Radiation Exposure during Fluoroscopy-Guided Ozone Chemonucleolysis for Lumbar Disc Herniation
Abstract
1. Introduction
2. Material and Methods
2.1. Radiation Exposure
2.2. Clinical and Demographic Data
3. Statistical Analysis
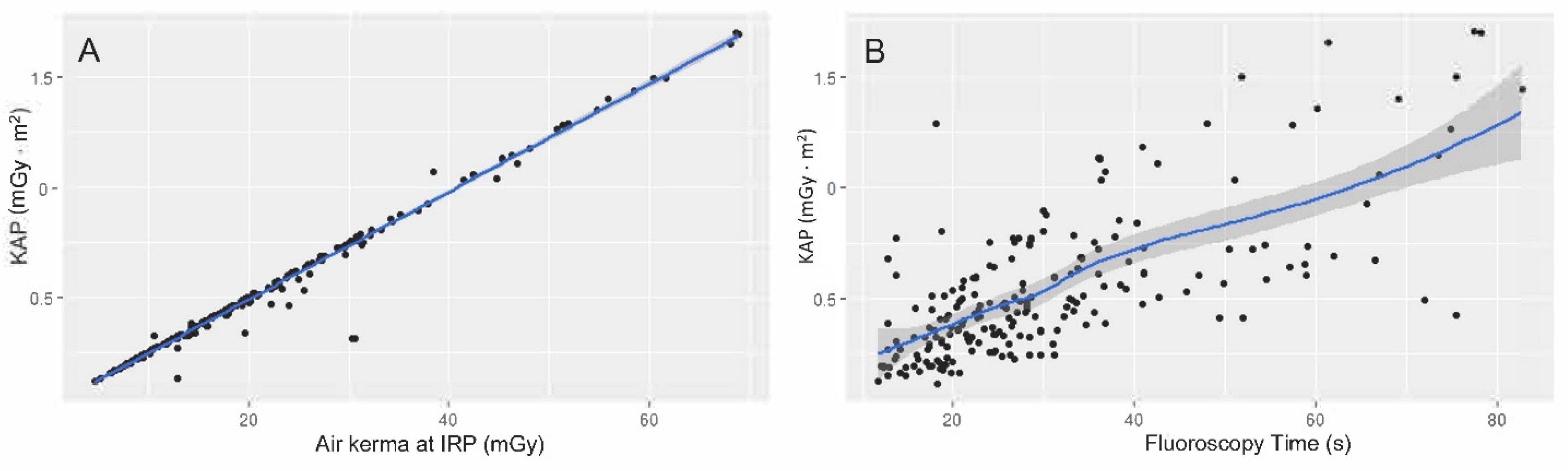
4. Results
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, R.M.; Andrade, N.S.; Neuman, B.J. Lumbar Disc Herniation. Curr. Rev. Musculoskelet. Med. 2017, 10, 507–516. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Steinbach, G.C.; Watenpaugh, D.E.; Hargens, A.R. Lumbar spine disc height and curvature responses to an axial load generated by a compression device compatible with magnetic resonance imaging. Spine 2001, 26, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [PubMed]
- Manchikanti, L.; Hirsch, J.A. Clinical management of radicular pain. Expert. Rev. Neurother. 2015, 15, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, P.A.; Verhagen, A.P.; Ostelo, R.W.; van Os, T.A.; Peul, W.C.; Koes, B.W. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: A systematic review. Eur. Spine J. 2007, 16, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V.; Jr LeQuang, J.A. Rehabilitation for Low Back Pain: A Narrative Review for Managing Pain and Improving Function in Acute and Chronic Conditions. Pain Ther. 2020, 9, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Alrwaily, M.; Timko, M.; Schneider, M.; Stevans, J.; Bise, C.; Hariharan, K.; Delitto, A. Treatment-Based Classification System for Low Back Pain: Revision and Update. Phys. Ther. 2016, 96, 1057–1066. [Google Scholar] [CrossRef]
- Ong, D.; Chua, N.H.; Vissers, K. Percutaneous Disc Decompression for Lumbar Radicular Pain: A Review Article. Pain Pract. 2016, 16, 111–126. [Google Scholar] [CrossRef]
- Buric, J.; Rigobello, L.; Hooper, D. Five and ten year follow-up on intradiscal ozone injection for disc herniation. Int. J. Spine Surg. 2014, 8, 17. [Google Scholar] [CrossRef]
- Narain, A.S.; Hijji, F.Y.; Yom, K.H.; Kudaravalli, K.T.; Haws, B.E.; Singh, K. Radiation exposure and reduction in the operating room: Perspectives and future directions in spine surgery. World J. Orthop. 2017, 8, 524–530. [Google Scholar] [CrossRef]
- Srinivasan, D.; Than, K.D.; Wang, A.C.; la Marca, F.; Wang, P.I.; Schermerhorn, T.C.; Park, P. Radiation safety and spine surgery: Systematic review of exposure limits and methods to minimize radiation exposure. World Neurosurg. 2014, 82, 1337–1343. [Google Scholar] [CrossRef]
- Chartier, H.; Fassier, P.; Leuraud, K.; Jacob, S.; Baudin, C.; Laurier, D.; Bernier, M. Occupational low-dose irradiation and cancer risk among medical radiation workers. Occup. Med. 2020, 70, 476–484. [Google Scholar] [CrossRef]
- Mulconrey, D.S. Fluoroscopic Radiation Exposure in Spinal Surgery: In Vivo Evaluation for Operating Room Personnel. Clin. Spine Surg. 2016, 29, E331–E335. [Google Scholar] [CrossRef]
