Abstract
Patients with stress urinary incontinence (SUI) may be afraid to increase intra-abdominal pressure to avoid incontinence. This could lead to weak expiratory muscles. The aim of this study was to investigate the association between respiratory muscle strength, physical function, and SUI in patients with SUI. A cross-sectional study was conducted in the Physical Medicine and Functional Rehabilitation Department. Thirty-one incontinent women (IG) and twenty-nine women in a control group (CG) were enrolled in this study. Anthropometric data, respiratory muscle strength (maximal inspiratory pressure; maximal expiratory pressure), SUI (Urogenital Distress Inventory-6; Incontinence Impact Questionnaire-7; Pad test), and physical function (waist circumference; timed-up-and-go test; abdominal muscle strength) were assessed. Body fat, body mass index, body weight, and waist circumference were higher in IG than CG (p < 0.01), while postural gait and abdominal muscles were lower (p < 0.001). Respiratory muscle strength displayed moderate correlations with SUI severity, especially for maximal expiratory pressure (p < 0.01). Maximal expiratory pressure was moderately associated with physical function. Deterioration in respiratory muscle strength is a characteristic of women with SUI. In this population, pelvic floor muscle training may be prescribed to improve continence. By feeling more confident about increasing intra-abdominal pressure, women with SUI would strengthen their expiratory muscles and eventually improve their physical function.
1. Introduction
Stress urinary incontinence (SUI) is the most prevalent type of urinary incontinence (UI) and is defined as the complaint of involuntary leakage on effort, exertion, sneezing, or coughing [1]. It is known that SUI primarily results from a pelvic floor muscles (PFM) dysfunction and is associated with an abnormal breathing pattern [2]. These physiological alterations likely affect the synergy between the diaphragm, the abdominal muscles, and the PFM during functional tasks [3].
During tasks requiring a forced expiratory effort (e.g., Valsalva maneuver, coughing, laughing, or sneezing), the combined actions of PFM, abdominal muscles, and the diaphragm help generate an expiratory force, increase intra-abdominal pressure (IAP), and maintain continence [4,5]. The trunk-stabilization muscles (i.e., composed of the abdominal, the pelvic floor, and the respiratory diaphragm muscles) work synchronously to maintain the abdominal lumbopelvic cavity stable by regulating IAP during a variety of daily life activities [6,7,8]. The action of these muscles can alter IAP and thereby influence PFM activity [9]. Recent evidence indicates that modified activation of trunk stabilization muscles could affect urinary continence regulatory mechanisms [10]. In addition, the coactivation of the trunk stabilization muscles could improve PFM-contracting behavior [11,12]. Altogether, the synchronized action between the PFM, the respiratory muscles, and the abdominal wall muscles participate in greater trunk stability that is in turn required to optimally perform tasks of daily life, including locomotor activities [13].
Since abdominal muscles work in synergy with PFM, participate in the respiratory mechanism, and cooperate to support breathing, alteration in one of these muscle groups may have an impact on the function of the other [14]. In light of this, we supposed that SUI may be associated with the changes in respiratory muscle performance.
To the best of the authors’ knowledge, there are few published data on the nature of the association between respiratory muscle strength (RMS) and impaired physical function (PF) in patients with SUI [15]. In addition, the relationship between RMS and SUI is unclear. Therefore, the aims of this comparative study were to (i) compare RMS and PF between SUI and healthy women, (ii) investigate the relationship between RMS and SUI severity, and (iii) evaluate the association of RMS with the PF of women with SUI. It was hypothesized that (i) RMS is compromised in SUI women compared to their healthy counterparts; (ii) RMS, and more particularly expiratory muscle pressure, is negatively correlated with SUI severity; and (iii) impaired PF is associated with both decreased RMS and SUI severity.
2. Materials and Methods
2.1. Study Design
This study is a subgroup analysis within the ongoing randomized controlled trial registered with the Pan African Clinical Trial Registry (PACTR) (identification number: PACTR202210746628521) to evaluate the impact of inspiratory muscle training on PFM strength and urinary leakage in patients with SUI. A cross-sectional, descriptive study was conducted following the guidelines established by the STROBE statement [16]. Testing occurred between January 2019 and June 2019 at Physical Medicine and Functional Rehabilitation Department, Habib Bourguiba Hospital (Sfax, Tunisia). Anthropometric measurement, RMS, PF, and SUI severity (Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire-7 (IIQ-7), and pad test) were assessed in the morning by the same clinical evaluator. All the participants signed an informed consent form approved by the institutional research ethics committee prior to enrollment in the study. The study was conducted in accordance with the Declaration of Helsinki 1975 and approved by the local ethics committee from the High Institute of Nursing, University of Sfax, Tunisia (Protection Committee Approval Registration code: CPP SUD N° 0355/2021).
2.2. Sample Size
A priori sample size was calculated using G∗Power [17] (version 3.1.9.6; University of Kiel, Franz Faul, Germany). Twenty-eight participants were deemed sufficient for an α error probability of 0.05 and power (1-β error probability) of 0.80 and an effect size of 0.8. A final sample size 60 women (31 women with UI (intervention group (IG)) and 29 healthy, age-matched healthy women (control group (CG)) was therefore recruited. Assumption of 56% for the non-inclusion- and exclusion- criteria gave a corrected total sample of 136 women [136 = 60/(1 − 0.56)].
