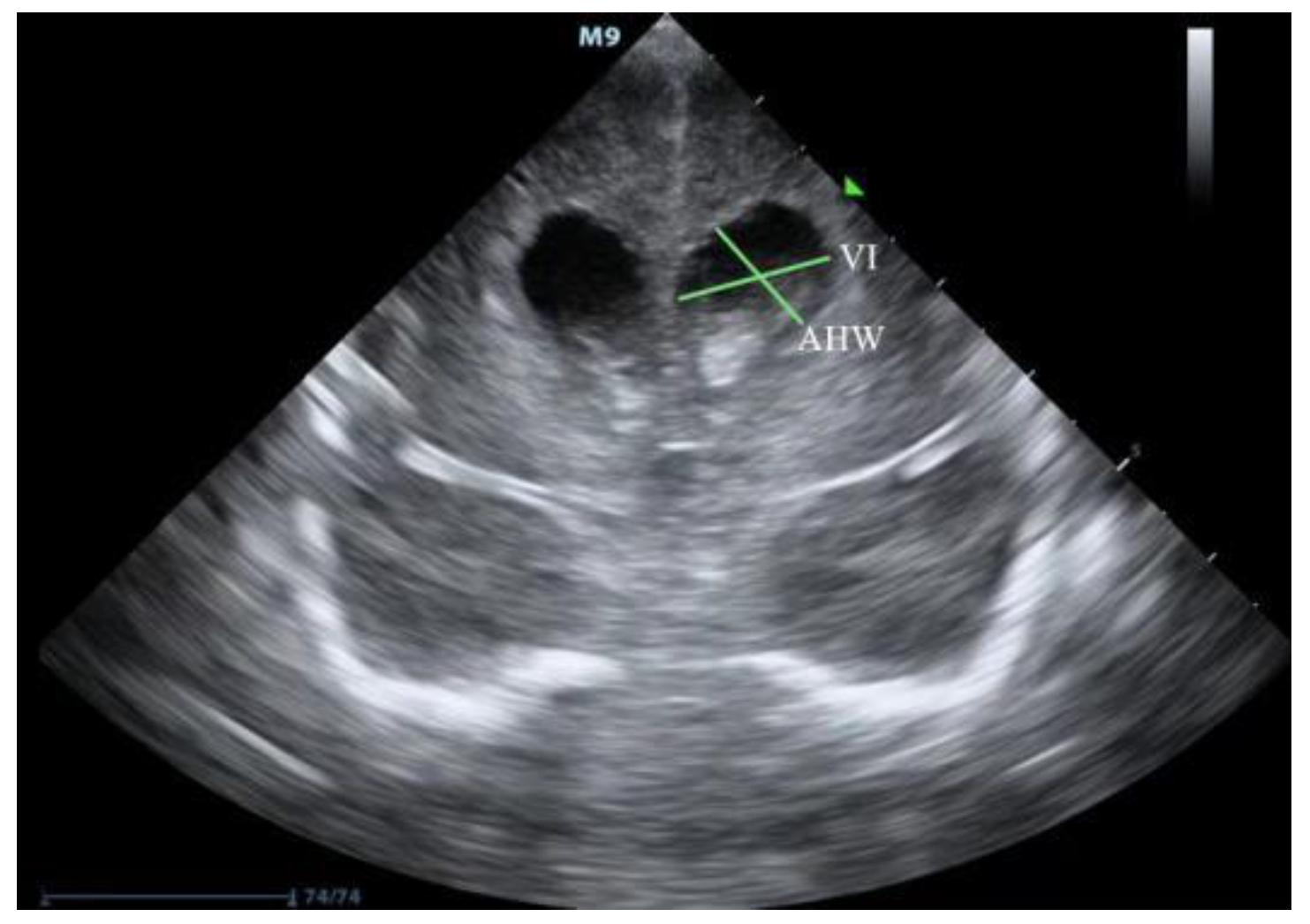
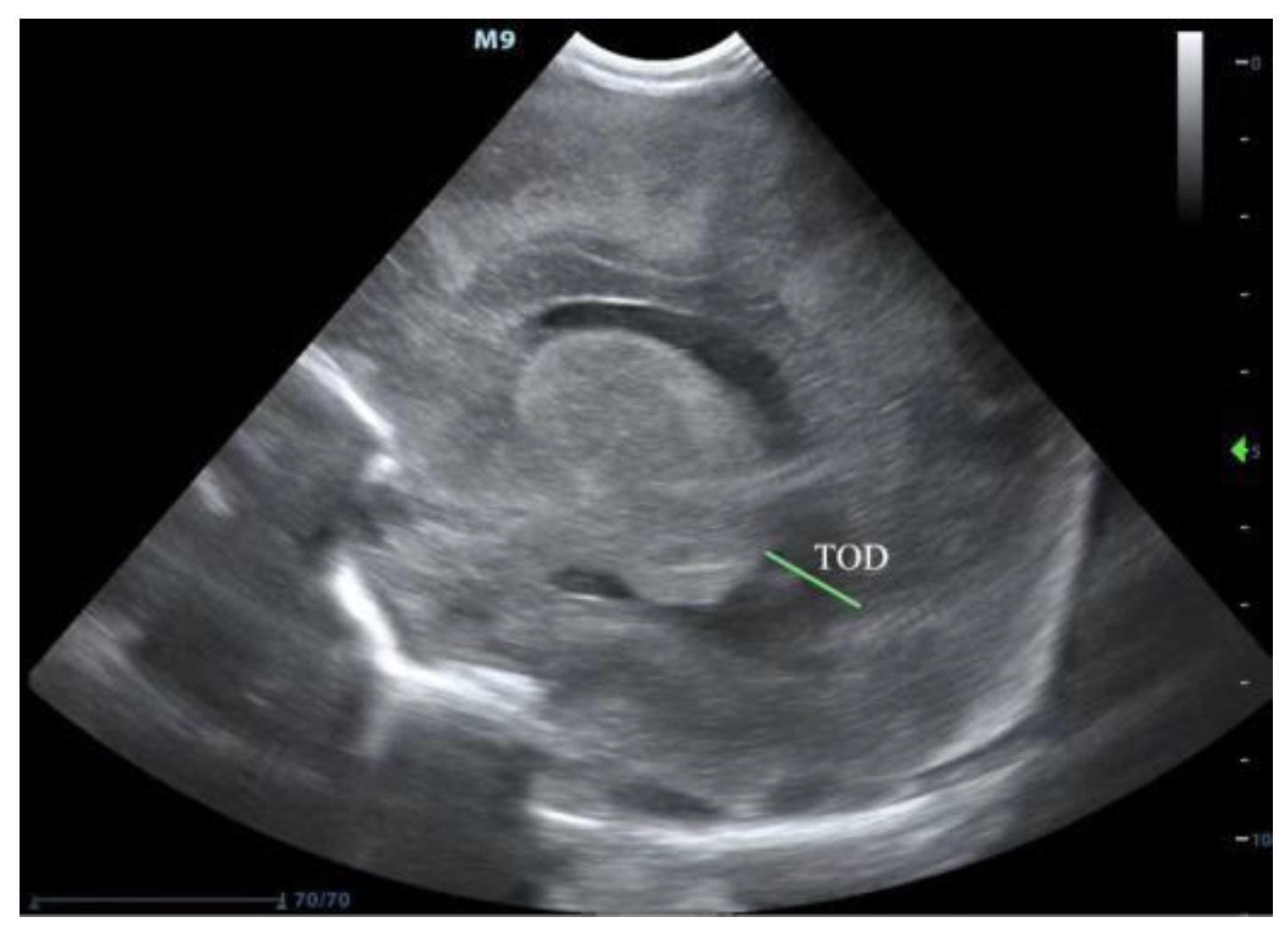
2. Ultrasound Diagnosis of PHVD
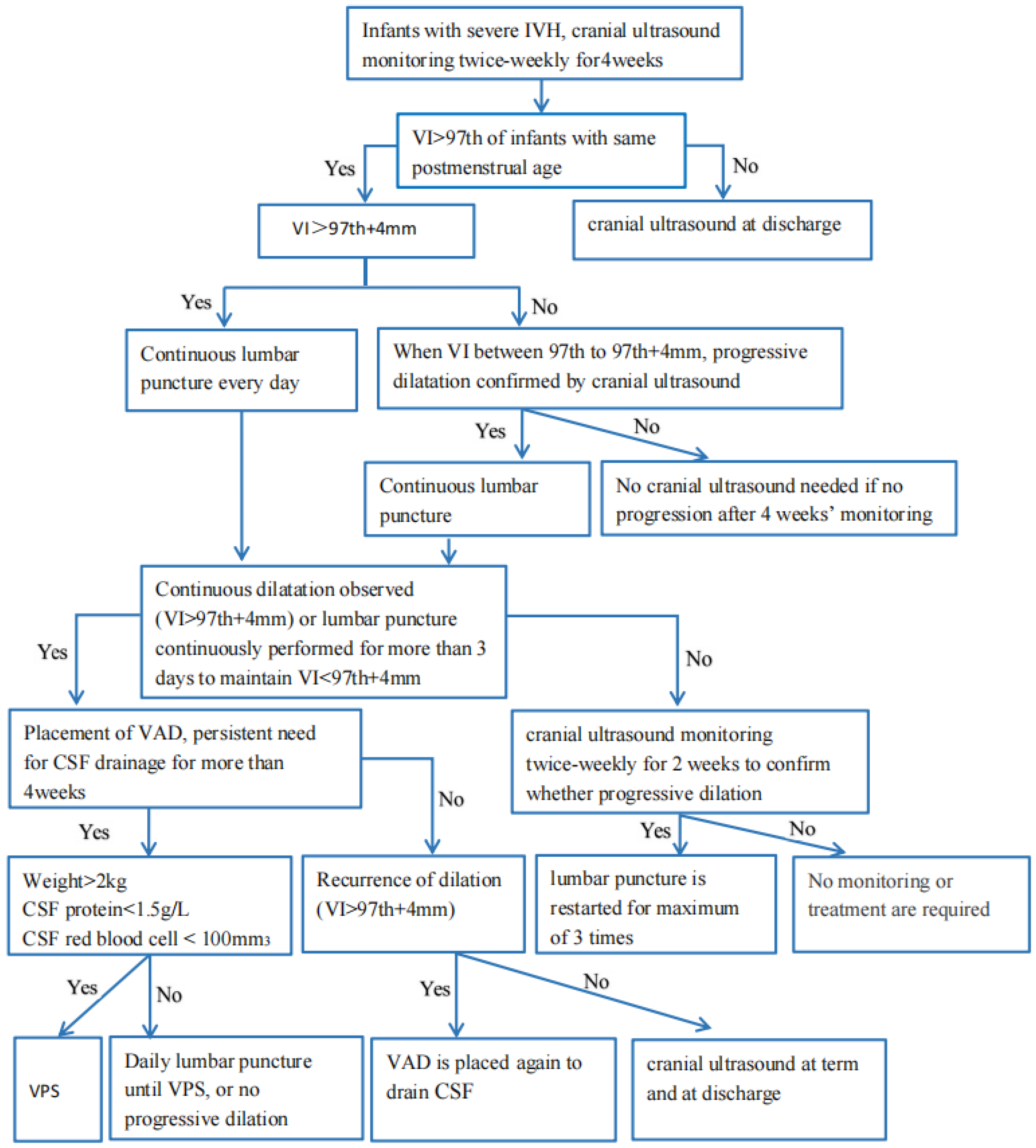
3. Treatment of PHVD
3.1. Continuous Lumbar Puncture
3.2. Drainage Intervention Fibrinolytic Therapy (DRIFT)
3.3. Ventricular Access Device (VAD)
3.4. Ventriculosubgaleal (VSG)
3.5. Ventriculoperitoneal Shunt (VPS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpe, J.J. Intraventricular hemorrhage in the premature infant-current concepts. Part II. Ann. Neurol. 1989, 25, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Intraventricular hemorrhage in the premature infant-current concepts. Part I. Ann. Neurol. 1989, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ramenghi, L.A. Germinal Matrix—Intraventricular Haemorrhage: Still a very important brain lesion in premature infants! J. Matern. Neonatal Med. 2015, 28 (Suppl. S1), 2259–2260. [Google Scholar] [CrossRef]
- Holwerda, J.C.; Van Braeckel, K.N.; Roze, E.; Hoving, E.W.; Maathuis, C.G.; Brouwer, O.F.; Martijn, A.; Bos, A.F. Functional outcome at school age of neonatal post-hemorrhagic ventricular dilatation. Early Hum. Dev. 2016, 96, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Leijser, L.M.; Miller, S.P.; van Wezel-Meijler, G.; Brouwer, A.J.; Traubici, J.; van Haastert, I.C.; Whyte, H.E.; Groenendaal, F.; Kulkarni, A.V.; Han, K.S.; et al. Posthemorrhagic ventricular dilatation in preterm infants: When best to intervene. Neurology 2018, 90, e698–e706. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.S.; Groenendaal, F.; Liem, K.D.; Heep, A.; Brouwer, A.J.; van ‘t Verlaat, E.; Benavente-Fernández, I.; van Straaten, H.L.; van Wezel-Meijler, G.; Smit, B.J.; et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F70–F75. [Google Scholar] [CrossRef] [PubMed]
- Leijser, L.M.; de Vries, L.S. Preterm brain injury: Germinal matrix-intraventricular hemorrhage and post-hemorrhagic ventricular dilatation. Handb. Clin. Neurol. 2019, 162, 173–199. [Google Scholar]
- Shankaran, S.; Bajaj, M.; Natarajan, G.; Saha, S.; Pappas, A.; Davis, A.S.; Hintz, S.R.; Adams-Chapman, I.; Das, A.; Bell, E.F.; et al. Outcomes Following Post-Hemorrhagic Ventricular Dilatation among Infants of Extremely Low Gestational Age. J. Pediatr. 2020, 226, 36–44.e3. [Google Scholar] [CrossRef]
- Levene, M.I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 1981, 56, 900–904. [Google Scholar] [CrossRef]
- Brouwer, A.; Groenendaal, F.; van Haastert, I.-L.; Rademaker, K.; Hanlo, P.; de Vries, L. Neurodevelopmental Outcome of Preterm Infants with Severe Intraventricular Hemorrhage and Therapy for Post-Hemorrhagic Ventricular Dilatation. J. Pediatr. 2008, 152, 648–654. [Google Scholar] [CrossRef]