- Miller, D.C.; Patel, J.; Smith, C.C.; Spine Intervention Society’s Patient Safety Comittee. Fact Finders for Patient Safety: Radiation Safety for Interventional Spine Procedures. Pain Med. 2018, 19, 629–630. [Google Scholar] [CrossRef]
- Kim, T.W.; Jung, J.H.; Jeon, H.J.; Yoon, K.B.; Yoon, D.M. Radiation exposure to physicians during interventional pain procedures. Korean J. Pain 2010, 23, 24–27. [Google Scholar] [CrossRef]
- Leoni, M.L.G.; Caruso, A.; Micheli, F. Factors Predicting Successful Outcome for Ozone Chemonucleolysis in Lumbar Disk Herniation. Pain Pract. 2021, 21, 653–661. [Google Scholar] [CrossRef]
- Skripochnik, E.; Loh, S.A. Fluoroscopy time is not accurate as a surrogate for radiation exposure. Vascular 2017, 25, 466–471. [Google Scholar] [CrossRef]
- Frobin, W.; Brinckmann, P.; Biggemann, M.; Tillotson, M.; Burton, K. Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin. Biomech. 1997, 12 (Suppl. S1), S1–S63. [Google Scholar] [CrossRef]
- Smuck, M.; Levin, J.; Zemper, E.; Ali, A.; Kennedy, D.J. A quantitative study of intervertebral disc morphologic changes following plasma-mediated percutaneous discectomy. Pain Med. 2014, 15, 1695–1703. [Google Scholar] [CrossRef][Green Version]
- Van Domelen, D.R.; Mitchell, E.M.; Perkins, N.J.; Schisterman, E.F.; Manatunga, A.K.; Huang, Y.; Lyles, R.H. Gamma models for estimating the odds ratio for a skewed biomarker measured in pools and subject to errors. Biostatistics 2021, 22, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Botwin, K.P.; Fuoco, G.S.; Torres, F.M.; Gruber, R.D.; Bouchlas, C.C.; Castellanos, R.; Rao, S. Radiation exposure to the spinal interventionalist performing lumbar discography. Pain Physician 2003, 6, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Min Young Lee, J.K.; Ryu, G.W.; Kim, K.H.; Nam, H.W.; Kim, K.P. Review of National Diagnostic Reference Levels for Interventional Procedures. Prog. Med. Phys. 2019, 30, 75–88. [Google Scholar]
- Musielak, B.; Kubicka, A.M.; Rychlik, M.; Czubak, J.; Czwojdziński, A.; Grzegorzewski, A.; Jóźwiak, M. Variation in pelvic shape and size in Eastern European males: A computed tomography comparative study. PeerJ 2019, 7, e6433. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.F.; Sparrey, C.J.; Been, E.; Kramer, P.A. Morphological and postural sexual dimorphism of the lumbar spine facilitates greater lordosis in females. J. Anat. 2016, 229, 82–91. [Google Scholar] [CrossRef]
- Gilsanz, V.; Boechat, M.I.; Gilsanz, R.; Loro, M.L.; Roe, T.F.; Goodman, W.G. Gender differences in vertebral sizes in adults: Biomechanical implications. Radiology 1994, 190, 678–682. [Google Scholar] [CrossRef]
- Smuck, M.; Zheng, P.; Chong, T.; Kao, M.C.; Geisser, M.E. Duration of fluoroscopic-guided spine interventions and radiation exposure is increased in overweight patients. PM&R 2013, 5, 291–296. [Google Scholar]
- Kukreja, S.; Haydel, J.; Nanda, A.; Sin, A.H. Impact of body habitus on fluoroscopic radiation emission during minimally invasive spine surgery. J. Neurosurg. Spine 2015, 22, 211–218. [Google Scholar] [CrossRef]
