Effects of Fluids on the Sublingual Microcirculation in Sepsis
Abstract
1. Introduction
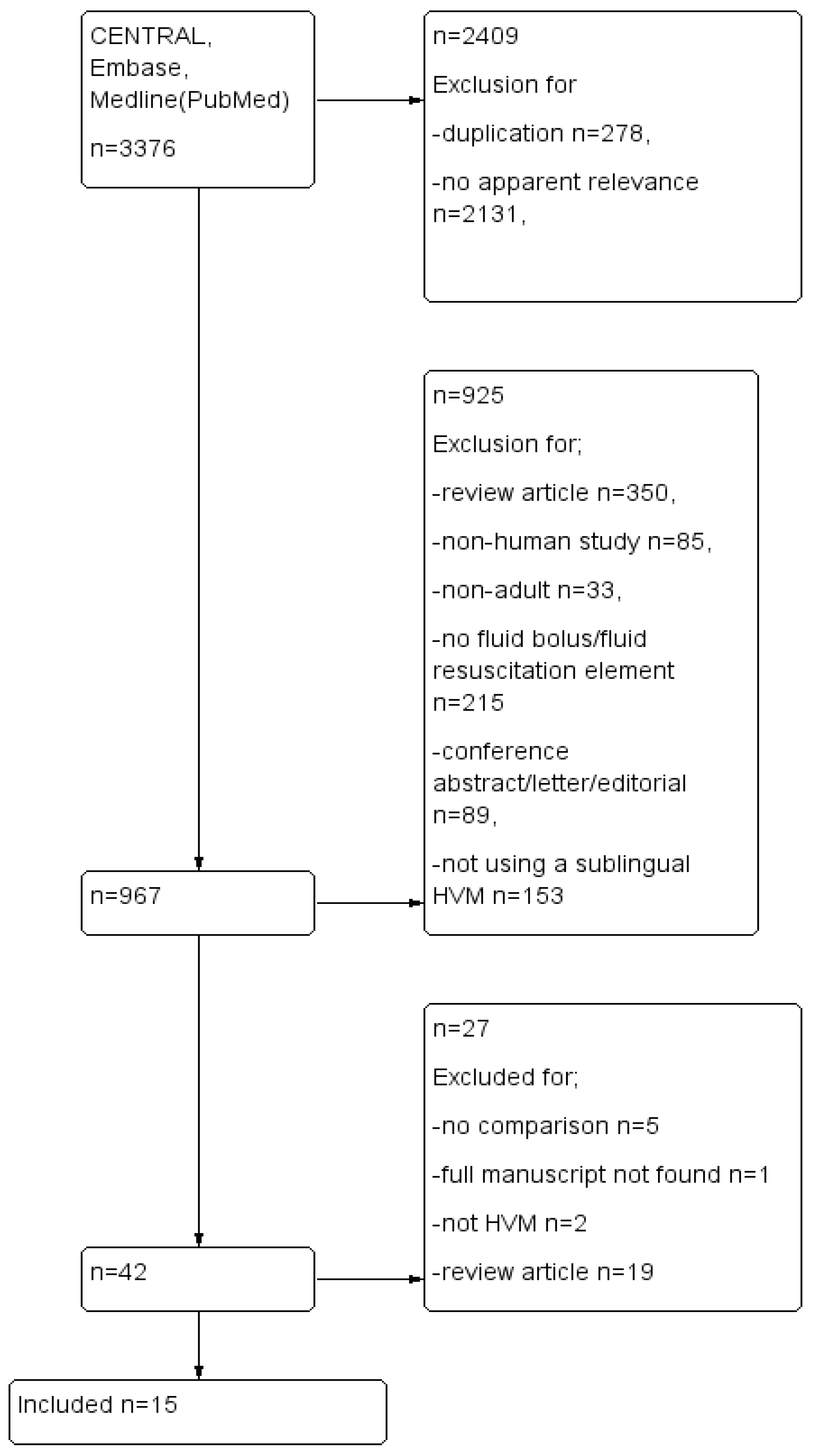
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Risk of Bias
3. Results
3.1. Data
3.2. Participants
3.3. Intervention
3.4. Comparator—Microcirculation Assessment and Outcome
| Author | Centre | Participants | Fluid (Type) | Fluid (Volume) | Microcirculation Measurement Device | Microcirculation Outcome Measure |
|---|---|---|---|---|---|---|
| Dubin, 2010 [32] | 2 Teaching ICUs Randomised controlled pilot study | Confirmed or suspected infection plus 2 ≤ signs of SIRS Signs of tissue hypoperfusion, MAP ≤ 65 mmHg despite fluid resuscitation or lactate ≤ 4 mmol/L n = 20 | 6% HES 130/0.4 in 0.9% NaCl vs. 0.9% NaCl | EGDT (CVP 8–12 mmHg, MAP ≥ 65 mmHg, ScVO2 ≥ 70%) Total fluid intake Saline group 8368 ± 2405 mL vs. 4682 ± 1371 mL p = 0.0008 | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | At 24 h 6% HES 130/0.4 group; ↑ MFI ↑ PPV ↑ FCD ↑ TVD Saline group; ↑ Heterogeneity Index |
| Edul, 2014 [35] | Single centre, surgical ICU Prospective observational | Post-operative severe sepsis n = 22 | 6% HES in 0.9% NaCl | 10 mL/kg | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | Before and 20 min after bolus ↑ RBC velocity Trend to ↑ PVD |
| Massey 2018 [36] | Multicentre, formal design sub-study of randomised control trial (ProCESS) | Adults with septic shock n = 207 | Not assigned, 96% received crystalloid | EGDT n = 439 (2254 ± 1472 mL) vs. Protocol-standard n = 446 (2226 ± 1363 mL) vs. Usual care (2083 ± 1405 mL) p = 0.15 | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | Reduced MFI in EGDT group, only stat significant difference Association of TVD, PVD and De Backer score with mortality |
| Ospina-Tascon, 2010 [40] | Single centre, ICU Prospective non-randomised observational | Severe sepsis, requiring fluid bolus n = 60 | 4% albumin (n = 31) vs. Ringer’s lactate (n = 29) | 400 mL 4% albumin vs. 1000 mL Ringer’s lactate, over 30 min | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | ↑ TVD ↑ PVD ↑ sPPV <24 h sepsis had more microvascular response to fluid |
| Pottecher, 2010 [33] | Medical and surgical University hospital ICU Prospective observational | Mechanically ventilated, severe sepsis or septic shock, requiring volume expansion n = 25 | Crystalloid (0.9% NaCl, n = 8) or colloid (6% HES 130/0.4, n = 17) | 500 mL over 30 min | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | PLR and volume expansion both resulted in the following microvascular changes; ↑ FCD ↑ MFI ↑ PPV ↓ FHI |
| Pranskunas, 2013 [34] | 22 bed mixed ICU, prospective observational | Patients with clinical signs of impaired organ perfusion n = 50 | 0.9% NaCl (crystalloid) or 6% HES 130/0.4 | 500 mL/30 min | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | Low MFI vs. High MFI Low MFI group response to FC; ↑ MFI ↓ Clinical signs of hypoperfusion |