2.3. Participants: Intervention and Control Groups
In the IG, women were initially selected from the Physical Medicine and Functional Rehabilitation Department database of the Habib Bourguiba Hospital (Sfax, Tunisia) based on a clinical assessment. The following inclusion criteria were applied: age between 35 and 65 years and any reported unintentional loss of urine during physical activity (walking, running, laughing, sneezing, or coughing) [1] (Figure 1). Participants were excluded if they met any of the following criteria: pelvic prolapse (i.e., a pelvic exam was conducted by a physician to evaluate the presence of prolapse), anal incontinence, urge incontinence, overactive bladder, presence of any concomitant pathology that may affect respiratory and pelvic floor function, history of surgery, recent trauma at the lumbopelvic or the abdominal or the thoracic regions or at the lower limbs, smoking, athletes (i.e., the international physical activity questionnaire short form (IPAQ-SF) was used to verify that tested population was only composed of sedentary women), and nulliparous women. In the CG, which did not report UI, participants were recruited among healthy nurses and from the local community.
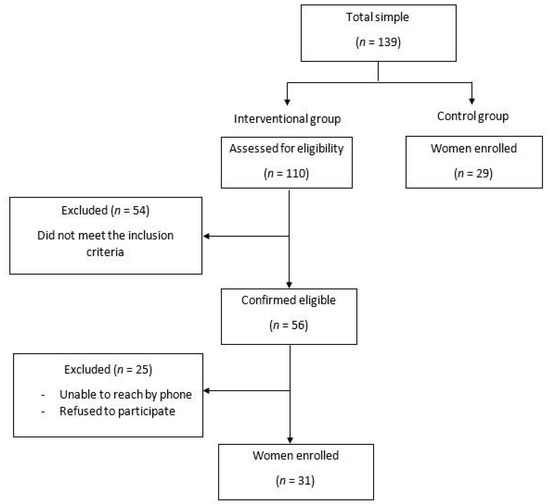
Figure 1.
Study design.
2.4. Anthropometric Measurements
The height was measured with a Harpenden stadiometer to the last complete 0.1 cm (Seca, Hamburg, Germany). A multi-frequency bioelectrical impedance meter (TBF-410GS, Tanita Co., Tokyo, Japan) was used to measure body weight, body fat, lean mass, and body mass index (BMI) [18]. This method was validated against the reference methods [19]. To obtain an accurate measurement of the patient’s body waist circumference (WC), a stretch-resistant tape that provides a constant tension of 100 g was used by only one trained investigator to minimize measurement errors and variability. In addition, the standard operating procedure was followed when conducting the measurements [18]. The WC was measured at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest [20]. The WC was utilized to determine central obesity beyond the women’s threshold value of 88 cm. Tested individuals remained calm, and measurements were taken at the end of a normal expiration. Each measurement was taken twice, and the average was taken if the two measurements were within 1 cm of each other. The measurements were repeated if the difference between the two measurements was more than 1 cm.
2.5. Urogenital Distress Inventory-6 (UDI-6)
The Arabic-validated short form of the UDI-6, which is reliable with an intraclass correlation coefficient (ICC) = 0.98, was used to measure UI severity [21]. Briefly, this self-administered questionnaire comprises six scored items, scaled from 0 to 3, to assess the prevalence, frequency, and severity of urinary leakage [22]. The total score ranges from 0 to 100, with the highest values demonstrating increased severity [23,24]. Items and questions were carefully explained by the interviewer.
2.6. Incontinence Impact Questionnaire-7 (IIQ-7)
The Arabic-validated version of the IIQ-7 was used to evaluate the quality of life of women with lower urinary tract symptoms [21]. This questionnaire is reliable (i.e., ICC = 0.98) for self-reported outcome measures [21]. The IIQ-7 level of validation, according to International Consultation on Incontinence (ICI) grades, is A [22]. It consists of seven items that are subdivided into four domains: physical activity (items 1 and 2), travel (items 3 and 4), social activities (item 5), and emotional health (items 6 and 7). The total score ranges from 0 to 100, with the highest values indicative of the worse quality of life [25].
2.7. The One-Hour Pad Test (Pad Test)
The pad test was conducted in accordance with International Continence Society (ICS) guidelines [26]. All participants were given pre-weighed pads to wear, and after that, they were told to consume 500 mL of plain water over the course of 15 min. Subsequently, women were required to carry out the typical ICS provocation activities [26]. All participants were asked not to urinate during that hour unless it was absolutely essential [26]. The total amount of urine lost may be calculated by reweighing the absorbent pad. The test outcomes are given in grams. When findings for the pad test surpass 2 g, it is termed positive. Greater losses indicate a more serious UI.