- Bindal, R.K.; Glaze, S.; Ognoskie, M.; Tunner, V.; Malone, R.; Ghosh, S. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J. Neurosurg. Spine 2008, 9, 570–573. [Google Scholar] [CrossRef]
- Iprenburg, M.; Wagner, R.; Godschalx, A.; Telfeian, A.E. Patient radiation exposure during transforaminal lumbar endoscopic spine surgery: A prospective study. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Ebraheim, N.A.; Karkare, N.; Liu, J.; Xu, R.; Werner, C.M. Angled posteroanterior fluoroscopy for L5-S1 discography: A technical note. Am. J. Orthop. 2007, 36, 380–381. [Google Scholar]
- Nie, H.F.; Zeng, J.C.; Liu, K.X. A simple technique of accessing the L5-S1 disc space for transforaminal endoscopic spine surgery. Surg. Technol. Int. 2012, 22, 302–306. [Google Scholar]
- Kumar, N.; Agorastides, I.D. The curved needle technique for accessing the L5/S1 disc space. Br. J. Radiol. 2000, 73, 655–657. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef]
- Pye, S.R.; Reid, D.M.; Lunt, M.; Adams, J.E.; Silman, A.J.; O’Neill, T.W. Lumbar disc degeneration: Association between osteophytes, end-plate sclerosis and disc space narrowing. Ann. Rheum. Dis. 2007, 66, 330–333. [Google Scholar] [CrossRef]
- Somma, F.; D’Agostino, V.; Negro, A.; Piscitelli, V.; Tamburrini, S.; Sicignano, C.; Fasano, F.; Peluso, S.; Villa, A.; Pace, G.; et al. Radiation exposure and clinical outcome in patients undergoing percutaneous intradiscal ozone therapy for disc herniation: Fluoroscopic versus conventional CT guidance. PLoS ONE 2022, 17, e0264767. [Google Scholar] [CrossRef]
- Choi, M.H.; Choi, B.G.; Jung, S.E.; Byun, J.Y. Factors Related to Radiation Exposure during Lumbar Spine Intervention. J. Korean Med. Sci. 2016, 31 (Suppl. S1), S55–S58. [Google Scholar] [CrossRef]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. In Annals of the ICRP; ICRP: Ottawa, ON, Canada, 2007; Volume 37, pp. 1–332. [Google Scholar]
- Malik, A.T.; Rai, H.H.; Lakdawala, R.H.; Noordin, S. Does surgeon experience influence the amount of radiation exposure during orthopedic procedures? A systematic review. Orthop. Rev. 2019, 11, 7667. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, N.; Abdi, S.; Wu, J.; Crawford, J.; Furgang, F.A. Fluoroscopy radiation safety for spine interventional pain procedures in university teaching hospitals. Pain Physician 2005, 8, 49–53. [Google Scholar]
- Hanu-Cernat, D.E.; Duarte, R.; Raphael, J.H.; Mutagi, H.; Kapur, S.; Senthil, L. Type of interventional pain procedure, body weight, and presence of spinal pathology are determinants of the level of radiation exposure for fluoroscopically guided pain procedures. Pain Pract. 2012, 12, 434–439. [Google Scholar] [CrossRef]
| Variable | Total OCN (n = 240) no. (%) |
|---|---|
| Age, mean ± SD | 53.9 ± 14.4 |
| Sex | |
| Male | 154 (64.2%) |
| Female | 86 (35.8%) |
| BMI | 25.7 (23.8–28.4) |
| <18.5 kg/m2 | 8 (3.3%) |
| 18.5–24.95 kg/m2 | 96 (40%) |
| 25–29.9 kg/m2 | 92 (38.3%) |
| >30 kg/m2 | 44 (18.4%) |