- Leijser, L.; Srinivasan, L.; Rutherford, M.; Counsell, S.; Allsop, J.M.; Cowan, F.M. Structural linear measurements in the newborn brain: Accuracy of cranial ultrasound compared to MRI. Pediatr. Radiol. 2007, 37, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Christian, E.A.; Jin, D.L.; Attenello, F.; Wen, T.; Cen, S.; Mack, W.J.; Krieger, M.D.; McComb, J.G. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J. Neurosurg. Pediatr. 2016, 17, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, A.; Evans, D.; Carter, M.; Thoresen, M.; Wroblewska, J.; Mandera, M.; Swietlinski, J.; Simpson, J.; Hajivassiliou, C.; Hunt, L.P.; et al. Randomized Clinical Trial of Prevention of Hydrocephalus After Intraventricular Hemorrhage in Preterm Infants: Brain-Washing Versus Tapping Fluid. Pediatrics 2007, 119, e1071–e1078. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, C.A.; Choudhri, A.F.; Auguste, K.I.; Limbrick, D.D., Jr.; Rogido, M.; Mitchell, L.; Flannery, A.M. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J. Neurosurg. Pediatr. 2014, 14 (Suppl. S1), 8–23. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, M.; Limbrick, D.D., Jr.; Inder, T.; Whitelaw, A.; Kulkarni, A.V.; Warf, B.; Volpe, J.J.; de Vries, L.S. Management of Post-hemorrhagic Ventricular Dilatation in the Infant Born Preterm. J. Pediatr. 2020, 226, 16–27.e3. [Google Scholar] [CrossRef]