2.8. Respiratory Muscle Strength (RMS)
RMS was evaluated while participants were seated at rest according to international guidelines [27]. Maximal inspiratory and expiratory pressures (MIP and MEP, respectively) were measured using a digital mouth pressure meter (MicroRPM, Micro Medical Ltd., Rochester, Kent, UK). This device produces adequate MIP and MEP reliability (i.e., ICC > 0.90) [28]. MIP was measured at the functional residual capacity, while MEP was measured following inspiration to maximum total lung capacity using the technique of Black and Hyatt [29]. Since all participants had no previous experience with these maneuvers, great care was taken to explain the procedures fully. Both inhalation and exhalation were performed as quickly as possible while maintaining maximum effort for at least 1 s. With the use of a nose clip, measurements were repeated until three successive trials with a difference of less than 10% between them were observed [27,30,31]. The highest value was used for further data analysis. Given the lack of Tunisian or North African norms for MIP and MEP [32], the latter was compared to the predictive value of Evans and Whitelaw [33]. Data were expressed as an absolute value (cmH2O) and as a percentage of the predicted value (% predicted) adjusted for age.
2.9. Physical Function (PF)
Functional mobility: The timed-up-and-go (TUG) test is a performance-based measure of functional mobility that was created initially to detect mobility and balance deficits [34]. Participants were required to stand up from a chair with armrests, walk three meters as fast as possible (without running), turn around, return to the chair, and sit down. The test was performed twice, and the best time in seconds to complete this task was used.
Abdominal muscle strength (AMS): The trunk extension and flexion modular (TEF) component attached to the Cybex® Norm II isokinetic dynamometer unit (Lumex, Inc., Ronkonkoma, NY, USA) was used to evaluate AMS at 60°/s and 90°/s. After the participant was positioned and secured in the TEF modular component, the range of motion was calculated. The zero anatomical position was established to be a vertical standing position. Setting the zero anatomical position provided the system with a fixed starting position to calculate the range of motion. There was a maximum range of motion of 50°, with 30° of flexion (−30°) and 20° of extension (+20°) of the trunk [35]. This trunk position represented the range of motion commonly observed during daily life activities [36]. Reductions in hip flexion and extension can be achieved by restricting trunk motion to less than 50°, according to Garcia-Vaquero et al. [37]. Furthermore, the placement of the dynamometer’s axis of rotation at the anterior superior iliac spine level and the use of the pad behind the sacrum and the pelvic strap reduced hip motion during the protocol. Concentric trunk flexion was assessed at 60°/s and 90°/s angular velocities. These angular velocities were selected for measuring mechanical work since they are considered safe and reliable [38]. Before testing, participants performed a standardized warm-up that consisted of cycling an ergometer for 10 min followed by a thorough explanation of the test procedure. To help the participants become acquainted with the equipment and the research protocol, a familiarization trial consisting of one set of five consecutive submaximal concentric trunk flexion and extension repetitions at 60°/s and 90°/s was conducted before the trunk muscles strength assessment. All participants performed three series of three maximum concentric contractions at 60°/s and five series of five maximum concentric contractions at 90°/s, as previously recommended [39]. Because fewer contractions are required for test evaluation at lower velocities (i.e., 60°/s), and more contractions are required for test evaluation at higher velocities (i.e., 90°/s), the difference in the amount of series between both velocities was adopted as recommended [40]. During a 10 s contraction, flexion and extension measurements were taken at 60° and 90° of trunk flexion. There was a 30 s rest period between each angular velocity recording. A one-minute rest period between the two measured velocities was observed. All trials were conducted at the same time of day and were overseen by the same researcher. Participants were allowed to view the Cybex NORM computer monitor, and they were encouraged through a standardized “stronger, faster” verbal command during the test session to exert maximal physical effort. The peak torque (N.m) was recorded. When the peak torque’s coefficient of variation (CV) was higher than 25%, the participants were allowed to rest, and the set was repeated [40,41].
2.10. Statistical Analysis
The Kolmogorov–Smirnov statistic was used to test the normality of the distribution of all variables. Data were presented as the mean and standard deviation (SD) or as the median and interquartile range (25th to 75th percentile). Anthropometric parameters, PF and RMS of IG and CG were compared between the two groups using Student’s t-test when the normality of distribution (Kolmogorov–Smirnov test) and the equality of variance (Levene median test) was verified. When these conditions were not obtained, a Mann–Whitney rank-sum test was used instead. Spearman’s rank correlation coefficients “r” were used to determine relationships between RMS, UI, functional mobility, and AMS in patients with UI. Magnitude of “r” values was considered as trivial (r < 0.1), small (0.1 < r < 0.3), moderate (0.3 < r < 0.5), large (0.5 < r < 0.7), very large (0.7 < r < 0.9), nearly perfect (r > 0.9), and perfect (r = 1.0) [42]. For the parametric tests, the effect sizes were given by Cohen’s d (mean 1 - mean 2)/pooled SD). For the non-parametric test, the effect size was calculated as r = . These effect sizes were interpreted as small: 0.2; medium: 0.5; large > 0.8 [43]. We applied multiple linear regression models to analyze associations between body composition, functional mobility, AMS, and SUI (pad test and UDI-6) with RMS. Unadjusted analyses were performed, and 95% confidence intervals (CI) were calculated. All statistical analysis was carried out using the IBM SPSS® Statistics version 25 (IBM Corp., Armonk, NY, USA). Differences were considered significant for p < 0.05.
