Characteristics of Diaphragmatic and Chest Wall Motion in People with Normal Pulmonary Function: A Study with Free-Breathing Dynamic MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Pulmonary Function Tests
2.3. MRI Protocol
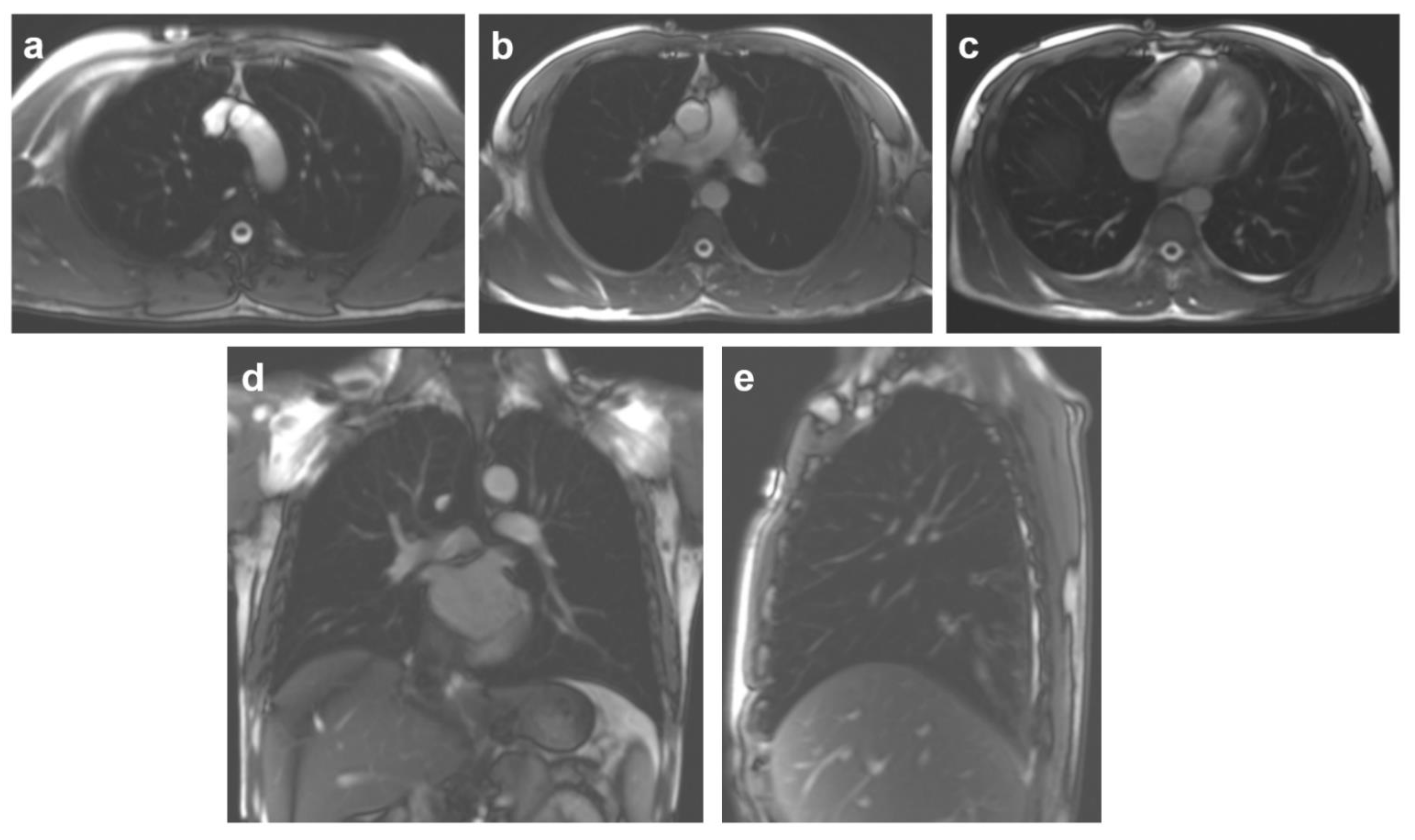
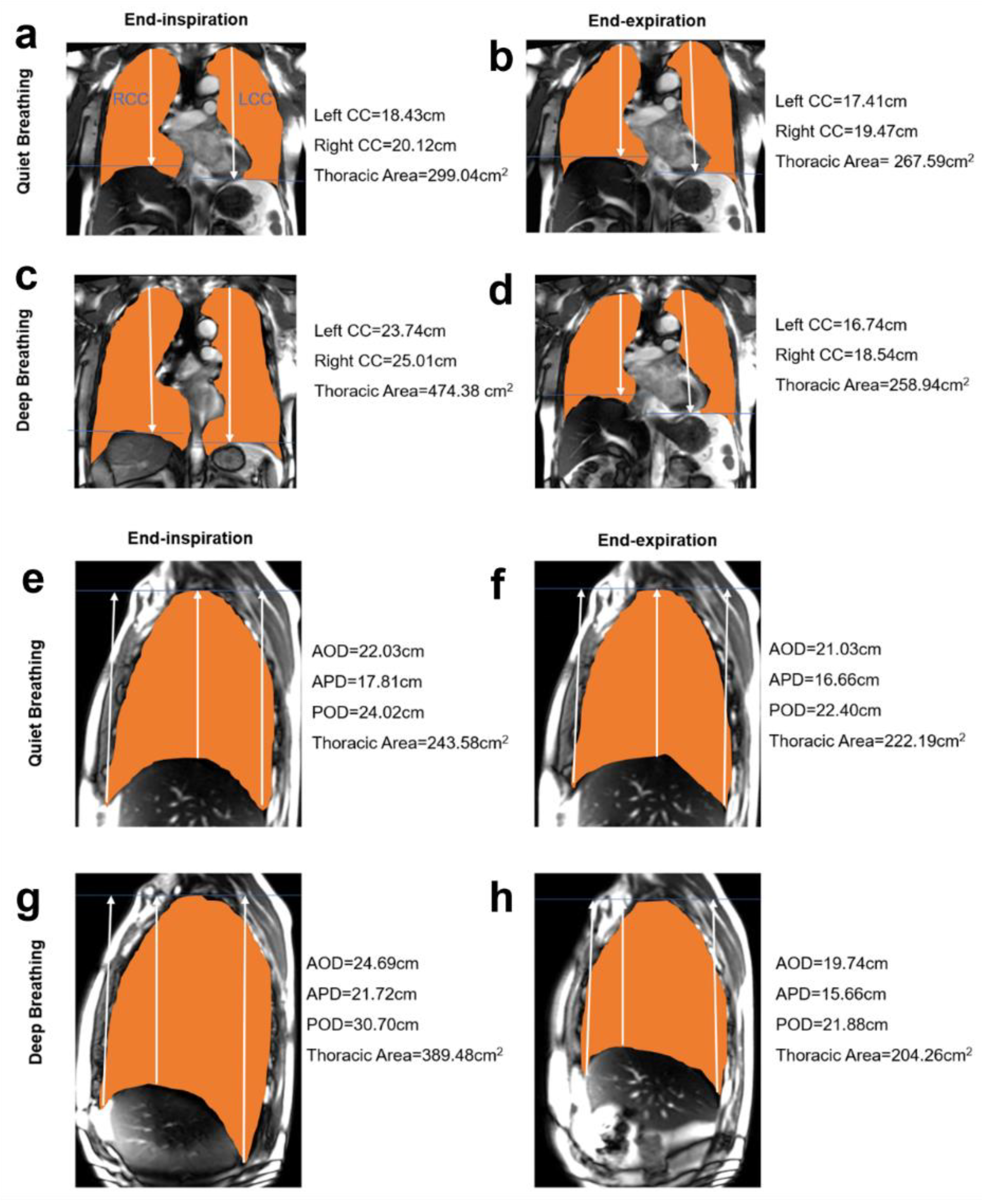
2.4. MR Image Processing and Measurements
2.5. Statistical Analysis
3. Results
3.1. The Demographic Characteristics
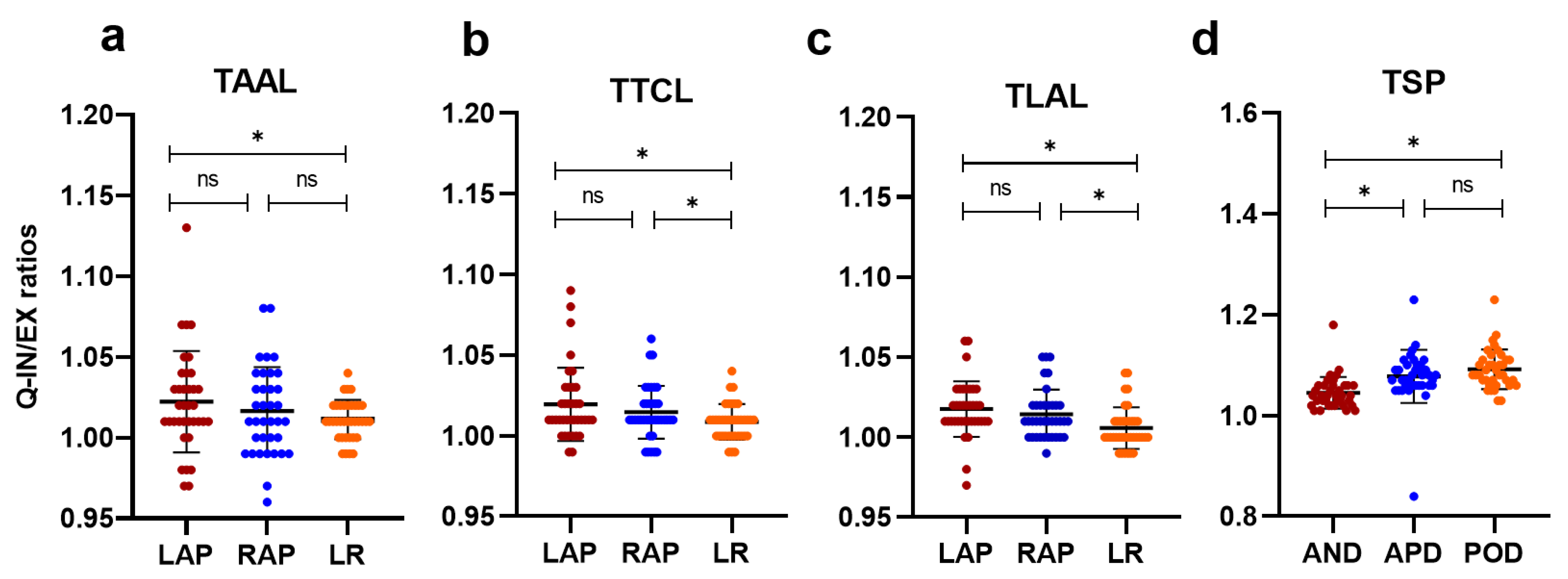
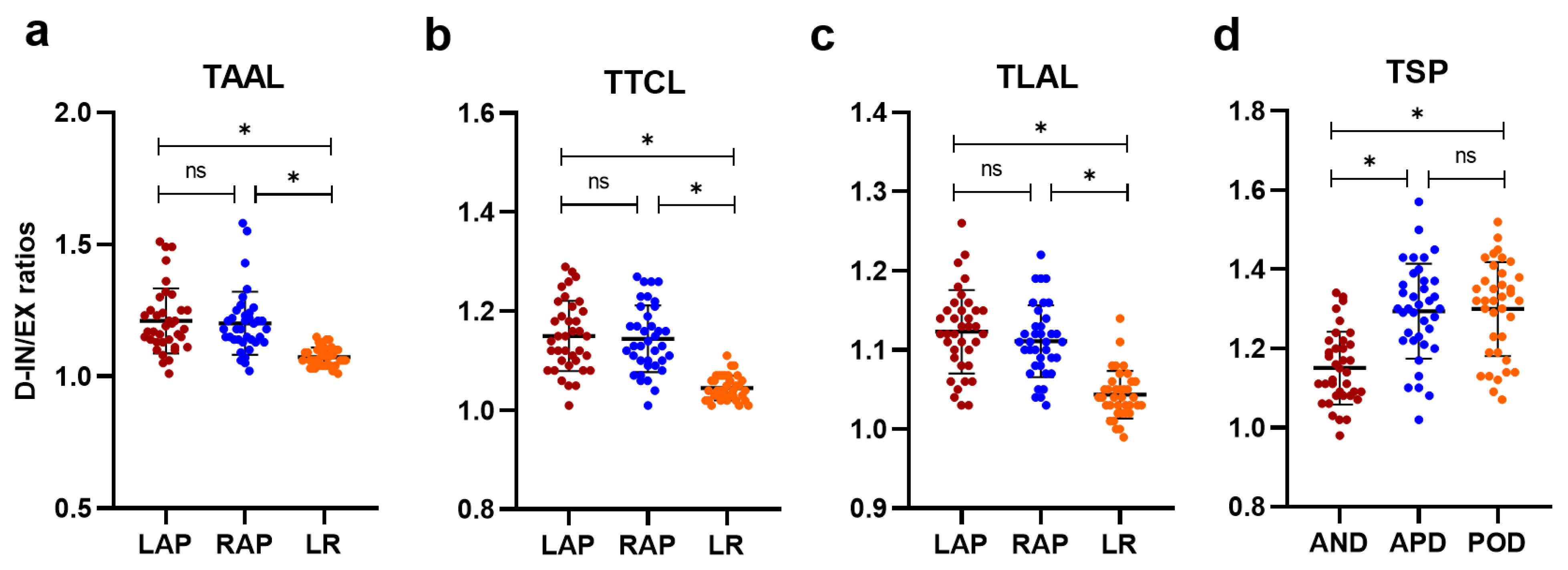
3.2. Quantitative Analysis of Chest Wall and Diaphragmatic Motion
3.3. Theeffect of Age and BMI on Chest Wall and Diaphragm Motion
3.4. The Influence of Smoking on Chest Wall and Diaphragm Motion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kondo, T.; Kobayashi, I.; Taguchi, Y.; Ohta, Y.; Yanagimachi, N. A dynamic analysis of chest wall motions with MRI in healthy young subjects. Respirology 2000, 5, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Takazakura, R.; Takahashi, M.; Nitta, N.; Sawai, S.; Tezuka, N.; Fujino, S.; Murata, K. Assessment of