- Maunu, J.; Parkkola, R.; Rikalainen, H.; Lehtonen, L.; Haataja, L.; Lapinleimu, H. Brain and Ventricles in Very Low Birth Weight Infants at Term: A Comparison Among Head Circumference, Ultrasound, and Magnetic Resonance Imaging. Pediatrics 2009, 123, 617–626. [Google Scholar] [CrossRef]
- Korobkin, R. The relationship between head circumference and the development of communicating hydrocephalus in infants following intraventricular hemmorrhage. Pediatrics 1975, 56, 74–77. [Google Scholar] [CrossRef]
- Whitelaw, A.; Lee-Kelland, R. Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage. Cochrane Database Syst. Rev. 2017, 2017, CD000216. [Google Scholar] [CrossRef]
- Whitelaw, A.; Pople, I.; Cherian, S.; Evans, D.; Thoresen, M. Phase 1 Trial of Prevention of Hydrocephalus After Intraventricular Hemorrhage in Newborn Infants by Drainage, Irrigation, and Fibrinolytic Therapy. Pediatrics 2003, 111, 759–765. [Google Scholar] [CrossRef]
- Whitelaw, A.; Jary, S.; Kmita, G.; Wroblewska, J.; Musialik-Swietlinska, E.; Mandera, M.; Hunt, L.; Carter, M.; Pople, I. Randomized Trial of Drainage, Irrigation and Fibrinolytic Therapy for Premature Infants with Posthemorrhagic Ventricular Dilatation: Developmental Outcome at 2 years. Pediatrics 2010, 125, e852–e858. [Google Scholar] [CrossRef]
