A Predictive Model for Dysphagia after Ventilator Liberation in Severe Pneumonia Patients Receiving Tracheostomy: A Single-Center, Observational Study
Abstract
1. Introduction
2. Materials and Methods
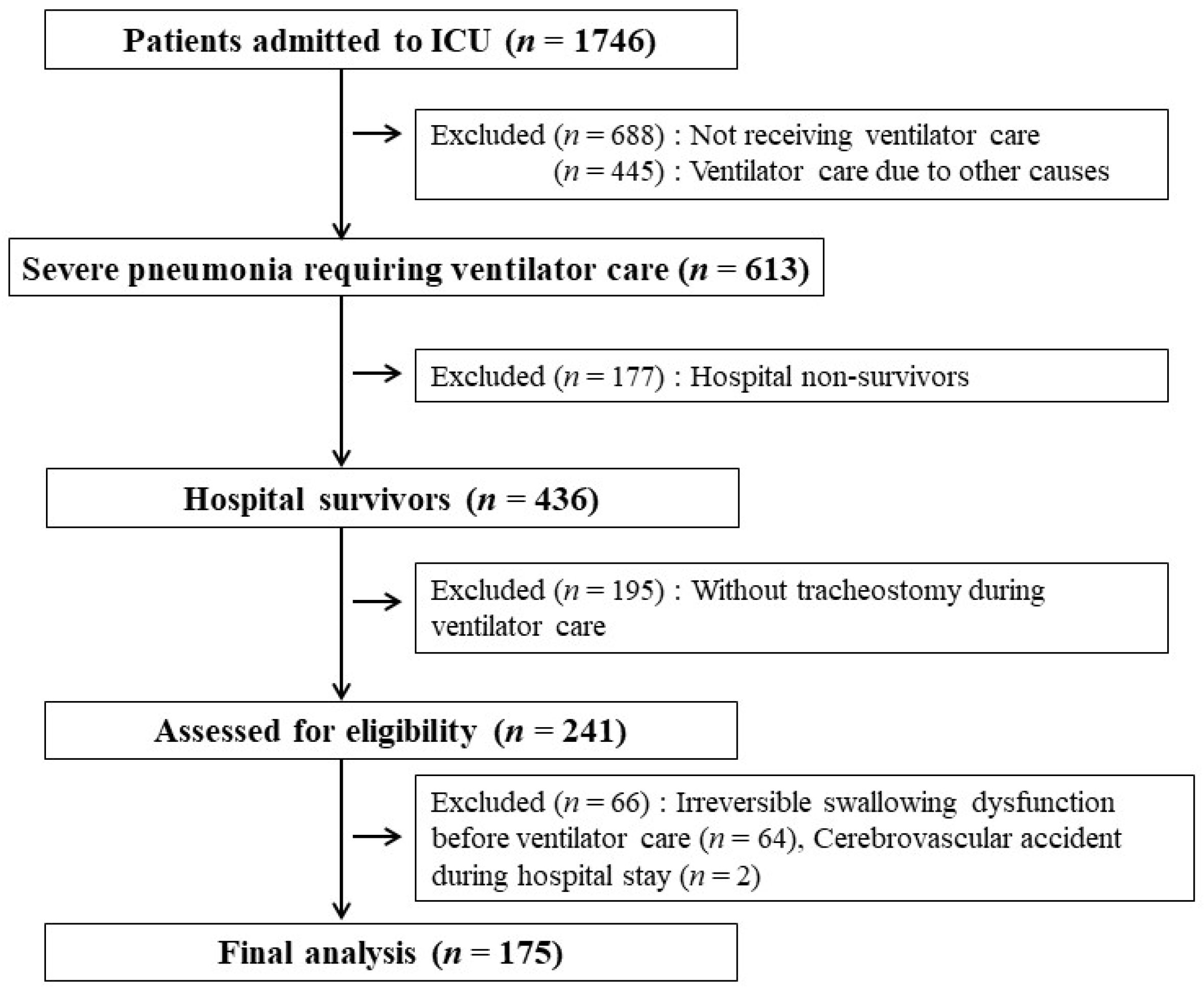
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Total Patients
3.2. Predictive Factors Associated with Dysphagia
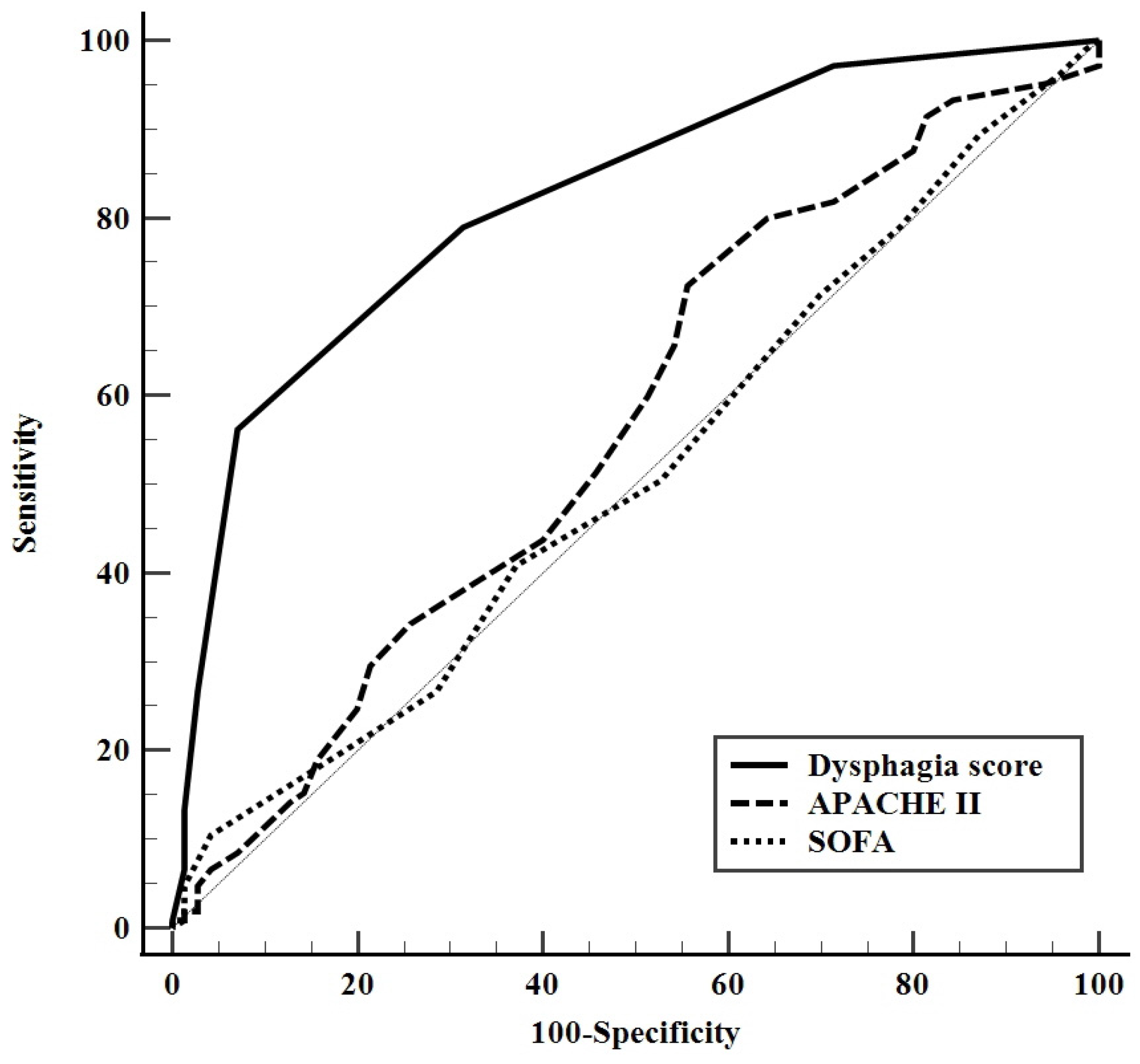
3.3. A predictive Model for Dysphagia at Hospital Discharge
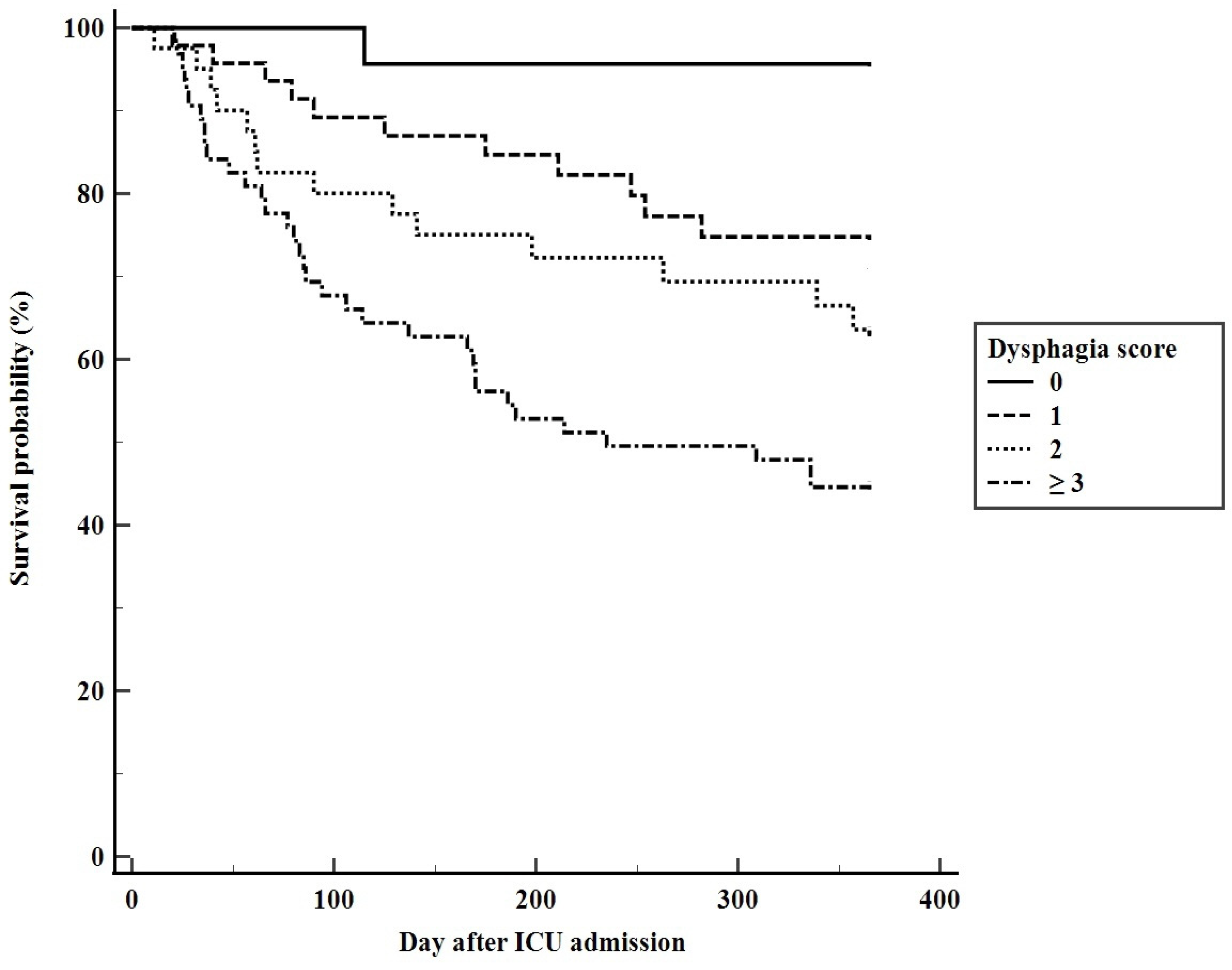
3.4. Predicting Long-Term Mortality Using the Dysphagia Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosokawa, K.; Nishimura, M.; Egi, M.; Vincent, J.L. Timing of tracheotomy in ICU patients: A systematic review of randomized controlled trials. Crit. Care 2015, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.H.; Napolitano, L.M. Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir. Care 2014, 59, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D.; Morris, P.E. Tracheostomy practice in adults with acute respiratory failure. Crit. Care Med. 2012, 40, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Ramos, F.J.; Añon, J.M.; Ortiz, R.; Colinas, L.; Masclans, J.R.; de Haro, C.; Ortega, A.; Peñuelas, O.; Cruz-Delgado, M.d.M.; et al. Early Tracheostomy for Managing ICU Capacity During the COVID-19 Outbreak: A Propensity-Matched Cohort Study. Chest 2022, 161, 121–129. [Google Scholar] [CrossRef]
- Hafner, G.; Neuhuber, A.; Hirtenfelder, S.; Schmedler, B.; Eckel, H.E. Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. Eur. Arch. Otorhinolaryngol. 2008, 265, 441–446. [Google Scholar] [CrossRef]
