Angioplasty of Dysfunctional Dialysis Fistula or Graft with Resveratrol-Excipient and Paclitaxel-Coated Balloon Improves Primary Patency Rates Compared to Plain Angioplasty Alone
Abstract
1. Introduction
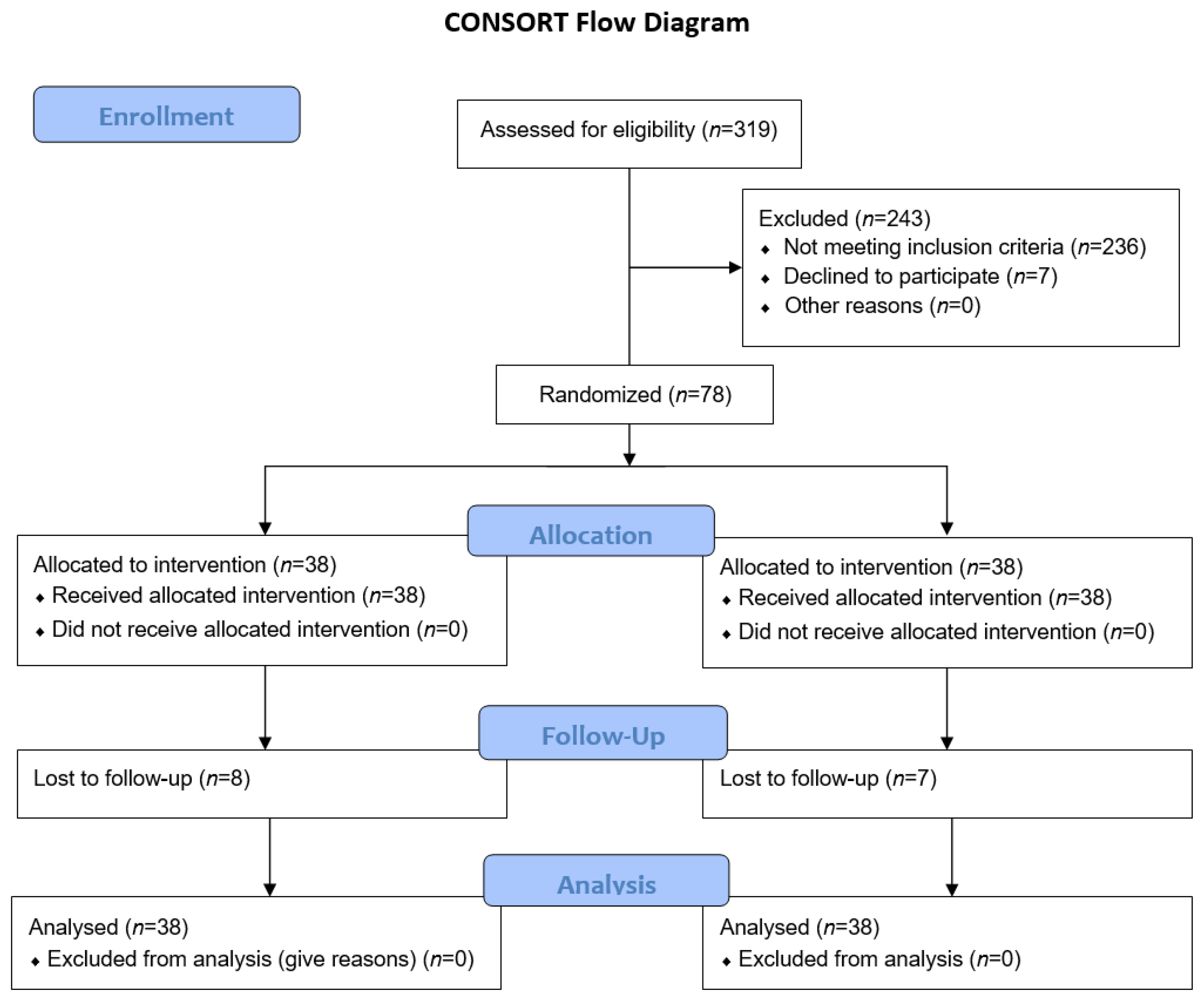
2. Materials and Methods
2.1. Trial Design
2.2. Trial Population, Inclusion/Exclusion Criteria
2.3. Trial Endpoints
2.4. Trial Device
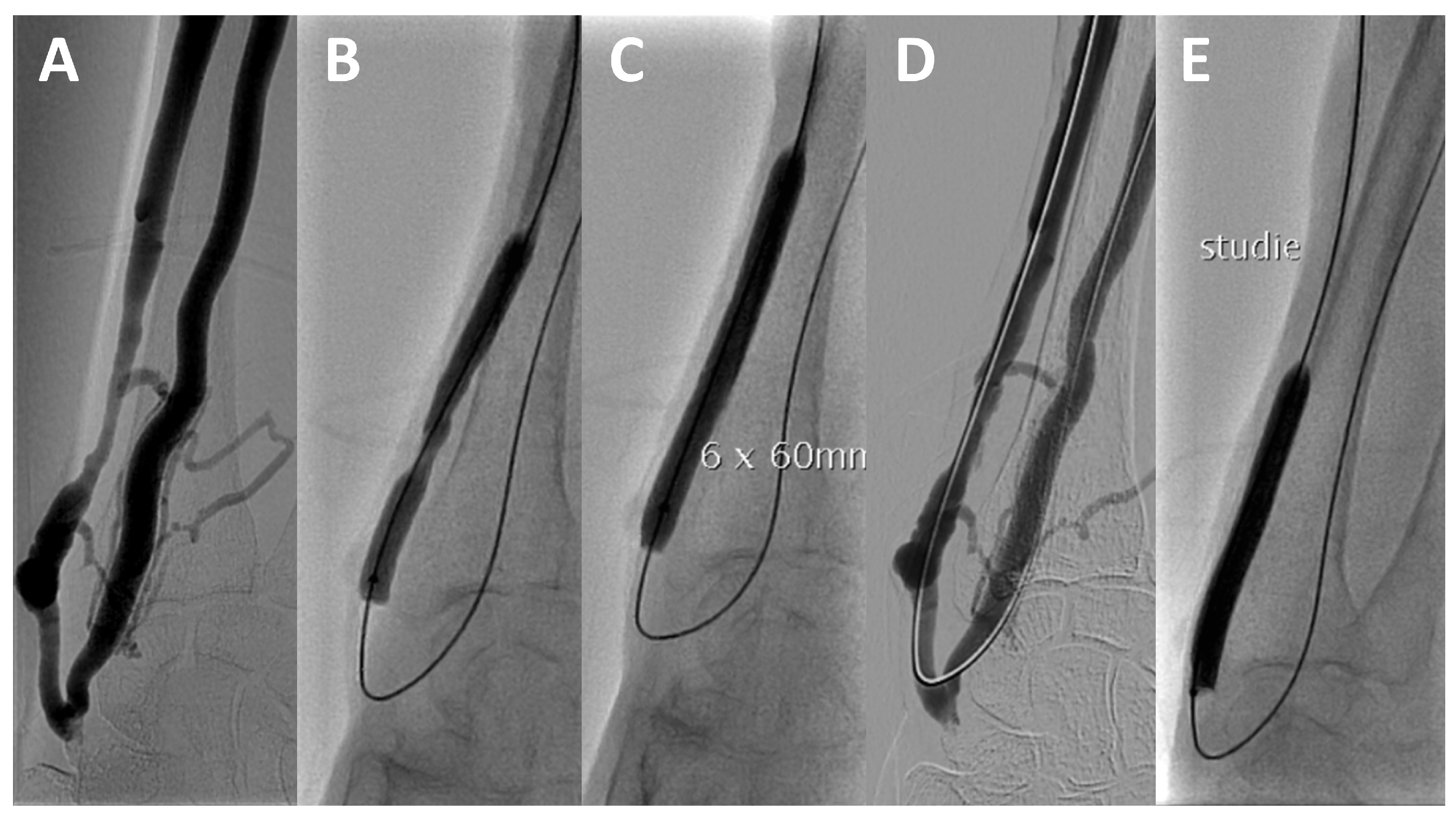
2.5. Trial Procedure
2.6. Follow-Up
2.7. Cost Analysis
2.8. Statistical Analysis
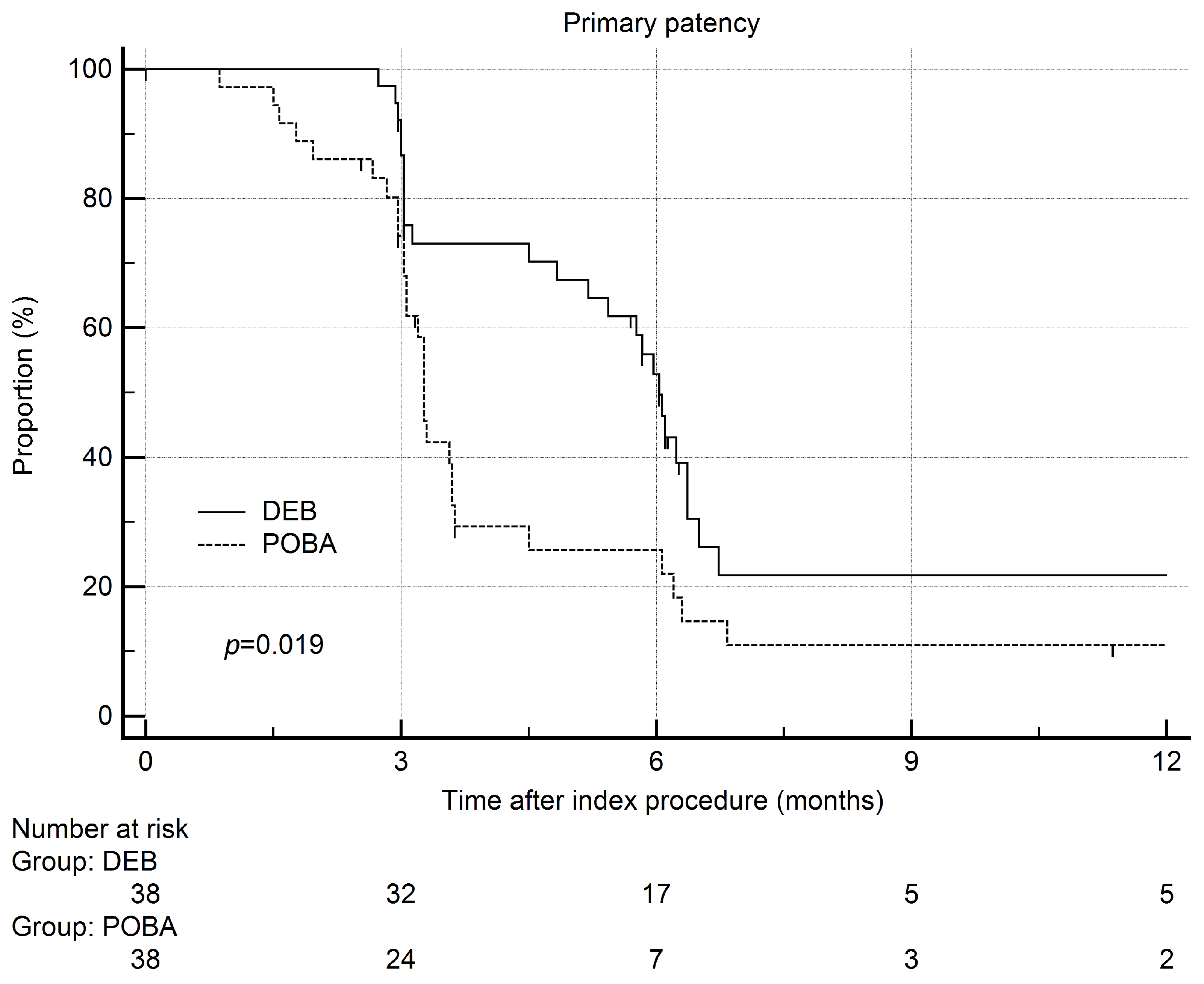
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and novel therapies for vascular access in haemodi-alysis. Nat. Rev. Nephrol. 2020, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaishi, A.A.; Oliver, M.J.; Thomas, S.M.; Lok, C.E.; Zhang, J.C.; Garg, A.X.; Kosa, S.D.; Quinn, R.R.; Moist, L.M. Patency Rates of the Arteriovenous Fistula for Hemodialysis: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2014, 63, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Park, T.; Kim, H.J.; Min, S.; Ha, J.; Min, S.-K. Editor’s Choice—Paclitaxel Coated Balloon Angioplasty vs. Plain Balloon Angioplasty for Haemodialysis Arteriovenous Access Stenosis: A Systematic Review and a Time to Event Meta-Analysis of Randomised Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liang, M.; Liu, Y.; Zheng, D.; He, Q.; Jin, J. Paclitaxel coated balloon versus conventional balloon angioplasty in dysfunctional dialysis arteriovenous fistula: A systematic review and meta-analysis of randomized controlled trials. Ren. Fail. 2022, 44, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lookstein, R.A.; Haruguchi, H.; Ouriel, K.; Weinberg, I.; Lei, L.; Cihlar, S.; Holden, A. Drug-Coated Balloons for Dysfunc-tional Dialysis Arteriovenous Fistulas. N. Engl. J. Med. 2020, 383, 733–742. [Google Scholar] [CrossRef]