- Luyt, K.; Jary, S.L.; Lea, C.L.; Young, G.J.; E Odd, D.; E Miller, H.; Kmita, G.; Williams, C.; Blair, P.; Hollingworth, W.; et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 466–473. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kotani, Y.; Kim, T.K.; Yokota, H.; Sugimoto, T.; Nakagawa, I.; Motoyama, Y.; Nakase, H. Efficacy and safety of intraventricular fibrinolytic therapy for post-intraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: A preliminary clinical study. Child’s Nerv. Syst. 2021, 37, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, P.V.; Rosales, P.H.; Hernández, D.G.Q.; Naranjo, E.A.C.; Navarro, V.G. Intraventricular hemorrhage and posthemorrhagic hydrocephalus in preterm infants: Diagnosis, classification, and treatment options. Child’s Nerv. Syst. 2019, 35, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-N.; Kong, X.-Y.; Han, T.-Y.; Chen, Y.; Huang, J.-J.; Feng, Z.-C. Therapeutic effect of Ommaya reservoir implantation on hydrocephalus in premature infants following intraventricular hemorrhage and factors associted with the therapeutic effect. Zhongguo Dang Dai Er Ke Za Zhi 2013, 15, 327–331. [Google Scholar] [PubMed]
- Zubair, A.; De Jesus, O. Ommaya Reservoir; StatPearls: Treasure Island, FL, USA, 2022.