- Warnecke, T.; Suntrup, S.; Teismann, I.K.; Hamacher, C.; Oelenberg, S.; Dziewas, R. Standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients. Crit. Care Med. 2013, 41, 1728–1732. [Google Scholar] [CrossRef]
- Zuercher, P.; Moret, C.S.; Dziewas, R.; Schefold, J.C. Dysphagia in the intensive care unit: Epidemiology, mechanisms, and clinical management. Crit. Care 2019, 23, 103. [Google Scholar] [CrossRef]
- Skoretz, S.A.; Riopelle, S.J.; Wellman, L.; Dawson, C. Investigating Swallowing and Tracheostomy Following Critical Illness: A Scoping Review. Crit. Care Med. 2020, 48, e141–e151. [Google Scholar] [CrossRef]
- Scheel, R.; Pisegna, J.M.; McNally, E.; Noordzij, J.P.; Langmore, S.E. Endoscopic Assessment of Swallowing After Prolonged Intubation in the ICU Setting. Ann. Otol. Rhinol. Laryngol. 2016, 125, 43–52. [Google Scholar] [CrossRef]
- Brodsky, M.B.; De, I.; Chilukuri, K.; Huang, M.; Palmer, J.B.; Needham, D.M. Coordination of Pharyngeal and Laryngeal Swallowing Events During Single Liquid Swallows After Oral Endotracheal Intubation for Patients with Acute Respiratory Distress Syndrome. Dysphagia 2018, 33, 768–777. [Google Scholar] [CrossRef]
- Langmore, S.E.; Krisciunas, G.P.; Warner, H.; White, S.D.; Dvorkin, D.; Fink, D.; McNally, E.; Scheel, R.; Higgins, C.; Levitt, J.E.; et al. Abnormalities of Aspiration and Swallowing Function in Survivors of Acute Respiratory Failure. Dysphagia 2021, 36, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Skoretz, S.A.; Flowers, H.L.; Martino, R. The incidence of dysphagia following endotracheal intubation: A systematic review. Chest 2010, 137, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Printza, A.; Tedla, M.; Frajkova, Z.; Sapalidis, K.; Triaridis, S. Dysphagia Severity and Management in Patients with COVID-19. Curr. Health Sci. J. 2021, 47, 147–156. [Google Scholar] [PubMed]
- Macht, M.; Wimbish, T.; Clark, B.J.; Benson, A.B.; Burnham, E.L.; Williams, A.; Moss, M. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit. Care 2011, 15, R231. [Google Scholar] [CrossRef] [PubMed]
- Schefold, J.C.; Berger, D.; Zürcher, P.; Lensch, M.; Perren, A.; Jakob, S.M.; Parviainen, I.; Takala, J. Dysphagia in Mechanically Ventilated ICU Patients (DYnAMICS): A Prospective Observational Trial. Crit. Care Med. 2017, 45, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Schenk, N.V.; Moret, C.; Berger, D.; Abegglen, R.; Schefold, J.C. Risk Factors for Dysphagia in ICU Patients After Invasive Mechanical Ventilation. Chest 2020, 158, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; de Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.S.; Kahn, J.M.; Hough, C.L.; Seeley, E.J.; White, D.B.; Douglas, I.S.; Cox, C.E.; Caldwell, E.; Bangdiwala, S.I.; Garrett, J.M.; et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit. Care Med. 2012, 40, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Validation of the Prognosis for Prolonged Ventilation (ProVent) score in patients receiving 14 days of mechanical ventilation. J. Crit. Care 