| Sadaka, 2011 [38] | 54 bed medical surgical, university affiliated ICU Prospective observational | Severe sepsis RCC transfusion for Hb < 7.0 or Hb 7.0–9.0 with lactic acidosis or ScVO2 < 70% n = 21 | Red Blood Cell (RBC) | 1 unit | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, Netherlands.) and Near Infrared Spectrometry (NIRS)(InSpectra Model 650; Hutchinson Technology Inc., Hutchinson, MN, USA) | No statistically significant change in PPV, MFI or PVD NIRS derived and SD derived variables changed in the same direction |
| Sakr, 2007 [39] | 31 bed medical surgical ICU in a university hospital Prospective observational | Severe sepsis requiring RBC transfusion n = 35 | RBC | 1 unit n = 31 2 units n = 4 Groups divided based on > or <8% increase in capillary perfusion | Cytoscan ARII (Cytometrics, Philadelphia, PA, USA) | ++Interindividual variability, overall, no impact on the microcirculation Improved microcirculation in patients with altered baseline, deterioration in patients with preserved baseline |
| Van Haren, 2012 [31] | 15 bed ICU, Prospective, double -blind RCT | Septic shock n = 24 | 7.2% NaCl/6% HES (HT) vs. 6% HES (IT) | 250 mL hypertonic vs. 500 mL IT | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | No significant changes found in any of the microcirculatory measurements compared to baseline or between groups ↑ Small vessel MFI in fluid responsive patients |
| Zhou, 2021, [42] | Emergency department and ICU Parallel group randomised prospective trial | Severe sepsis and septic shock n = 31 | Not reported | POEM score guided vs. POEM score measured but did not guide resuscitation | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | Microcirculation guided resuscitation does not affect perfusion and organ function but does result in significantly lower fluid intake |
| Van der Voort, 2015 [41] | 18 bed mixed medical surgical ICU, single-centre, open-labelled, randomised controlled pilot study | Severe sepsis and septic shock n = 90 | Crystalloid and colloid (gelatin or albumin) | EGDT vs. resuscitation guided by microcirculation monitoring | Cytoscan (Cytometrics, Philadelphia, Pennsylvania, PA, USA) | No difference in SOFA between groups at Day 4 |
| Donati, 2014 [29] | 12 bed ICU, prospective randomised study | Sepsis, severe sepsis, septic shock requiring blood transfusion n = 20 | RBC | Leukodepleted vs. non-leukodepleted RBC | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.), NIRS, PBR | PPV, DeBacker Score, MFI, HI Leukodepleted RCC = ↑ PPV, PVD, DeBacker Score&Blood velocity |
| Damiani, 2015 [30] | 12 bed ICU, prospective randomised study | Sepsis, severe sepsis, septic shock requiring blood transfusion n = 20 | RBC | Fresh leukodepleted vs. old leukodepleted vs. non-leukodepleted RBC | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | Change in fHB negatively correlated with TVD, DeBacker score, PVD change inverse correlation with fHB |