3. Results
3.1. Participant Characteristics
One hundred and thirty-nine women were recruited (110 with SUI and 29 CG). After applying the inclusion and exclusion criteria, the final sample consisted of 60 women (IG (n = 31), CG (n = 29)) (Figure 1).
Anthropometric data, SUI tests, PF, and RMS for CG (n = 29) and IG (n = 31) are shown in Table 1. No significant differences were found between the two groups for age and body height (p > 0.05); however, body weight, fat mass, BMI, and WC scores were significantly higher for IG than CG (p = 0.01; p < 0.01; p < 0.001, and p < 0.001, respectively). PF was deteriorated for IG compared to CG (p < 0.001), as evidenced by results for TUG (p < 0.001) and AMS at both 60°/s and 90°/s (p < 0.001). Furthermore, IG had a lower RMS (i.e., MIP and MEP, p < 0.001) compared to CG. Finally, IG showed respiratory muscle weakness when compared with normal predictive values (MIP: p < 0.001 and MEP: p = 0.001).

Table 1.
Characteristics of the participants.
3.2. Correlation of RMS (MIP and MEP) with SUI (UDI-6, IIQ-7, and Pad Test), PF (TUG, AMS at 60°/s, and AMS at 90°/s), WC, and BMI
A large correlation was observed with MEP for UDI-6, IIQ-7, TUG, AMS at 60°/s, and AMS at 90°/s (Table 2). A moderate correlation was observed with MEP for the pad test and WC, whereas MIP had a moderate correlation with IIQ-7 and AMS at 60°/s. However, no correlation was found between RMS and BMI. (Table 2).

Table 2.
Spearman correlation coefficient “r” analysis for relationships between respiratory muscle strength (RMS), stress urinary incontinence (SUI), waist circumference (WC), body mass index (BMI), and physical function (PF) in incontinent women (n = 31).
3.3. Correlation of SUI (UDI-6, IIQ-7, and Pad Test) with PF (TUG, AMS at 60°/s, and AMS at 90°/s), WC, and BMI
UDI-6 showed a very large correlation with IIQ-7, pad test, and TUG and a large correlation with WC, BMI, AMS at 60°/s, and AMS at 90°/s (Table 2). IIQ-7 showed a very large correlation with the pad test, TUG, and AMS at 60°/s; a large correlation with AMS at 90°/s; and a moderate correlation with WC (Table 2). The pad test showed a large correlation with WC, TUG, and AMS at 60°/s and a moderate correlation with AMS at 90°/s (Table 2).
3.4. Correlation of WC and BMI with PF (TUG, AMS at 60°/s, and AMSat 90°/s)
WC significantly correlated with TUG and AMS at 60°/s (Table 2), whereas BMI had a moderate correlation with TUG.
3.5. Associations between PF (TUG, AMS at 60°/s), WC, BMI, Fat Mass, and SUI (UDI-6 and Pad Test) with RMS
The linear regression model results are presented in Table 3. We showed that women having a higher fat mass perform more efficiently in the MEP assessment (β coefficient = 0.463, p = 0.034). MEP was significantly affected by BMI. Each point increase in BMI leads to alter MEP performance by −1.135.

Table 3.
Regression analysis summary for factors impacting on MEP and MIP for women with SUI.
The results showed that an increasing score of UDI-6 is associated with worse MEP. Indeed, for each point increase in the mean overall UDI-6 score, the MEP will decrease by 0.354. In addition, MEP score increased with the increase of AMS (β coefficient = 0.161, p = 0.041).
However, our results showed that MIP does not present a significant association with SUI, BMI, WC, TUG, or AMS.
4. Discussion
This study showed that respiratory muscle weakness leads to a higher degree of UI severity and a deterioration in functional mobility. In particular, expiratory muscle weakness had a negative effect on SUI and PF. This study provides important novel insight into the relationships among RMS, UDI-6, IIQ-7, PF, AMS, WC, and BMI of patients with SUI. These findings are confirmed by the functional connection between the diaphragm, abdominal muscles, and PFM [9], which participate in postural control and breathing [7,44].
The present results support the hypothesis that expiratory muscle contractions during sneezing or coughing generate a rapid increase in IAP, which pulls up the diaphragm and elevates IAP, resulting in high expiratory flow rates [7,45]. Therefore, SUI emerges when powerful contractions of the PFM fail or cannot sustain the increasing pressure resulting from expiratory muscle activity [15]. Consequently, if the expiratory muscle strength is compromised because of its synergic activity, the actions of PFM may also be negatively impacted, potentially leading to SUI. Additionally, the relationship between abdominal muscle and SUI as well as the relationship between SUI and functional mobility could be explained by expiratory muscular strength. The present study produced results that corroborate the findings by Aguilar-Zafra et al. (2022) [15], who found that expiratory muscular weakness had a similar decrease in SUI function.