- Kamann, S.; Haase, T.; Stolzenburg, N.; Löchel, M.; Peters, D.; Schnorr, J. Resveratrol-Coated Balloon Catheters in Porcine Coronary and Peripheral Arteries. Int. J. Mol. Sci. 2019, 20, 2285. [Google Scholar] [CrossRef]
- Speck, U.; Häckel, A.; Schellenberger, E.; Kamann, S.; Löchel, M.; Clever, Y.P.; Peters, D.; Scheller, B.; Trog, S.; Bettink, S. Drug Distribution and Basic Pharmacology of Paclitaxel/Resveratrol-Coated Balloon Catheters. Cardiovasc. Interv. Radiol. 2018, 41, 1599–1610. [Google Scholar] [CrossRef]
- Shenoy, S.; Allon, M.; Beathard, G.; Brouwer-Maier, D.; Dember, L.M.; Glickman, M.; Lee, C.; Litchfield, T.; Lok, C.; Huber, T.; et al. Clinical Trial End Points for Hemodialysis Vascular Access. Clin. J. Am. Soc. Nephrol. 2018, 13, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Gögebakan, O.; Redlich, U.; Tautenhahn, J.; Ricke, J.; Halloul, Z.; Meyer, D.-R.; Waliszewski, M.; Schnorr, B.; Zeller, T.; et al. Angiographic and Clinical Outcomes After Treatment of Femoro-Popliteal Lesions with a Novel Paclitaxel-Matrix-Coated Balloon Catheter. Cardiovasc. Interv. Radiol. 2017, 40, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Manns, B.; Tonelli, M.; Yilmaz, S.; Lee, H.; Laupland, K.; Klarenbach, S.; Radkevich, V.; Murphy, B. Establishment and Maintenance of Vascular Access in Incident Hemodialysis Patients: A Prospective Cost Analysis. J. Am. Soc. Nephrol. 2004, 16, 201–209. [Google Scholar] [CrossRef]