| Trzeciak, 2008 [16] | Single centre ED and ICU, prospective observational study | Septic patients treated with EGDT n = 33 | Not reported | EGDT | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | MFI improved after EGDT in SOFA-improvers, but MFI did not improve in SOFA-non-improvers |
| Vellinga, 2013 [43] | Post hoc analysis of a single centre prospective observational study | Sepsis n = 70 | Crystalloids, colloids, blood products | EGDT, 250 mL boluses, groups divided according to CVP > 12 mmHg vs. CVP < 12 mmHg | Sidestream Dark Field Device (Microscan, Microvision Medical, Amsterdam, The Netherlands.) | MFI increased after EGDT, MFI and PPV lower in CVP > 12 mmHg group |
3.5. Outcomes—Randomised Trials of Fluid Resuscitation and the Microcirculation in Sepsis
3.6. Outcome Evidence from Prospective Observational Studies
3.7. Evidence for Red Blood Cells and the Microcirculation in Sepsis
3.8. End goal Directed Therapy
3.9. Excluded Studies
4. Discussion
4.1. Allocation (Selection Bias)
4.2. Blinding (Performance Bias and Detection Bias)
4.3. Incomplete Outcome Data (Attrition Bias) and Selective Reporting (Reporting Bias)
4.4. Other Potential Sources of Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Executive summary: Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46, 1–9. [Google Scholar] [CrossRef]
- Maconochie, I.K.; Aickin, R.; Hazinski, M.F.; Atkins, D.L.; Bingham, R.; Couto, T.B.; Guerguerian, A.-M.; Nadkarni, V.M.; Ng, K.-C.; Nuthall, G.A.; et al. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020, 142, S140–S184. [Google Scholar] [CrossRef]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9 (Suppl. S4), S13–S19. [Google Scholar] [CrossRef]
- De Backer, D.; Creteur, J.; Preiser, J.-C.; Dubois, M.-J.; Vincent, J.-L. Microvascular Blood Flow Is Altered in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 98–104. [Google Scholar] [CrossRef]
- Vellinga, N.A.R.; Boerma, E.C.; Koopmans, M.; Donati, A.; Dubin, A.; Shapiro, N.I.; Pearse, R.M.; Machado, F.R.; Fries, M.; Akarsu-Ayazoglu, T.; et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit. Care Med. 2015, 43, 48–56. [Google Scholar] [CrossRef]
- Moore, J.P.R.; Dyson, A.; Singer, M.; Fraser, J. Microcirculatory dysfunction and resuscitation: Why, when, and how. Br. J. Anaesth. 2015, 115, 366–375. [Google Scholar] [CrossRef]
- Poole, D.C.; Pittman, R.N.; Musch, T.I.; Østergaard, L. August Krogh’s theory of muscle microvascular control and oxygen delivery: A paradigm shift based on new data. J. Physiol. 2020, 598, 4473–4507. [Google Scholar] [CrossRef] [PubMed]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19, 14726. [Google Scholar] [CrossRef]
- Fang, X.; Tang, W.; Sun, S.; Huang, L.; Chang, Y.-T.; Castillo, C.; Weil, M.H. Comparison of buccal microcirculation between septic and hemorrhagic shock. Crit. Care Med. 2006, 34, S447–S453. [Google Scholar] [CrossRef] [PubMed]
