Prevalence and Persistence of Symptoms in Adult COVID-19 Survivors 3 and 18 Months after Discharge from Hospital or Corona Hotels
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures
3. Outcomes of the Study
4. Statistical Analysis
5. Results
6. Baseline Characteristics
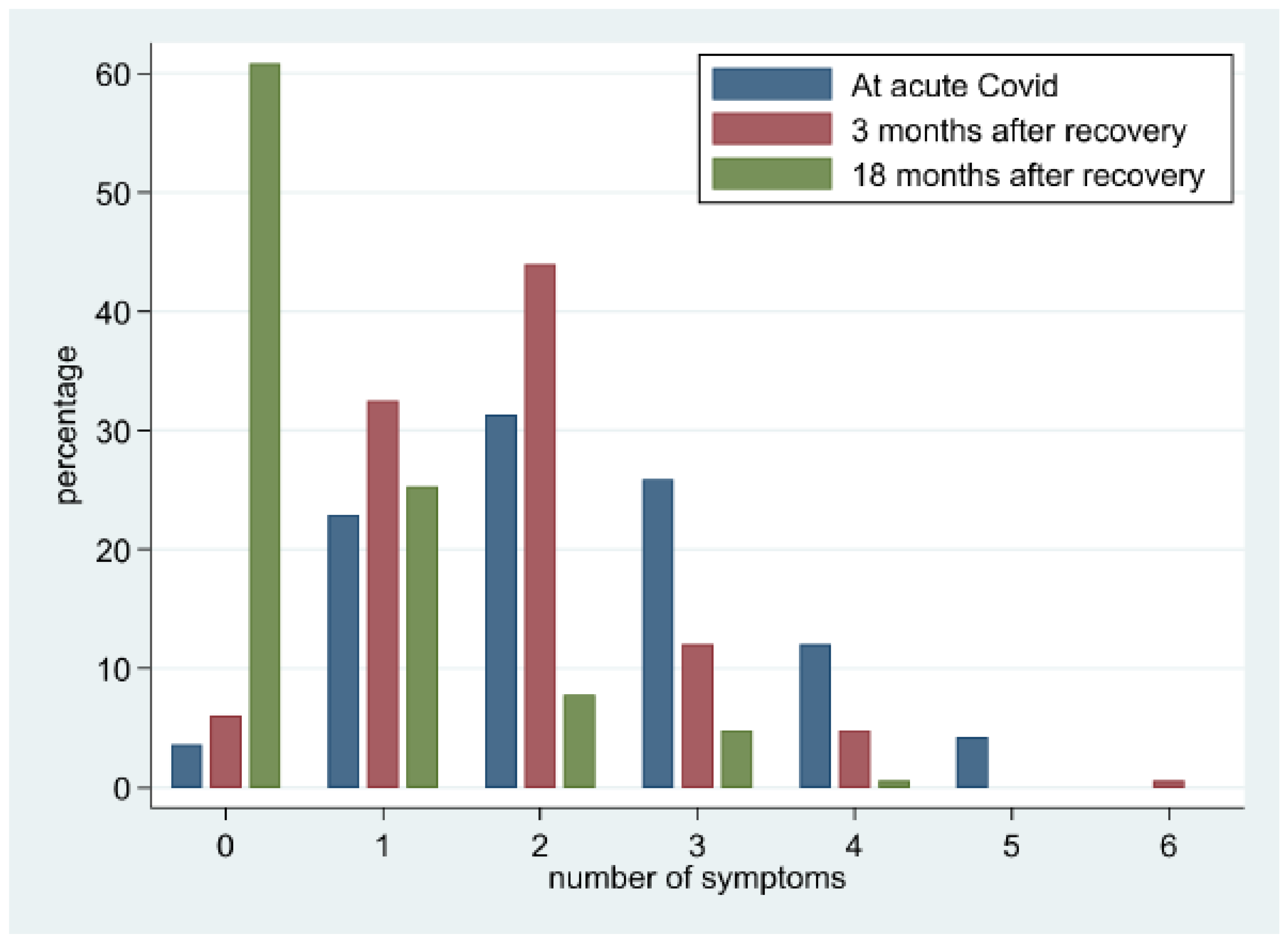
7. Symptom Evaluation
7.1. Predictors of the Number of Symptoms
7.2. Symptoms and Health-Related Quality of Life
7.3. Predictors of Selected Symptoms
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms among Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- The Writing Committee for the COMEBAC Study Group. Four-Month Clinical Status of a Cohort of Patients after Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Herck, M.V.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent Symptoms 3 Months after a SARS-CoV-2 Infection: The Post-COVID-19 Syndrome? ERJ Open Res. 2020, 6, 542–2020. [Google Scholar] [CrossRef]
- Ghosn, J.; Piroth, L.; Epaulard, O.; Le Turnier, P.; Mentré, F.; Bachelet, D.; Laouénan, C. Persistent COVID-19 Symptoms Are Highly Prevalent 6 Months after Hospitalization: Results from a Large Prospective Cohort. Clin. Microbiol. Infect. 2021, 27, 1041.e1. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, Y.; Song, W.; Li, Q.; Xie, H.; Xu, Q.; Jia, J.; Li, L.; Mao, H.; Zhou, X.; et al. Follow-up Study of the Pulmonary Function and Related Physiological Characteristics of COVID-19 Survivors Three Months after Recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Patel, A.; Jernigan, D.B.; Abdirizak, F.; Abedi, G.; Aggarwal, S.; Albina, D.; Allen, E.; Andersen, L.; Anderson, J.; Anderson, M.; et al. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak—United States, December 31, 2019–February 4, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 140–146. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E.; Rachel Lu, J.F.; Sherbourne, C.D. The MOS 36-Ltem Short-Form Health Survey (SF-36): III. Tests of Data Quality, Scaling Assumptions, and Reliability across Diverse Patient Groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- Tuty Kuswardhani, R.A.; Henrina, J.; Pranata, R.; Anthonius Lim, M.; Lawrensia, S.; Suastika, K. Charlson Comorbidity Index and a Composite of Poor Outcomes in COVID-19 Patients: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2103–2109. [Google Scholar] [CrossRef]
- Austin, S.R.; Wong, Y.-N.; Uzzo, R.G.; Beck, J.R.; Egleston, B.L. Why Summary Comorbidity Measures such as the Charlson Comorbidity Index and Elixhauser Score Work. Med. Care 2015, 53, e65–e72. [Google Scholar] [CrossRef]