diaphragmatic motion after lung resection using magnetic resonance imaging. Radiat. Med. 2007, 25, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Embarak, S.; Walaa, M.; Lutfy, S.M. Role of diaphragmatic rapid shallow breathing index in predicting weaning outcome in patients with acute exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Mekov, E.; Yanev, N.; Kurtelova, N.; Mihalova, T.; Tsakova, A.; Yamakova, Y.; Miravitlles, M.; Petkov, R. Diaphragmatic Movement at Rest After Exertion: ANon-Invasive Easy to Obtain Prognostic Marker in, C.O.P.D. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 1041–1050. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.-P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- Gierada, D.S.; Curtin, J.J.; Erickson, S.J.; Prost, R.W.; Strandt, J.A.; Goodman, L.R. Diaphragmatic motion: Fast gradient-recalled-echo MR imaging in healthy subjects. Radiology 1995, 194, 879–884. [Google Scholar] [CrossRef]
- De Groote, A.; Wantier, M.; Cheron, G.; Estenne, M.; Paiva, M. Chest wall motion during tidal breathing. J. Appl. Physiol. 1997, 83, 1531–1537. [Google Scholar] [CrossRef]
- Traser, L.; Özen, A.C.; Burk, F.; Burdumy, M.; Bock, M.; Richter, B.; Echternach, M. Respiratory dynamics in phonation and breathing—A real-time MRI study. Respir. Physiol. Neurobiol. 2017, 236, 69–77. [Google Scholar] [CrossRef]
- Traser, L.; Schwab, C.; Burk, F.; Özen, A.C.; Burdumy, M.; Bock, M.; Richter, B.; Echternach, M. The influence of gravity on respiratory kinematics during phonation measured by dynamic magnetic resonance imaging. Sci. Rep. 2021, 11, 22965. [Google Scholar] [CrossRef]
- Hellebrandová, L.; Chlumský, J.; Vostatek, P.; Novák, D.; Ryznarova, Z.; Bunc, V. Airflow Limitation Is Accompanied by Diaphragm Dysfunction. Physiol. Res. 2016, 65, 469–479. [Google Scholar] [CrossRef]
- Shimada, A.; Kawata, N.; Sato, H.; Ikari, J.; Suzuki, E.; Anazawa, R.; Suzuki, M.; Masuda, Y.; Haneishi, H.; Tatsumi, K. Dynamic Quantitative Magnetic Resonance Imaging Assessment of Areas of the Lung During Free-Breathing of Patients with Chronic Obstructive Pulmonary Disease. Acad. Radiol. 2022, 29 (Suppl. 2), S215–S225. [Google Scholar] [CrossRef] [PubMed]