Our findings identified that expiratory muscle strength was inversely correlated with the WC. In other words, linear regression model results indicate that MEP is associated with BMI. Previous research has linked WC to SUI in the elderly [46,47], but the relationship between BMI and SUI is inconsistent [48]. This could be explained by the fact that a high BMI, which could be due to muscle weight or edema, does not represent abdominal adiposity. However, WC can negatively affect the mechanism of continence through increasing IAP and exerting direct pressure on the pelvic and urethral structures [49,50]. Although WC and BMI are correlated, in practice, measuring and interpreting WC is easier than measuring BMI. As a result, we believe WC is a more accurate clinical predictor of SUI than BMI.
Another key finding was that RMS was associated with AMS, especially for expiratory muscles. This observation supports the role of the contraction of the abdominal muscles during forced expiration maneuvers, which contribute greatly to making expiration more rapid and more efficient. Their actions result in pulling down the ribcage and facilitating the upward movement of the diaphragm [7]. During efforts that increase IAP (e.g., coughing, sneezing, jumping), the PFM is engaged to support the position of the bladder neck and assist in maintaining continence. Several studies support the association between trunk flexor strength and the PFM and suggest that contractions of the PFM are related to abdominal muscle activity [9,51]. From this perspective, it is compelling that trunk or AMS and coordination can generate an appropriate contraction of the PFM, which directly prevents SUI episodes [52,53].
The present study reveals a positive correlation between SUI and expiratory muscle strength in incontinent women for the first time. By measuring expiratory muscle strength, clinicians can detect the deterioration of PF in patients with SUI. Thus, it is recommended to integrate RMS measurements in patients with SUI to prevent some comorbidities [54] (i.e., urinary tract infections, constipation, chronic obstructive pulmonary disease, depression), stop SUI’s negative effect on lifestyle, and reduce the impairment of quality of life. In this vein, some researchers have found that PFM exercise improves pulmonary function and posture [44,55,56], especially when paired with abdominal muscle training [57]. The ability to perform activities of daily living without assistance is commonly used to assess functional capacity. Incontinent women had impairment in functional capacity, and the state of the expiratory muscles can be suggested as an appropriate variable for estimating functional capacity.
The poor performance on TUG tests may be a risk factor for SUI. The objective of the TUG test is to evaluate the dynamic balance between sitting, standing, and walking. The reduction in mobility associated with the functional decline of TUG may be the cause of the development of SUI because of uncoordinated PFM, inability to rush to the toilet in time, or other multifactorial factors [53,58].
In addition, as determined by TUG performance, functional mobility impairment was statistically associated with weaker AMS, abdominal obesity, and poorer expiratory muscle strength but not with the inspiratory muscles. These findings, however, could also be attributed to expiratory muscle strength directly interfering with the trunk stabilizing system [44] given that the trunk stabilizing system is associated with balance and walking ability [59]. In support, functional capacity is negatively affected by weakness in the abdominal muscles [59] and a lower balance capacity [60] compared to continent women. As a result, it is reasonable to expect that when patients have difficulty performing activities requiring a significant contribution from abdominal muscles, deficiencies in their ability to perform these activities will be a sign of expiratory muscle weakness. This weakness is associated with alteration in the activity of the PFM, resulting in a shift in the pelvic position, which can impair breathing and posture [61].
Some strengths of the present study should be highlighted. First, we used validated questionnaires to assess the SUI severity. Second, this is the first study that investigated the association between SUI and PF and RMS in North African women with SUI. Future studies with more heterogeneous samples of women (i.e., athletes, pregnant) are warranted.
This study presents some limitations. First, our study has a cross-sectional design with a relatively small number of recruited participants. Studies with a larger sample size to confirm our findings are necessary. Second, SUI evaluated by the one-hour pad test as suggested by ICS, which is secondary to fluid intake, is sadly unreliable, as patients will have different degrees of dehydration. Therefore, the pad testing needs to be either 48 h or should be with a fixed volume instilled in the bladder. Third, it was not possible to isolate the participation of other muscles that have helped with trunk flexion during the isokinetic dynamometer assessment. Even though the abdominal group is the primary trunk flexor, other muscles, such as the iliopsoas, contribute to trunk flexion and may have influenced strength measurements and, by extension, correlational analysis. Fourth, it would be interesting to determine if respiratory muscle weakness influences the ability to walk short distances by implementing functional tests (e.g., 6 min walk test). Finally, we did not determine the parity of the participants [62]. Parity apparently influences some lung function data, including MIP and MEP [63,64].
5. Conclusions
The study showed that incontinent women have lower RMS, mainly within the expiratory muscles, and deteriorated PF (functional ability, AMS, abdominal obesity) compared to healthy individuals. Furthermore, the patients’ urinary and PF were moderately to significantly affected by expiratory muscle strength. Since SUI may decrease PF and RMS, it is highly recommended to encourage women to train their PFM. PFM training could effectively improve RMS and AMS in patients with SUI. By feeling more confident about increasing IAP, women with SUI would strengthen their expiratory muscles and improve PF.