- Peretta, P.; Ragazzi, P.; Carlino, C.F.; Gaglini, P.; Cinalli, G. The role of Ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Child’s Nerv. Syst. 2007, 23, 765–771. [Google Scholar] [CrossRef]
- Lin, Z.-L.; Yu, B.; Liang, Z.-Q.; Chen, X.-W.; Liu, J.-Q.; Chen, S.-Q.; Zhang, Z.-Y.; Zhang, N. Role of Ommaya reservoir in the management of neonates with post-hemorrhagic hydrocephalus. Zhonghua Er Ke Za Zhi 2009, 47, 140–145. [Google Scholar]
- Richard, E.; Cinalli, G.; Assis, D.; Pierre-Kahn, A.; Lacaze-Masmonteil, T. Treatment of post-haemorrhage ventricular dilatation with an Ommaya’s reservoir: Management and outcome of 64 preterm infants. Childs Nerv. Syst. 2001, 17, 334–340. [Google Scholar]
- Eid, S.; Iwanaga, J.; Oskouian, R.J.; Loukas, M.; Oakes, W.J.; Tubbs, R.S. Ventriculosubgaleal shunting—A comprehensive review and over two-decade surgical experience. Child’s Nerv. Syst. 2018, 34, 1639–1642. [Google Scholar] [CrossRef]
- Sil, K.; Ghosh, S.K.; Chatterjee, S. Ventriculo-subgaleal shunts—Broadening the horizons: An institutional experience. Child’s Nerv. Syst. 2021, 37, 1113–1119. [Google Scholar] [CrossRef]
- Wellons, J.C.; Shannon, C.N.; Kulkarni, A.V.; Simon, T.D.; Riva-Cambrin, J.; Whitehead, W.E.; Oakes, W.J.; Drake, J.M.; Luerssen, T.G.; Walker, M.L.; et al. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J. Neurosurg. Pediatr. 2009, 4, 50–55. [Google Scholar] [CrossRef]
- Kutty, R.; Sreemathyamma, S.; Korde, P.; Prabhakar, R.; Peethambaran, A.; Libu, G. Outcome of Ventriculosubgaleal shunt in the management of infectious and non-infectious Hydrocephalus in pre-term infants. J. Pediatr. Neurosci. 2018, 13, 322–328. [Google Scholar] [CrossRef]
- Fountain, D.M.; Chari, A.; Allen, D.; James, G. Comparison of the use of ventricular access devices and ventriculosubgaleal shunts in posthaemorrhagic hydrocephalus: Systematic review and meta-analysis. Child’s Nerv. Syst. 2016, 32, 259–267. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, S.; Harischandra, L. Cerebrospinal fluid shunts—How they work: The basics. Neurol. India 2018, 66, 24. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Riva-Cambrin, J.; Rozzelle, C.J.; Naftel, R.P.; Alvey, J.S.; Reeder, R.W.; Holubkov, R.; Browd, S.R.; Cochrane, D.D.; Limbrick, D.D.; et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: A prospective study by the Hydrocephalus Clinical Research Network. J. Neurosurg. Pediatr. 2018, 21, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells Prevent Hydrocephalus After Severe Intraventricular Hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Im, G.H.; Choi, S.J.; Park, W.S. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS ONE 2015, 10, e0132919. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, S.I.; Park, W.S. Mesenchymal Stem Cells for Severe Intraventricular Hemorrhage in Preterm Infants: Phase I Dose-Escalation Clinical Trial. STEM CELLS Transl. Med. 2018, 7, 847–856. [Google Scholar] [CrossRef]
- Strahle, J.M.; Garton, T.; Bazzi, A.A.; Kilaru, H.; Garton, H.J.; Maher, C.O.; Muraszko, K.M.; Keep, R.F.; Xi, G. Role of Hemoglobin and Iron in Hydrocephalus After Neonatal Intraventricular Hemorrhage. Neurosurgery 2014, 75, 696–705. [Google Scholar] [CrossRef]
- Fowler, J.B.; De Jesus, O.; Mesfin, F.B. Ventriculoperitoneal Shunt; StatPearls: Treasure Island, FL, USA, 2022.