- den Uil, C.A.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.D.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2010, 31, 3032–3039. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Dubois, M.-J.; De Backer, D.; Creteur, J.; Vincent, J.-L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004, 32, 1825–1831. [Google Scholar] [CrossRef]
- Trzeciak, S.; McCoy, J.V.; Dellinger, R.P.; Arnold, R.C.; Rizzuto, M.; Abate, N.L.; Shapiro, N.I.; Parrillo, J.E.; Hollenberg, S.M. Early Increases in Microcirculatory Perfusion During Protocol-Directed Resuscitation are Associated with Reduced Multi-Organ Failure at 24 h in Patients with Sepsis. Intensive Care Med. 2008, 34, 2210–2217. [Google Scholar] [CrossRef]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.-L. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar] [CrossRef]
- Goedhart, P.T.; Khalilzada, M.; Bezemer, R.; Merza, J.; Ince, C. Sidestream Dark Field (SDF) imaging: A novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt. Express 2007, 15, 15101–15114. [Google Scholar] [CrossRef]
- van Elteren, H.A.; Ince, C.; Tibboel, D.; Reiss, I.K.M.; de Jonge, R.C.J. Cutaneous microcirculation in preterm neonates: Comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J. Clin. Monit. Comput. 2015, 29, 543–548. [Google Scholar] [CrossRef]
- Potter, E.K.; Hodgson, L.; Creagh-Brown, B.; Forni, L.G. Manipulating the Microcirculation in Sepsis—The Impact of Vasoactive Medications on Microcirculatory Blood Flow: A Systematic Review. Shock 2019, 52, 5–12. [Google Scholar] [CrossRef]
- Riversa, E.P.; Jaehne, A.K.; Eichhorn-Wharry, L.; Brown, S.; Amponsah, D. Fluid therapy in septic shock. Curr. Opin. Crit. Care 2010, 16, 297–308. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Davis, A.L.; Zaritsky, A. Role of early fluid resuscitation in pediatric septic shock. JAMA 1991, 266, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Meyhoff, T.S.; Hjortrup, P.B.; Møller, M.H.; Wetterslev, J.; Lange, T.; Kjaer, M.-B.N.; Jonsson, A.B.; Hjortsø, C.J.S.; Cronhjort, M.; Laake, J.H.; et al. Conservative vs liberal fluid therapy in septic shock (CLASSIC) trial-Protocol and statistical analysis plan. Acta Anaesthesiol. Scand. 2019, 63, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Silversides, J.A.; Perner, A.; Malbrain, M.L.N.G. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019, 45, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Boerma, E.C.; Koopmans, M.; Konijn, A.; Kaiferova, K.; Bakker, A.J.; Van Roon, E.N.; Buter, H.; Bruins, N.; Egbers, P.H.; Gerritsen, R.T.; et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: A double-blind randomized placebo controlled trial. Crit. Care Med. 2010, 38, 93–100. [Google Scholar] [CrossRef]
- ROBINS-I Tool. Cochrane Methods. Available online: https://methods.cochrane.org/methods-cochrane/robins-i-tool (accessed on 10 August 2022).