Author Contributions
Conceptualization, S.A. (Sirine Abidi)., S.G., M.C. and M.H.E.; methodology, S.A. (Sirine Abidi)., A.G., S.G, M.C. and S.A. (Said Ahmaidi); formal analysis, S.A. (Sirine Abidi). and M.C.; data curation, S.A. (Sirine Abidi).; writing—original draft preparation, S.A. (Sirine Abidi)., and A.G.; writing—review and editing, S.A. (Sirine Abidi)., A.G., O.G., T.P., J.L., H.B.S., B.K., K.W. and M.C.; visualization, S.A. (Sirine Abidi) and A.G.; supervision, M.H.E. and S.A. (Said Ahmaidi); project administration, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki 1975 and approved by the local ethics committee from the High Institute of Nursing, University of Sfax, Tunisia (Protection Committee Approval Registration code: CPP SUD N° 0355/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy restrictions.
Acknowledgments
The authors would like to thank all patients and healthy controls who participated in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecology J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, N.K.; Neumann, P. Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manouevre. Neurourol. Urodyn. 2006, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Hwang, U.J.; Lee, M.S.; Jung, S.H.; Ahn, S.H.; Kwon, O.Y. Effect of pelvic floor electrical stimulation on diaphragm excursion and rib cage movement during tidal and forceful breathing and coughing in women with stress urinary incontinence: A randomized controlled trial. Medicine 2021, 100, e24158. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man. Ther. 2004, 9, 3–12. [Google Scholar] [CrossRef]
- Navarro Brazalez, B.; Sanchez Sanchez, B.; Prieto Gomez, V.; De La Villa Polo, P.; McLean, L.; Torres Lacomba, M. Pelvic floor and abdominal muscle responses during hypopressive exercises in women with pelvic floor dysfunction. Neurourol. Urodyn. 2020, 39, 793–803. [Google Scholar] [CrossRef]
- Hodges, P.W.; Gandevia, S.C. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J. Appl. Physiol. 2000, 89, 967–976. [Google Scholar] [CrossRef]
- Talasz, H.; Kofler, M.; Kalchschmid, E.; Pretterklieber, M.; Lechleitner, M. Breathing with the pelvic floor? Correlation of pelvic floor muscle function and expiratory flows in healthy young nulliparous women. Int. Urogynecology J. 2010, 21, 475–481. [Google Scholar] [CrossRef]
- Talasz, H.; Kremser, C.; Kofler, M.; Kalchschmid, E.; Lechleitner, M.; Rudisch, A. Phase-locked parallel movement of diaphragm and pelvic floor during breathing and coughing—A dynamic MRI investigation in healthy females. Int. Urogynecology J. 2011, 22, 61–68. [Google Scholar] [CrossRef]
- Neumann, P.; Gill, V. Pelvic Floor and Abdominal Muscle Interaction: EMG Activity and Intra-abdominal Pressure. Int. Urogynecology J. 2002, 13, 125–132. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, S.Y.; Oh, D.W. Pelvic floor muscle exercises utilizing trunk stabilization for treating postpartum urinary incontinence: Randomized controlled pilot trial of supervised versus unsupervised training. Clin. Rehabil. 2012, 26, 132–141. [Google Scholar] [CrossRef]
- Sapsford, R.R.; Hodges, P.W.; Richardson, C.A.; Cooper, D.H.; Markwell, S.J.; Jull, G.A. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol. Urodyn. 2001, 20, 31–42. [Google Scholar] [CrossRef]
- Leitner, M.; Moser, H.; Taeymans, J.; Kuhn, A.; Radlinger, L. Pelvic floor muscle displacement during voluntary and involuntary activation in continent and incontinent women: A systematic review. Int. Urogynecology J. 2015, 26, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.W.; Rath, D.; Hodges, P.W. Postural and respiratory activation of the trunk muscles changes with mode and speed of locomotion. Gait Posture 2004, 20, 280–290. [Google Scholar] [CrossRef]
- Azevedo, I.G.; Sousa, S.L.D.O.; Viana, E.D.S.R.; Dantas, D.D.S.; Maciel, Á.C.C.; Da Câmara, S.M.A. Relationship between symptomatic pelvic organ prolapse and respiratory muscle strength in middle-aged and older women in Northeast Brazil: A cross-sectional study. Physiother. Theory Pract. 2021, 37, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zafra, S.; Del Corral, T.; Montero-Gonzalez, N.; de-Gabriel-Hierro, A.; Lopez-de-Uralde-Villanueva, I. Urinary incontinence and impaired physical function are associated with expiratory muscle weakness in patients with multiple sclerosis. Disabil. Rehabil. 2022, 44, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical status: The use and interpretation of anthropometry. In Report of a WHO Expert Committee; World Health Organ: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Bosy-Westphal, A.; Later, W.; Hitze, B.; Sato, T.; Kossel, E.; Glüer, C.-C.; Heller, M.; Müller, M.J. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes. Facts 2008, 1, 319–324. [Google Scholar] [CrossRef]