- Reddy, G.K.; Bollam, P.; Shi, R.; Guthikonda, B.; Nanda, A. Management of Adult Hydrocephalus with Ventriculoperitoneal Shunts: Long-term Single-Institution Experience. Neurosurgery 2011, 69, 774–780. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Hong, C.J.; Nassiri, F.; Hong, B.Y.; Riva-Cambrin, J.; Kulkarni, A.V. Treatment of posthemorrhagic ventricular dilation in preterm infants: A systematic review and meta-analysis of outcomes and complications. J. Neurosurg. Pediatr. 2015, 16, 545–555. [Google Scholar] [CrossRef]
- Brouwer, A.J.; Brouwer, M.J.; Groenendaal, F.; Benders, M.J.; Whitelaw, A.; De Vries, L.S. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F50–F55. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; Hutchison, A.; Bundy, A.; Machin, J.; Thieme, G.; Stahlman, M.; James, A. Serial sonography of posthemorrhagic ventricular dilatation and porencephaly after intracranial hemorrhage in the preterm neonate. Am. J. Roentgenol. 1983, 141, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.G.; Peter, J.C. Advantages of delayed VP shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Child’s Nerv. Syst. 2001, 17, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, D.H.; Vachhrajani, S.; Bohnstedt, B.N.; Patel, N.B.; Patel, A.J.; Fox, B.D.; Jea, A.; Boaz, J.C. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J. Neurosurg. Pediatr. 2011, 7, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Emami-Naeini, P.; Nejat, F.; El Khashab, M. Incidence of hydrocephalus and the need to ventriculoperitoneal shunting in premature infants with intraventricular hemorrhage: Risk factors and outcome. Child’s Nerv. Syst. 2011, 27, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Kestle, J.R.; Riva-Cambrin, J.; Wellons, J.C., 3rd; Kulkarni, A.V.; Whitehead, W.E.; Walker, M.L.; Oakes, W.J.; Drake, J.M.; Luerssen, T.G.; Simon, T.D.; et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: The Hydrocephalus Clinical Research Network Quality Improvement Initiative. J. Neurosurg. Pediatr. 2011, 8, 22–29. [Google Scholar] [CrossRef]
- Limbrick, D.D., Jr.; Mathur, A.; Johnston, J.M.; Munro, R.; Sagar, J.; Inder, T.; Park, T.S.; Leonard, J.L.; Smyth, M.D. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: A 10-year single-institution study. J. Neurosurg. Pediatr. 2010, 6, 224–230. [Google Scholar] [CrossRef]
- Mwachaka, P.M.; Obonyo, N.G.; Mutiso, B.K.; Ranketi, S.; Mwang’Ombe, N. Ventriculoperitoneal Shunt Complications: A Three-Year Retrospective Study in a Kenyan National Teaching and Referral Hospital. Pediatr. Neurosurg. 2010, 46, 1–5. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Nie, C. Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review. J. Clin. Med. 2022, 11, 7468. https://doi.org/10.3390/jcm11247468
Liu G, Nie C. Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review. Journal of Clinical Medicine. 2022; 11(24):7468. https://doi.org/10.3390/jcm11247468
Chicago/Turabian StyleLiu, Gengying, and Chuan Nie. 2022. "Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review" Journal of Clinical Medicine 11, no. 24: 7468. https://doi.org/10.3390/jcm11247468
APA StyleLiu, G., & Nie, C. (2022). Ultrasonic Diagnosis and Management of Posthemorrhagic Ventricular Dilatation in Premature Infants: A Narrative Review. Journal of Clinical Medicine, 11(24), 7468. https://doi.org/10.3390/jcm11247468