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Donati, A.; Damiani, E.; Luchetti, M.M.; Domizi, R.; Scorcella, C.; Carsetti, A.; Gabbanelli, V.; Carletti, P.; Bencivenga, R.; Vink, H.; et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: A pilot study. Crit. Care 2014, 18, R33. [Google Scholar] [CrossRef]
- Damiani, E.; Adrario, E.; Luchetti, M.M.; Scorcella, C.; Carsetti, A.; Mininno, N.; Pierantozzi, S.; Principi, T.; Strovegli, D.; Bencivenga, R.; et al. Plasma free hemoglobin and microcirculatory response to fresh or old blood transfusions in sepsis. PLoS ONE 2015, 10, e0122655. [Google Scholar] [CrossRef]
- van Haren, F.M.P.; Sleigh, J.; Boerma, E.C.; La Pine, M.; Bahr, M.; Pickkers, P.; van der Hoeven, J.G. Hypertonic Fluid Administration in Patients With Septic Shock: A Prospective Randomized Controlled Pilot Study. Shock 2012, 37, 268–275. [Google Scholar] [CrossRef]
- Dubin, A.; Pozo, M.O.; Casabella, C.A.; Murias, G.; Pálizas, F.; Moseinco, M.C.; Kanoore Edul, V.S.; Pálizas, F.; Estenssoro, E.; Ince, C. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J. Crit. Care 2010, 25, 659.e1–659.e8. [Google Scholar] [CrossRef]
- Pottecher, J.; Deruddre, S.; Teboul, J.-L.; Georger, J.-F.; Laplace, C.; Benhamou, D.; Vicaut, E.; Duranteau, J. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010, 36, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Pranskunas, A.; Koopmans, M.; Koetsier, P.M.; Pilvinis, V.; Boerma, E.C. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013, 39, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.K.; Ince, C.; Navarro, N.; Previgliano, L.; Risso-Vazquez, A.; Rubatto, P.N.; Dubin, A. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann. Intensive Care 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Massey, M.J.; Hou, P.C.; Filbin, M.; Wang, H.; Ngo, L.; Huang, D.T.; Aird, W.C.; Novack, V.; Trzeciak, S.; Yealy, D.M.; et al. Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit. Care 2018, 22, 308. [Google Scholar] [CrossRef]
- ProCESS Investigators; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Sadaka, F.; Aggu-Sher, R.; Krause, K.; O’Brien, J.; Armbrecht, E.S.; Taylor, R.W. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann. Intensive Care 2011, 1, 46. [Google Scholar] [CrossRef]
- Sakr, Y.; Chierego, M.; Piagnerelli, M.; Verdant, C.; Dubois, M.-J.; Koch, M.; Creteur, J.; Gullo, A.; Vincent, J.-L.; De Backer, D. Microvascular response to red blood cell transfusion in patients with severe sepsis*. Crit. Care Med. 2007, 35, 1639–1644. [Google Scholar] [CrossRef]
- Ospina-Tascon, G.; Neves, A.P.; Occhipinti, G.; Donadello, K.; Büchele, G.; Simion, D.; Chierego, M.-L.; Silva, T.O.; Fonseca, A.; Vincent, J.-L.; et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010, 36, 949–955. [Google Scholar] [CrossRef]
- van der Voort, P.H.J.; van Zanten, M.; Bosman, R.J.; van Stijn, I.; Wester, J.P.J.; van Raalte, R.; Oudemans-van Straaten, H.M.; Zandstra, D.F. Testing a conceptual model on early opening of the microcirculation in severe sepsis and septic shock: A randomised controlled pilot study. Eur. J. Anaesthesiol. 2015, 32, 189–198. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Dai, C.; Zhu, Y.; Han, T.; Zhou, J.; Zhao, L.; Wang, X.; Liu, H.; Qu, J.; Li, W. The effectiveness and feasibility of fluid resuscitation directed by microcirculation monitoring in patients with septic shock: A randomized controlled trial. Ann. Palliat. Med. 2021, 10, 9069–9077. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, N.A.; Ince, C.; Boerma, E.C. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: A hypothesis generating post hoc analysis. BMC Anesthesiol. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Watchorn, J.C.; Fargaly, H.; Gilani, M.; Assadi, J.; Deitchman, A.R.; Naumann, D.N.; Wollborn, J.; Goebel, U.; McCurdy, M.T.; Hutchings, S.D. The Reproducibility of the Point of Care Microcirculation (POEM) Score When Used to Assess Critically Ill Patients: A Multicenter Prospective Observational Study. Shock 2020, 54, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Kattan, E.; Ferri, G.; Pairumani, R.; Valenzuela, E.D.; Alegría, L.; Oviedo, V.; Pavez, N.; Soto, D.; Vera, M.; et al. Effects of capillary refill time-vs. lactate-targeted fluid resuscitation on regional, microcirculatory and hypoxia-related perfusion parameters in septic shock: A randomized controlled trial. Ann. Intensive Care 2020, 10, 150. [Google Scholar] [CrossRef]