- Mason, C.; Katzmarzyk, P. Variability in Waist Circumference Measurements According to Anatomic Measurement Site. Obesity 2009, 17, 1789–1795. [Google Scholar] [CrossRef]
- Ghroubi, S.; El Fani, N.; Elarem, S.; Alila, S.; Ben Ayed, H.; Borgi, O.; Chmak, J.; Elleuch, M.H. Arabic (Tunisian) translation and validation of the urogenital distress inventory short form (UDI-6) and incontinence impact questionnaire short form (IIQ-7). Arab. J. Urol. 2020, 18, 27–33. [Google Scholar] [CrossRef]
- Castro-Diaz, D.; Robinson, D.; Bosch, R.; Costantini, E.; Cotterill, N.; Espuna-Pons, M.; Kocjancic, E.; Lemos, N.; Tarcan, T.; Yoshida, M. Patient-reported outcome assessment. In Incontinence: Proceedings of the Sixth International Consultation on Incontinence, 6th ed.; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; Health Publications Limited: Tokyo, Japan, 2017; pp. 541–598. [Google Scholar]
- Shumaker, S.A.; Wyman, J.F.; Uebersax, J.; McClish, D.; Fantl, J.A. Health-related quality of life measures for women with urinary incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual. Life Res. 1994, 3, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, K.; Grzybowska, M.E.; Kubik-Komar, A.; Rechberger, T.; Miotla, P. Identification of the Urogenital Distress Inventory-6 and the Incontinence Impact Questionnaire-7 cutoff scores in urinary incontinent women. Health Qual. Life Outcomes 2021, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, J.S.; Wyman, J.F.; Shumaker, S.A.; McClish, D.K. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol. Urodyn. 1995, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dube, B.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- Dimitriadis, Z.; Kapreli, E.; Konstantinidou, I.; Oldham, J.; Strimpakos, N. Test/retest reliability of maximum mouth pressure measurements with the MicroRPM in healthy volunteers. Respir. Care 2011, 56, 776–782. [Google Scholar] [CrossRef]
- Black, L.F.; Hyatt, R.E. Maximal respiratory pressures: Normal values and relationship to age and sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar]
- Harik-Khan, R.I.; Wise, R.A.; Fozard, J.L. Determinants of maximal inspiratory pressure. The Baltimore Longitudinal Study of Aging. Am. J. Respir. Crit. Care Med. 1998, 158, 1459–1464. [Google Scholar] [CrossRef]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Kammoun, R.; Ben Saad, H. From deficiency to handicap in the respiratory field: Lung function tests (LFT) norms and quality of life (QOL) questionnaires validated for the Tunisian population. La Tunis. Med. 2020, 98, 378–395. [Google Scholar]
- Evans, J.A.; Whitelaw, W.A. The assessment of maximal respiratory mouth pressures in adults. Respir. Care 2009, 54, 1348–1359. [Google Scholar] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Grabiner, M.D.; Jeziorowski, J.J. Isokinetic trunk extension and flexion strength-endurance relationships. Clin. Biomech. 1991, 6, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Leardini, A.; Biagi, F.; Merlo, A.; Belvedere, C.; Benedetti, M.G. Multi-segment trunk kinematics during locomotion and elementary exercises. Clin. Biomech. 2011, 26, 562–571. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.P.; Barbado, D.; Juan-Recio, C.; Lopez-Valenciano, A.; Vera-Garcia, F.J. Isokinetic trunk flexion-extension protocol to assess trunk muscle strength and endurance: Reliability, learning effect, and sex differences. J. Sport Health Sci. 2020, 9, 692–701. [Google Scholar] [CrossRef]
- Karatas, G.K.; Gogus, F.; Meray, J. Reliability of isokinetic trunk muscle strength measurement. Am. J. Phys. Med. Rehabil. 2002, 81, 79–85. [Google Scholar] [CrossRef]
- Karatas, M.; Cetin, N.; Bayramoglu, M.; Dilek, A. Trunk muscle strength in relation to balance and functional disability in unihemispheric stroke patients. Am. J. Phys. Med. Rehabil. 2004, 83, 81–87. [Google Scholar] [CrossRef]
- Sole, G.; Hamrén, J.; Milosavljevic, S.; Nicholson, H.; Sullivan, S.J. Test-retest reliability of isokinetic knee extension and flexion. Arch. Phys. Med. Rehabil. 2007, 88, 626–631. [Google Scholar] [CrossRef]
- Madsen, O.R. Trunk extensor and flexor strength measured by the Cybex 6000 dynamometer. Assessment of short-term and long-term reproducibility of several strength variables. Spine 1996, 21, 2770–2776. [Google Scholar] [CrossRef]
- Hopkins, W.G. Linear models and effect magnitudes for research, clinical and practical applications. Sportscience 2010, 14, 49–59. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor and Francis: Hoboken, NJ, USA, 2013; pp. 23–26. [Google Scholar] [CrossRef]