| Level of herniated disc | |
| L1–L2 | 4 (1.7%) |
| L2–L3 | 11 (4.6%) |
| L3–L4 | 24 (10%) |
| L4–L5 | 85 (35.4%) |
| L5–S1 | 116 (48.3%) |
| Disc height | |
| Type A (≤50% reduction) | 118 (49.2%) |
| Type B (>50% reduction) | 122 (50.8%) |
| Side of disc herniation | |
| Left | 129 (53.8%) |
| Right | 111 (46.2%) |
| KAP (mGy · m2), median (IQR) | 0.46 (0.33–0.68) |
| Time(s), median (IQR) | 26.3 (19.4–35.9) |
| Dose at IRP (mGy), median (IQR) | 19.3 (13.2–27.3) |
| Level of Herniated Disc | Fluoroscopy Time (S) Median (IQR) | KAP (mGy m2) Median (IQR) | Air Kerma at IRP (mGy) Median (IQR) |
|---|---|---|---|
| L1–L2 | 24.6 (21.9–30.2) | 0.37 (0.29–0.44) | 17.8 (15.3–24.2) |
| L2–L3 | 25.3 (19.7–32.5) | 0.38 (0.17–0.49) | 14.3 (9.6–19.6) |
| L3–L4 | 18.6 (17.4–30.3) | 0.25 (0.20–0.32) | 10 (8–13.2) |
| L4–L5 | 21.4 (17.1–26.2) | 0.43 (0.30–0.60) | 17.8 (12–24.2) |
| L5–S1 | 30.9 (25.4–41) | 0.54 (0.42–0.75) | 22.5 (17.1–30.5) |
| Level of Herniated Disc | β | OR (95% CI) | p Value |
|---|---|---|---|
| Intercept | −1.66 | 0.19 (0.13–0.28) | 0 |
| Age | 0.003 | 1.003 (0.99–1.007) | 0.07 |
| Gender (male) | 0.137 | 1.15 (1.03–1.28) | 0.01 |
| BMI | |||
| <18.5 kg/m2 | −0.06 | 0.94 (0.86–1.04) | 0.27 |
| 18.5–24.95 kg/m2 | Ref. | - | - |
| 25–29.9 kg/m2 | 0.04 | 1.04 (0.99–1.09) | 0.10 |
| >30 kg/m2 | 0.14 | 1.14 (1.05–1.25) | 0.002 |
| Level of herniated disc | |||
| L1–L2 | −0.40 | 1.49 (0.42–1.06) | 0.09 |
| L2–L3 | −0.29 | 0.75 (0.58–0.96) | 0.02 |
| L3–L4 | −0.46 | 0.63 (0.53–0.75) | <0.001 |
| L4–L5 | −0.29 | 0.75 (0.67–0.84) | <0.001 |
| L5–S1 | Ref. | - | - |
| Disc height (type B) | 0.58 | 1.79 (1.60–2.00) | <0.001 |
| Side of disc herniation (right) | −0.0063 | 0.99 (0.90–1.01) | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoni, M.L.G.; Vitali, S.; Micheli, F.; Mercieri, M.; Varrassi, G.; Casale, R.; Occhigrossi, F.; Giordano, C. Radiation Exposure during Fluoroscopy-Guided Ozone Chemonucleolysis for Lumbar Disc Herniation. J. Clin. Med. 2022, 11, 7424. https://doi.org/10.3390/jcm11247424
Leoni MLG, Vitali S, Micheli F, Mercieri M, Varrassi G, Casale R, Occhigrossi F, Giordano C. Radiation Exposure during Fluoroscopy-Guided Ozone Chemonucleolysis for Lumbar Disc Herniation. Journal of Clinical Medicine. 2022; 11(24):7424. https://doi.org/10.3390/jcm11247424
Chicago/Turabian StyleLeoni, Matteo Luigi Giuseppe, Sara Vitali, Fabrizio Micheli, Marco Mercieri, Giustino Varrassi, Roberto Casale, Felice Occhigrossi, and Carlo Giordano. 2022. "Radiation Exposure during Fluoroscopy-Guided Ozone Chemonucleolysis for Lumbar Disc Herniation" Journal of Clinical Medicine 11, no. 24: 7424. https://doi.org/10.3390/jcm11247424
APA StyleLeoni, M. L. G., Vitali, S., Micheli, F., Mercieri, M., Varrassi, G., Casale, R., Occhigrossi, F., & Giordano, C. (2022). Radiation Exposure during Fluoroscopy-Guided Ozone Chemonucleolysis for Lumbar Disc Herniation. Journal of Clinical Medicine, 11(24), 7424. https://doi.org/10.3390/jcm11247424