- Craighero, S.; Promayon, E.; Baconnier, P.; LeBas, J.F.; Coulomb, M. Dynamic echo-planar MR imaging of the diaphragm for a 3D dynamic analysis. Eur. Radiol. 2005, 15, 742–748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotani, T.; Minami, S.; Takahashi, K.; Isobe, K.; Nakata, Y.; Takaso, M.; Inoue, M.; Maruta, T.; Akazawa, T.; Ueda, T.; et al. An analysis of chest wall diaphragm motions in patients with idiopathic scoliosis using dynamic breathing, M.R.I. Spine 2004, 29, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.C.W.; Li, A.M.; Ng, B.K.W.; Chan, D.F.Y.; Lam, T.-P.; Lam, W.W.M.; Cheng, J.C.Y. Dynamic Magnetic Resonance Imaging in Assessing Lung Volumes, Chest Wall, and Diaphragm Motions in Adolescent Idiopathic Scoliosis Versus Normal Controls. Spine 2006, 31, 2243–2249. [Google Scholar] [CrossRef]
- Chu, W.C.W.; Ng, B.K.W.; Li, A.M.; Lam, T.-P.; Lam, W.W.M.; Cheng, J.C.Y. Dynamic magnetic resonance imaging in assessing lung function in adolescent idiopathic scoliosis: A pilot study of comparison before and after posterior spinal fusion. J. Orthop. Surg. Res. 2007, 2, 20. [Google Scholar] [CrossRef]