2018, 44, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Ambler, M.; Mahalingasivam, V.; Jones, A.; Rowan, K.; Rubenfeld, G.D. Evidence for a causal link between sepsis and long-term mortality: A systematic review of epidemiologic studies. Crit. Care 2016, 20, 101. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Kothari, M.; Bjerrum, K.; Nielsen, L.H.; Jensen, J.; Nielsen, J.F. Influence of External Subglottic Air Flow on Dysphagic Tracheotomized Patients With Severe Brain Injury. Ann. Otol. Rhinol. Laryngol. 2017, 126, 199–204. [Google Scholar] [CrossRef]
- Ledl, C.; Ullrich, Y.Y. Occlusion of Tracheostomy Tubes Does Not Alter Pharyngeal Phase Kinematics But Reduces Penetration by Enhancing Pharyngeal Clearance: A Prospective Study in Patients With Neurogenic Dysphagia. Am. J. Phys. Med. Rehabil. 2017, 96, 268–272. [Google Scholar] [CrossRef]
- Suntrup, S.; Marian, T.; Schröder, J.B.; Suttrup, I.; Muhle, P.; Oelenberg, S.; Hamacher, C.; Minnerup, J.; Warnecke, T.; Dziewas, R. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: A randomized controlled trial. Intensive Care Med. 2015, 41, 1629–1637. [Google Scholar] [CrossRef]
- Sutt, A.L.; Cornwell, P.; Mullany, D.; Kinneally, T.; Fraser, J.F. The use of tracheostomy speaking valves in mechanically ventilated patients results in improved communication and does not prolong ventilation time in cardiothoracic intensive care unit patients. J. Crit. Care 2015, 30, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.; McAuley, D.F.; Walshe, M.; McGaughey, J.; Anand, R.; Fallis, R.; Blackwood, B. Interventions