- Zhao, M.; Li, A.; Zhuang, H.; Dong, L.; Li, J.; Liu, C.; Weng, Y.; Zhang, S.; Duan, M. The clinical significance of determining the severity and prognosis by monitoring the changes in sublingual microcirculation in patients with severe sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue Chin. Crit. Care Med. Zhongguo Weizhongbing Jijiuyixue 2012, 24, 158–161. [Google Scholar]
- Lu, Y.; Liu, L.; Qiu, X.; Yu, Q.; Yang, Y.; Qiu, H. Effect of early goal directed therapy on tissue perfusion in patients with septic shock. World J. Emerg. Med. 2013, 4, 117–122. [Google Scholar] [CrossRef][Green Version]
- Veenstra, G.; Ince, C.; Barendrecht, B.W.; Zijlstra, H.W.; Boerma, E.C. Differences in capillary recruitment between cardiac surgery and septic patients after fluid resuscitation. Microvasc. Res. 2019, 123, 14–18. [Google Scholar] [CrossRef]
- Ince, C.; Boerma, E.C.; Cecconi, M.; De Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.-L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef]
- Valerio, L.; Peters, R.J.; Zwinderman, A.H.; Pinto-Sietsma, S.-J. Reproducibility of sublingual microcirculation parameters obtained from sidestream darkfield imaging. PLoS ONE 2019, 14, e0213175. [Google Scholar] [CrossRef]
- Gilbert-Kawai, E.; Coppel, J.; Bountziouka, V.; Ince, C.; Martin, D.; Caudwell Xtreme Everest and Xtreme Everest 2 Research Groups. A comparison of the quality of image acquisition between the incident dark field and sidestream dark field video-microscopes. BMC Med. Imaging 2016, 16, 10. [Google Scholar] [CrossRef]
- Boerma, E.C.; van der Voort, P.H.J.; Spronk, P.E.; Ince, C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit. Care Med. 2007, 35, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Sinaasappel, M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit. Care Med. 1999, 27, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Calfee, C.S. Phenotypes in ARDS: Moving Towards Precision Medicine. Curr. Opin. Crit. Care 2019, 25, 12–20. [Google Scholar] [CrossRef]
- Ince, C. The rationale for microcirculatory guided fluid therapy. Curr. Opin. Crit. Care 2014, 20, 301–308. [Google Scholar] [CrossRef]
- Roy, T.K.; Secomb, T.W. Effects of impaired microvascular flow regulation on metabolism-perfusion matching and organ function. Microcirculation 2021, 28, 12673. [Google Scholar] [CrossRef] [PubMed]
- Magnin, M.; Foulon, É.; Lurier, T.; Allaouchiche, B.; Bonnet-Garin, J.-M.; Junot, S. Evaluation of microcirculation by Sidestream Dark Field imaging: Impact of hemodynamic status on the occurrence of pressure artifacts—A pilot study. Microvasc. Res. 2020, 131, 104025. [Google Scholar] [CrossRef]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; de Pont, A.C.; Sakr, Y.; Spies, C.; Reinhart, K.; Vincent, J.L.; Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care 2008, 12, R74. [Google Scholar] [CrossRef]
- Silva, J.M.; de Oliveira, A.M.R.R.; Nogueira, F.A.M.; Vianna, P.M.M.; Pereira Filho, M.C.; Dias, L.F.; Maia, V.P.L.; de Suoza Neucamp, C.; Amendola, C.P.; Carmona, M.J.C.; et al. The effect of excess fluid balance on the mortality rate of surgical patients: A multicenter prospective study. Crit. Care 2013, 17, R288. [Google Scholar] [CrossRef]
- Kissoon, N.R.; Mandrekar, J.N.; Fugate, J.E.; Lanzino, G.; Wijdicks, E.F.M.; Rabinstein, A.A. Positive Fluid Balance Is Associated With Poor Outcomes in Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2015, 24, 2245–2251. [Google Scholar] [CrossRef]
- EMA PRAC Recommends Suspending Hydroxyethyl-Starch Solutions for Infusion from the Market. Available online: https://www.ema.europa.eu/en/news/prac-recommends-suspending-hydroxyethyl-starch-solutions-infusion-market-0 (accessed on 4 May 2022).