- Ayez, N.; Fioole, B.; Aarts, R.A.; van den Dorpel, M.A.; Akkersdijk, G.P.; Dinkelman, M.K.; de Smet, A.A. Secondary interven-tions in patients with autologous arteriovenous fistulas strongly improve patency rates. J. Vasc. Surg. 2011, 54, 1095–1099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voorzaat, B.M.; Janmaat, C.J.; van der Bogt, K.E.; Dekker, F.W.; Rotmans, J.I. Patency Outcomes of Arteriovenous Fistulas and Grafts for Hemodialysis Access: A Trade-Off between Nonmaturation and Long-Term Complications. Kidney360 2020, 1, 916–924. [Google Scholar] [CrossRef] [PubMed]
- MacRae, J.M.; Dipchand, C.; Oliver, M.; Moist, L.; Lok, C.; Clark, E.; Hiremath, S.; Kappel, J.; Kiaii, M.; Luscombe, R.; et al. Arteriovenous Access Failure, Stenosis, and Thrombosis. Can. J. Kidney Health. Dis. 2016, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-R.; Zou, L.; Xing, Y.; Tan, Y.-C.; Xu, G.-J.; He, Z.-J.; Cao, J.-Q.; Wu, J.-Y.; Liang, X.-X.; Zhang, H.-P.; et al. Predictors of primary patency after percutaneous bal-loon angioplasty for stenosis of Brescia-Cimino hemodialysis arteriovenous fistula. Br. J. Radiol. 2020, 93, 20190505. [Google Scholar] [CrossRef]
- Kim, W.S.; Pyun, W.B.; Kang, B.C. The Primary Patency of Percutaneous Transluminal Angioplasty in Hemodialysis Patients With Vascular Access Failure. Korean Circ. J. 2011, 41, 512–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kavan, J.; Kudlicka, J.; Malik, J.; Chytilova, E.; Lambert, L.; Slavikova, M.; Matras, P.; Burgetova, A. Treatment of failing arterio-venous dialysis graft by angioplasty, stent, and stent graft, Two-years analysis of patency rates and cost-effectiveness. Exp. Ther. Med. 2019, 18, 4144–4150. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Pacheco, E.; Yahagi, K.; Mori, H.; Ladich, E.; Virmani, R. Comparison of Particulate Embolization after Femoral Artery Treatment with IN.PACT Admiral versus Lutonix 035 Paclitaxel-Coated Balloons in Healthy Swine. J. Vasc. Interv. Radiol. 2016, 27, 1676–1685.e2. [Google Scholar] [CrossRef]
- Mori, M.; Sakamoto, A.; Kawakami, R.; Sato, Y.; Jinnouchi, H.; Kawai, K.; Cornelissen, A.; Virmani, R.; Finn, A.V. Paclitaxel- and Sirolimus-coated Balloons in Peripheral Artery Disease Treatment: Current Perspectives and Concerns. Vasc. Endovasc. Rev. 2021, 4, e03. [Google Scholar] [CrossRef]
- Caradu, C.; Lakhlifi, E.; Colacchio, E.C.; Midy, D.; Bérard, X.; Poirier, M.; Ducasse, E. Systematic review and updated me-ta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease. J. Vasc. Surg. 2019, 70, 981–995.e10. [Google Scholar] [CrossRef]
- Katsanos, K.; Karnabatidis, D.; Kitrou, P.; Spiliopoulos, S.; Christeas, N.; Siablis, D. Paclitaxel-coated balloon angio-plasty vs. plain balloon dilation for the treatment of failing dialysis access, 6-month interim results from a prospec-tive randomized controlled trial. J. Endovasc. Ther. 2012, 19, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, S.O.; Lawson, J.; Roy-Chaudhury, P.; Saad, T.F. Drug Coated Balloon Angioplasty in Failing AV Fistulas. Clin. J. Am. Soc. Nephrol. 2018, 13, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, M.K.; Christaina, E.; Antoniou, G.A.; Argyriou, C.; Trypsianis, G.; Georgiadis, G.S. Plain versus paclitax-el-coated balloon angioplasty in arteriovenous fistula and graft stenosis, An umbrella review. J. Vasc. Access. 