- Szczygiel, E.; Blaut, J.; Zielonka-Pycka, K.; Tomaszewski, K.; Golec, J.; Czechowska, D.; Maslon, A.; Golec, E. The Impact of Deep Muscle Training on the Quality of Posture and Breathing. J. Mot. Behav. 2018, 50, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, F.; Lee, A.H.; Binns, C.W.; Nishimura, K.; Taniguchi, H. Association of impaired respiratory function with urinary incontinence. Respirology 2009, 14, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Sebastianelli, A.; Salvi, M.; De Nunzio, C.; Tubaro, A.; Gravas, S.; Moncada, I.; Serni, S.; Maggi, M.; Vignozzi, L. The impact of central obesity on storage luts and urinary incontinence after prostatic surgery. Curr. Urol. Rep. 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, C.; Schreiner, L.; Morsch, T.; Saadi, R.; Figueiredo, M.; Padoin, A. Urinary Incontinence and Quality of Life in Female Patients with Obesity. Rev. Bras. Ginecol. Obs. RBGO Gynecol. Obstet. 2018, 40, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.K.; Danforth, K.N.; Rosner, B.; Curhan, G.C.; Resnick, N.M.; Grodstein, F. Body Mass Index, Weight Gain, and Incident Urinary Incontinence in Middle-Aged Women. Obstet. Gynecol. 2007, 110, 346–353. [Google Scholar] [CrossRef]
- Lamerton, T.J.; Torquati, L.; Brown, W.J. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1735–1745. [Google Scholar] [CrossRef]
- Richter, H.E.; Creasman, J.M.; Myers, D.L.; Wheeler, T.L.; Burgio, K.L.; Subak, L.L. Urodynamic characterization of obese women with urinary incontinence undergoing a weight loss program: The Program to Reduce Incontinence by Diet and Exercise (PRIDE) trial. Int. Urogynecology J. Pelvic. Floor Dysfunct. 2008, 19, 1653–1658. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; Da Roza, T.; Mochizuki, L.; Arbieto, E.R.M.; Tonon da Luz, S.C. Assessment of abdominal and pelvic floor muscle function among continent and incontinent athletes. Int. Urogynecology J. 2019, 30, 693–699. [Google Scholar] [CrossRef]
- Hung, H.-C.; Hsiao, S.-M.; Chih, S.-Y.; Lin, H.-H.; Tsauo, J.-Y. An alternative intervention for urinary incontinence: Retraining diaphragmatic, deep abdominal and pelvic floor muscle coordinated function. Man. Ther. 2010, 15, 273–279. [Google Scholar] [CrossRef]
- Madill, S.J.; McLean, L. Relationship between abdominal and pelvic floor muscle activation and intravaginal pressure during pelvic floor muscle contractions in healthy continent women. Neurourol. Urodyn. 2006, 25, 722–730. [Google Scholar] [CrossRef]
- Van Gerwen, M.; Schellevis, F.; Lagro-Janssen, T. Comorbidities associated with urinary incontinence: A case-control study from the Second Dutch National Survey of General Practice. J. Am. Board Fam. Med. 2007, 20, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Ha, M. Effect of pelvic floor muscle exercises on pulmonary function. J. Phys. Ther. Sci. 2015, 27, 3233–3235. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hwang, B.; Kim, Y. The impact of the pelvic floor muscles on dynamic ventilation maneuvers. J. Phys. Ther. Sci. 2015, 27, 3155–3157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kucukkaya, B.; Kahyaoglu Sut, H. Effectiveness of pelvic floor muscle and abdominal training in women with stress urinary incontinence. Psychol. Health Med. 2021, 26, 779–786. [Google Scholar] [CrossRef]
- Chiu, A.F.; Huang, M.H.; Hsu, M.H.; Liu, J.L.; Chiu, J.F. Association of urinary incontinence with impaired functional status among older people living in a long-term care setting. Geriatr. Gerontol. Int. 2015, 15, 296–301. [Google Scholar] [CrossRef]
- Müller, R.; Ertelt, T.; Blickhan, R. Low back pain affects trunk as well as lower limb movements during walking and running. J. Biomech. 2015, 48, 1009–1014. [Google Scholar] [CrossRef]
- Smith, M.D.; Coppieters, M.W.; Hodges, P.W. Is balance different in women with and without stress urinary incontinence? Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2008, 27, 71–78. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, M.-H.; Chen, T.-W.; Weng, M.-C.; Lee, C.-L.; Wang, G.-J. Relationship between ankle position and pelvic floor muscle activity in female stress urinary incontinence. Urology 2005, 66, 288–292. [Google Scholar] [CrossRef]
- Triki, L.; Ben Saad, H. The impacts of parity on spirometric parameters: A systematic review. Expert Rev. Respir. Med. 2021, 15, 1169–1185. [Google Scholar] [CrossRef]
- Ben Saad, H.; Tfifha, M.; Harrabi, I.; Tabka, Z.; Guenard, H.; Hayot, M.; Zbidi, A. [Factors influencing pulmonary function in Tunisian women aged 45 years and more]. Rev. Mal. Respir. 2006, 23, 324–338. [Google Scholar] [CrossRef]
- Lemos, A.; Souza, A.I.; Andrade, A.D.; Figueiroa, J.N.; Cabral-Filho, J.E. Respiratory muscle strength: Comparison between primigravidae and nulligravidae. J. Bras. Pneumol. 2011, 37, 193–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).