- NIH. Clinical Spectrum. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 7 May 2022).
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of Sex and Gender on COVID-19 Outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; KC, R.; Dua, K.; Patel, R.P.; Peterson, G.M.; O’Toole, R.F. Smoking and COVID-19: What We Know so Far. Respir. Med. 2021, 176, 106237. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of Post-COVID-19 Symptoms in Hospitalized and Non-Hospitalized COVID-19 Survivors: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients One Year after COVID-19: A Prospective Cohort Study. Clin. Infect. Dis. 2021, 74, 1191–1198. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Non-Critical COVID-19 Two Months after Symptoms’ Onset. Clin. Microbiol. Infect. 2020, 27, 258–263. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID Syndrome in Non-Hospitalised Patients with COVID-19: A Longitudinal Prospective Cohort Study. Lancet Reg. Health-Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Pellicer-Valero, O.J.; Navarro-Pardo, E.; Palacios-Ceña, D.; Florencio, L.L.; Guijarro, C.; Martín-Guerrero, J.D. Symptoms Experienced at the Acute Phase of SARS-CoV-2 Infection as Risk Factor of Long-Term Post-COVID Symptoms: The LONG-COVID-EXP-CM Multicenter Study. Int. J. Infect. Dis. 2022, 116, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.M.H.; Rajebi, H.; Moghaddas, F.; Ghasemiadl, M.; Talari, H. Chest CT in COVID-19 Pneumonia: What Are the Findings in Mid-Term Follow-Up? Emerg. Radiol. 2020, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising Long COVID: A Living Systematic Review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Zhang, H.; Rostami, M.R.; Leopold, P.L.; Mezey, J.G.; O’Beirne, S.L.; Strulovici-Barel, Y.; Crystal, R.G. Expression of the SARS-CoV-2 ACE2 Receptor in the Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 2020, 202, 219–229. [Google Scholar] [CrossRef]
- Cai, G.; Bossé, Y.; Xiao, F.; Kheradmand, F.; Amos, C.I. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 201, 1557–1559. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-Related Risk Factors of COVID-19: A Systematic Review and Meta-Analysis of 42 Studies and 423,117 Patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Van Zyl-Smit, R.N.; Richards, G.; Leone, F.T. Tobacco Smoking and COVID-19 Infection. Lancet Respir. Med. 2020, 8, 664–665. [Google Scholar] [CrossRef]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, Hypertension, Body Mass Index, Smoking and COVID-19-Related Mortality: A Systematic Review and Meta-Analysis of Observational Studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The Impact of COPD and Smoking History on the Severity of COVID-19: A Systemic Review and Meta-Analysis. J. Med. Virol. 2020, 92, 1915–1921. [Google Scholar] [CrossRef]