2021, 23, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Wang, J.; Zhao, J.; Singh, N.; Zhang, W.W. A systematic review and meta-analysis of the risk of death and patency after application of paclitaxel-coated balloons in the hemodialysis access. J. Vasc. Surg. 2020, 72, 2186–2196.e3. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.; Limmer, A.M.; Paravastu, S.C.V.; Thomas, S.D.; Bennett, M.H.; Holden, A.; Schneider, P.A.; Varcoe, R.L. Mortality After Paclitax-el-Coated Device Use in Dialysis Access, A Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2019, 26, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, S.O.; Saad, T.F.; Roy-Chaudhury, P.; Lutonix AV Clinical Trial Investigators. The Lutonix AV Random-ized Trial of Paclitaxel-Coated Balloons in Arteriovenous Fistula Stenosis, 2-Year Results and Subgroup Analysis. J. Vasc. Interv. Radiol. 2020, 31, 1.e5–14.e5. [Google Scholar] [CrossRef] [PubMed]
| DCB Group (n = 38) | POBA Group (n = 38) | p | |
|---|---|---|---|
| age | 71 (IQR 65, 78) | 69 (IQR 64, 76) | 0.557 |
| male sex | 21 (55%) | 18 (47%) | 0.647 |
| antipletelet therapy | 22 (58%) | 26 (68%) | 0.476 |
| anticoagulation therapy | 15 (39%) | 12 (32%) | 0.632 |
| diabetes | 25 (66%) | 23 (61%) | 0.812 |
| ischemic heart disease | 13 (34%) | 13 (34%) | 1.0 |
| AVF | 26 (68%) | 28 (74%) | 0.800 |
| AVG | 12 (32%) | 10 (26%) | |
| median time since access creation (years) | 1.56 (IQR 0.89, 2.91) | 1.42 (IQR 0.92, 2.74) | 0.934 |
| previous PTA on vascular access | 29 (76%) | 21 (82%) | 0.779 |
| stenosis location | 0.687 | ||
| graft | 2 (5%) | 1 (3%) | |
| outflow vein | 11 (29%) | 8 (21%) | |