for oropharyngeal dysphagia in acute and critical care: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Ponfick, M.; Linden, R.; Nowak, D.A. Dysphagia—A common, transient symptom in critical illness polyneuropathy: A fiberoptic endoscopic evaluation of swallowing study. Crit. Care Med. 2015, 43, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ceriana, P.; Carlucci, A.; Schreiber, A.; Fracchia, C.; Cazzani, C.; Dichiarante, M.; Cattani, B.; Fassio, C.; Segagni, D.; Nava, S. Changes of swallowing function after tracheostomy: A videofluoroscopy study. Minerva Anestesiol. 2015, 81, 389–397. [Google Scholar] [PubMed]
- Phua, J.; Faruq, M.O.; Kulkarni, A.P.; Redjeki, I.S.; Detleuxay, K.; Mendsaikhan, N.; Sann, K.K.; Shrestha, B.R.; Hashmi, M.; Palo, J.E.M.; et al. Critical Care Bed Capacity in Asian Countries and Regions. Crit. Care Med. 2020, 48, 654–662. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 175) | |
|---|---|
| Male | 126 (72.0%) |
| Age (years) | 71.3 ± 10.6 |
| BMI (kg/m2) | 21.6 ± 3.7 |
| ICU LOS (days) | 17.4 ± 9.8 |
| MV LOS (days) | 15.7 ± 9.4 |
| Hospital LOS (days) | 35 (15–54) |
| APACHE II score * | 20.8 ± 6.3 |
| SOFA score * | 7.5 ± 3.1 |
| Charlson’s comorbidity index | 2.3 ± 1.6 |
| Total medical expenditures (USD) † | 27,737 (4832–100,065) |
| Out-of-pocket medical expenditures (USD) † | 6634 (518–65,591) |
| Period from endotracheal intubation to tracheostomy (days) | 6.2 ± 3.4 |
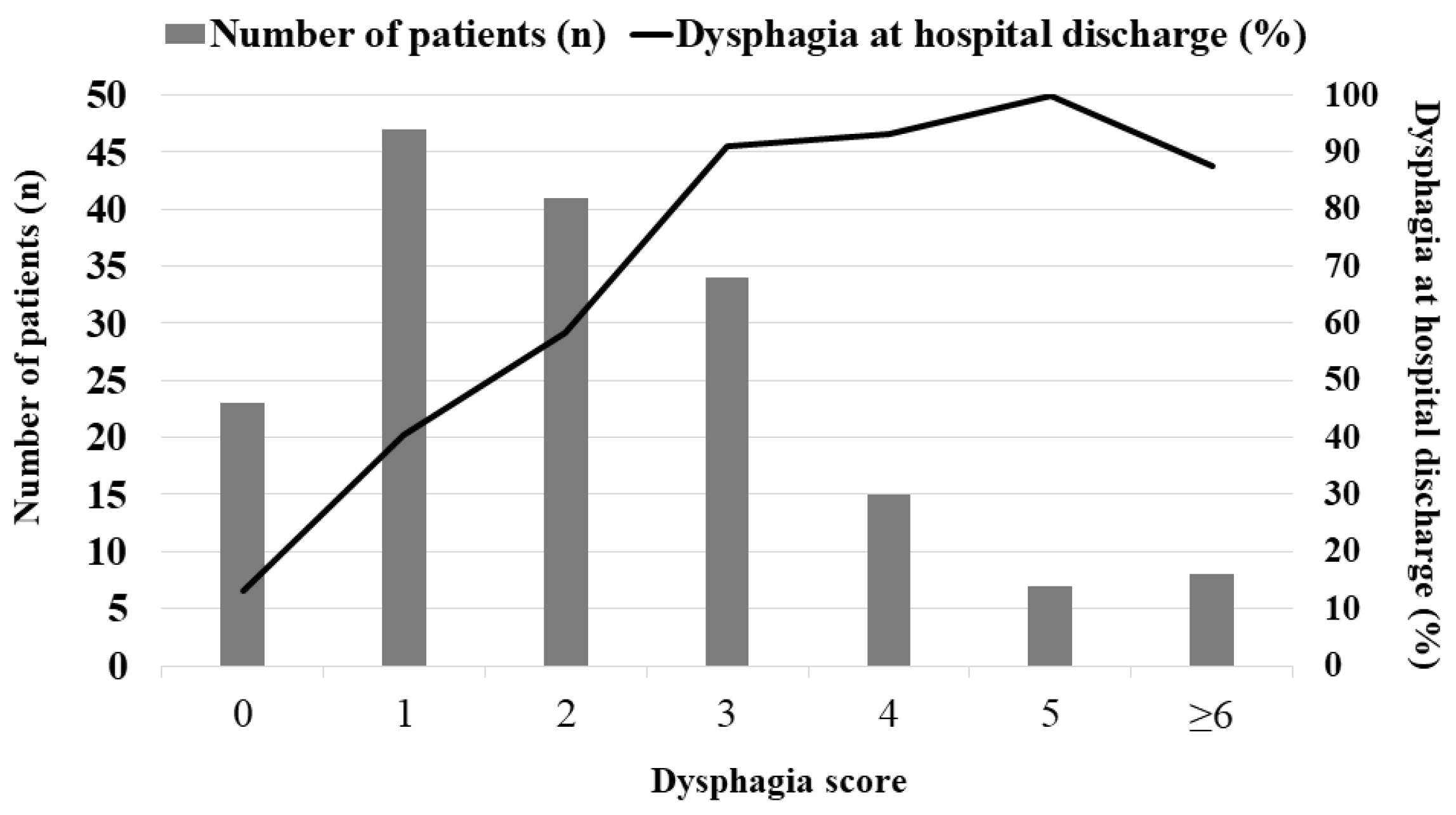