| Parameters | n = 166 |
|---|---|
| Age, yrs. (mean, SD) | 52.1 ± 16.8 |
| 19–86 |
| Male n (%) | 83 (50) |
| Female n (%) | 83 (50) |
| BMI kg/m2 (mean, SD) | 28.1 ± 5.8 |
| Smoking Status | |
| 5 (3.0) |
| 25 (15.1) |
| 136 (81.9) |
| Comorbidities (mean, SD) | 1.8 ± 2.1 |
| 39 (23.5) |
| 28 (16.9) |
| 11 (6.6) |
| 10 (6.0) |
| 9 (5.4) |
| 8 (4.8) |
| 6 (3.6) |
| 4 (2.4) |
| 3 (1.8) |
| 3 (3.8) |
| 2 (1.2) |
| 1 (0.6) |
| 1 (0.6) |
| 4 (2.4) |
| Charlson Comorbidity Index (mean, SD) | 1.6 ± 1.9 |
| Disease Severity NIH scale | |
| 51 (30.7) |
| 37 (22.3) |
| 67 (40.4) |
| 11 (6.6) |
| Treatment | |
| Oxygen n (%) | 64 (38.6) |
| HFNC n (%) *$ | 29 (17.5) |
| 2.24 ± 3.7 |
| ICU admission n (%) | 15 (9.0) |
| Intubationn (%) | 9 (5.4) |
| 0.9 ± 4.0 |
| Drug Treatment | |
| 39 (23.5) |
| 27 (16.3) |
| 15 (9.0) |
| 34 (20.5) |
| 83 (50.0) |
| 19 (11.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalak, G.; Jarjou’i, A.; Bohadana, A.; Wild, P.; Rokach, A.; Amiad, N.; Abdelrahman, N.; Arish, N.; Chen-Shuali, C.; Izbicki, G. Prevalence and Persistence of Symptoms in Adult COVID-19 Survivors 3 and 18 Months after Discharge from Hospital or Corona Hotels. J. Clin. Med. 2022, 11, 7413. https://doi.org/10.3390/jcm11247413
Kalak G, Jarjou’i A, Bohadana A, Wild P, Rokach A, Amiad N, Abdelrahman N, Arish N, Chen-Shuali C, Izbicki G. Prevalence and Persistence of Symptoms in Adult COVID-19 Survivors 3 and 18 Months after Discharge from Hospital or Corona Hotels. Journal of Clinical Medicine. 2022; 11(24):7413. https://doi.org/10.3390/jcm11247413
Chicago/Turabian StyleKalak, George, Amir Jarjou’i, Abraham Bohadana, Pascal Wild, Ariel Rokach, Noa Amiad, Nader Abdelrahman, Nissim Arish, Chen Chen-Shuali, and Gabriel Izbicki. 2022. "Prevalence and Persistence of Symptoms in Adult COVID-19 Survivors 3 and 18 Months after Discharge from Hospital or Corona Hotels" Journal of Clinical Medicine 11, no. 24: 7413. https://doi.org/10.3390/jcm11247413
APA StyleKalak, G., Jarjou’i, A., Bohadana, A., Wild, P., Rokach, A., Amiad, N., Abdelrahman, N., Arish, N., Chen-Shuali, C., & Izbicki, G. (2022). Prevalence and Persistence of Symptoms in Adult COVID-19 Survivors 3 and 18 Months after Discharge from Hospital or Corona Hotels. Journal of Clinical Medicine, 11(24), 7413. https://doi.org/10.3390/jcm11247413