| juxta-anastomotic outflow vein | 21 (55%) | 26 (68%) | |
| venous anastomosis | 4 (11%) | 3 (8%) | |
| balloon diameter | 0.786 | ||
| 5 mm | 5 (13%) | 5 (13%) | |
| 6 mm | 18 (47%) | 21 (52%) | |
| 7 mm | 12 (32%) | 10 (26%) | |
| 8 mm | 3 (8%) | 2 (5%) | |
| degree of stenosis | 67% ± 11% | 69% ± 10% | 0.360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, M.; Matras, P.; Kavan, J.; Lambert, L.; Burgetova, A. Angioplasty of Dysfunctional Dialysis Fistula or Graft with Resveratrol-Excipient and Paclitaxel-Coated Balloon Improves Primary Patency Rates Compared to Plain Angioplasty Alone. J. Clin. Med. 2022, 11, 7405. https://doi.org/10.3390/jcm11247405
Novak M, Matras P, Kavan J, Lambert L, Burgetova A. Angioplasty of Dysfunctional Dialysis Fistula or Graft with Resveratrol-Excipient and Paclitaxel-Coated Balloon Improves Primary Patency Rates Compared to Plain Angioplasty Alone. Journal of Clinical Medicine. 2022; 11(24):7405. https://doi.org/10.3390/jcm11247405
Chicago/Turabian StyleNovak, Matej, Patrik Matras, Jan Kavan, Lukas Lambert, and Andrea Burgetova. 2022. "Angioplasty of Dysfunctional Dialysis Fistula or Graft with Resveratrol-Excipient and Paclitaxel-Coated Balloon Improves Primary Patency Rates Compared to Plain Angioplasty Alone" Journal of Clinical Medicine 11, no. 24: 7405. https://doi.org/10.3390/jcm11247405
APA StyleNovak, M., Matras, P., Kavan, J., Lambert, L., & Burgetova, A. (2022). Angioplasty of Dysfunctional Dialysis Fistula or Graft with Resveratrol-Excipient and Paclitaxel-Coated Balloon Improves Primary Patency Rates Compared to Plain Angioplasty Alone. Journal of Clinical Medicine, 11(24), 7405. https://doi.org/10.3390/jcm11247405