| Dysphagia at hospital discharge | 105 (60.0%) |
| One-year cumulative mortality after ICU admission | 77 (44.0%) |
| Dysphagia at Hospital Discharge | |||
|---|---|---|---|
| Yes (n = 105) | No (n = 70) | p-Value | |
| Male | 73 (69.5) | 53 (75.7) | 0.396 |
| Age, yr | 72.7 ± 10.1 | 69.1 ± 11.0 | 0.003 |
| BMI, kg/m2 | 20.8 ± 3.6 | 23.0 ± 3.6 | <0.001 |
| APACHE II score * | 21.3 ± 6.2 | 20.0 ± 6.4 | 0.167 |
| SOFA score * | 7.6 ± 3.1 | 7.4 ± 3.0 | 0.726 |
| MV LOS | 17.2 ± 10.1 | 13.6 ± 7.8 | 0.013 |
| Comorbidities | |||
| Chronic lung diseases † | 41 (39.0) | 23 (32.9) | 0.427 |
| Cardiovascular diseases | 36 (34.3) | 20 (28.6) | 0.509 |
| Chronic neurological diseases ‡ | 41 (39.0) | 15 (21.4) | 0.020 |
| Diabetes | 26 (24.8) | 23 (32.9) | 0.303 |
| Solid malignant tumors | 11 (10.5) | 14 (20.0) | 0.121 |
| Chronic kidney diseases | 10 (9.7) | 9 (12.9) | 0.621 |
| Requirement for neuromuscular blocking agents during the first 24 h after mechanical ventilation | 69 (65.7) | 53 (75.7) | 0.181 |
| Requirement for vasopressors during the first 24 h after mechanical ventilation | 64 (61.0) | 40 (57.1) | 0.640 |
| Period from endotracheal intubation to tracheostomy (days) | 6.4 ± 3.5 | 5.8 ± 3.1 | 0.225 |
| Dysphagia therapy programs during hospital stay § | 68 (64.8) | 64 (91.4) | <0.001 |
| One-year cumulative mortality | 60 (57.1) | 17 (24.3) | <0.001 |
| Variables | Adjusted OR * (95% CI) | p-Value | β Value |
|---|---|---|---|
| BMI < 18.5 kg/m2 | 11.219 (2.917–43.142) | <0.001 | 2.418 |
| No dysphagia therapy programs | 5.546 (2.000–15.380) | 0.001 | 1.713 |