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R.; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar] [CrossRef]
- Lat, I.; Coopersmith, C.M.; De Backer, D. The Surviving Sepsis Campaign: Fluid Resuscitation and Vasopressor Therapy Research Priorities in Adult Patients. Crit. Care Med. 2021, 49, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Ince, C.; Orlando, F.; Pierpaoli, E.; Cirioni, O.; Giacometti, A.; Mocchegiani, F.; Pelaia, P.; Provinciali, M.; Donati, A. Effects of the Infusion of 4% or 20% Human Serum Albumin on the Skeletal Muscle Microcirculation in Endotoxemic Rats. PLoS ONE 2016, 11, e0151005. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef]
- The Effect of Three Different Fluids(Albumin 5%, Normal Saline, Hydroxyethyl Starch 130 kD) on Microcirculation in Severe Sepsis/Septic Shock Patients—No Study Results Posted—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01319630 (accessed on 1 December 2021).
- Sturm, T.; Leiblein, J.; Clauß, C.; Erles, E.; Thiel, M. Bedside determination of microcirculatory oxygen delivery and uptake: A prospective observational clinical study for proof of principle. Sci. Rep. 2021, 11, 24516. [Google Scholar] [CrossRef]
- Naumann, D.N.; Mellis, C.; Smith, I.M.; Mamuza, J.; Skene, I.; Harris, T.; Midwinter, M.J.; Hutchings, S.D. Safety and feasibility of sublingual microcirculation assessment in the emergency department for civilian and military patients with traumatic haemorrhagic shock: A prospective cohort study. BMJ Open 2016, 6, e014162. [Google Scholar] [CrossRef]
- Dekker, N.A.; Veerhoek, D.; Koning, N.J.; van Leeuwen, A.L.I.; Elbers, P.W.G.; van den Brom, C.E.; Vonk, A.B.A.; Boer, C. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019, 74, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef]
- Alegría, L.; Vera, M.; Dreyse, J.; Castro, R.; Carpio, D.; Henriquez, C.; Gajardo, D.; Bravo, S.; Araneda, F.; Kattan, E.; et al. A hypoperfusion context may aid to interpret hyperlactatemia in sepsis-3 septic shock patients: A proof-of-concept study. Ann. Intensive Care 2017, 7, 29. [Google Scholar] [CrossRef]
- de Miranda, A.C.; de Menezes, I.A.C.; Junior, H.C.; Luy, A.M.; do Nascimento, M.M. Monitoring peripheral perfusion in sepsis associated acute kidney injury: Analysis of mortality. PLoS ONE 2020, 15, e0239770. [Google Scholar] [CrossRef] [PubMed]
- Zafrani, L.; Ince, C. Microcirculation in Acute and Chronic Kidney Diseases. Am. J. Kidney Dis. 2015, 66, 1083–1094. [Google Scholar] [CrossRef]
- Raia, L.; Gabarre, P.; Bonny, V.; Urbina, T.; Missri, L.; Boelle, P.-Y.; Baudel, J.-L.; Guidet, B.; Maury, E.; Joffre, J.; et al. Kinetics of capillary refill time after fluid challenge. Ann. Intensive Care 2022, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Lemoinne, S.; Boelle, P.Y.; Galbois, A.; Baudel, J.L.; Lemant, J.; Joffre, J.; Margetis, D.; Guidet, B.; Maury, E.; et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011, 37, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Ltaief, Z.; Schneider, A.G.; Liaudet, L. Pathophysiology and clinical implications of the veno-arterial PCO2 gap. Crit. Care 2021, 25, 318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusack, R.; O’Neill, S.; Martin-Loeches, I. Effects of Fluids on the Sublingual Microcirculation in Sepsis. J. Clin. Med. 2022, 11, 7277. https://doi.org/10.3390/jcm11247277
Cusack R, O’Neill S, Martin-Loeches I. Effects of Fluids on the Sublingual Microcirculation in Sepsis. Journal of Clinical Medicine. 2022; 11(24):7277. https://doi.org/10.3390/jcm11247277
Chicago/Turabian StyleCusack, Rachael, Susan O’Neill, and Ignacio Martin-Loeches. 2022. "Effects of Fluids on the Sublingual Microcirculation in Sepsis" Journal of Clinical Medicine 11, no. 24: 7277. https://doi.org/10.3390/jcm11247277
APA StyleCusack, R., O’Neill, S., & Martin-Loeches, I. (2022). Effects of Fluids on the Sublingual Microcirculation in Sepsis. Journal of Clinical Medicine, 11(24), 7277. https://doi.org/10.3390/jcm11247277