| MV LOS ≥ 15 days † | 3.776 (1.766–8.075) | 0.001 | 1.329 |
| Age ≥ 74 years † | 3.445 (1.608–7.382) | 0.001 | 1.237 |
| Chronic neurologic diseases as comorbidities ‡ | 2.485 (1.096–5.636) | 0.029 | 0.910 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, W.; Jang, M.H.; Kim, S.H.; Yoon, J.A.; Jang, H.; Kim, S.; Lee, K. A Predictive Model for Dysphagia after Ventilator Liberation in Severe Pneumonia Patients Receiving Tracheostomy: A Single-Center, Observational Study. J. Clin. Med. 2022, 11, 7391. https://doi.org/10.3390/jcm11247391
Yoo W, Jang MH, Kim SH, Yoon JA, Jang H, Kim S, Lee K. A Predictive Model for Dysphagia after Ventilator Liberation in Severe Pneumonia Patients Receiving Tracheostomy: A Single-Center, Observational Study. Journal of Clinical Medicine. 2022; 11(24):7391. https://doi.org/10.3390/jcm11247391
Chicago/Turabian StyleYoo, Wanho, Myung Hun Jang, Sang Hun Kim, Jin A. Yoon, Hyojin Jang, Soohan Kim, and Kwangha Lee. 2022. "A Predictive Model for Dysphagia after Ventilator Liberation in Severe Pneumonia Patients Receiving Tracheostomy: A Single-Center, Observational Study" Journal of Clinical Medicine 11, no. 24: 7391. https://doi.org/10.3390/jcm11247391
APA StyleYoo, W., Jang, M. H., Kim, S. H., Yoon, J. A., Jang, H., Kim, S., & Lee, K. (2022). A Predictive Model for Dysphagia after Ventilator Liberation in Severe Pneumonia Patients Receiving Tracheostomy: A Single-Center, Observational Study. Journal of Clinical Medicine, 11(24), 7391. https://doi.org/10.3390/jcm11247391