- Wens, S.C.A.; Ciet, P.; Perez-Rovira, A.; Logie, K.; Salamon, E.; Wielopolski, P.; De Bruijne, M.; Kruijshaar, M.E.; Tiddens, H.A.W.M.; Van Doorn, P.A.; et al. Lung MRI and impairment of diaphragmatic function in Pompe disease. BMC Pulm. Med. 2015, 15, 54. [Google Scholar] [CrossRef]
- Mogalle, K.; Perez-Rovira, A.; Ciet, P.; Wens, S.C.A.; van Doorn, P.A.; Tiddens, H.A.W.M.; van der Ploeg, A.T.; de Bruijne, M. Quantification of Diaphragm Mechanics in Pompe Disease Using Dynamic 3D MRI. PLoS ONE 2016, 11, e0158912. [Google Scholar] [CrossRef]
- Mankodi, A.; Kovacs, W.; Norato, G.; Hsieh, N.; Bandettini, W.P.; Bishop, C.A.; Shimellis, H.; Newbould, R.D.; Kim, E.; Fischbeck, K.H.; et al. Respiratory magnetic resonance imaging biomarkers in Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2017, 4, 655–662. [Google Scholar] [CrossRef]
- Harlaar, L.; Ciet, P.; van Tulder, G.; Pittaro, A.; Van Kooten, H.A.; Van der Beek, N.A.M.E.; Brusse, E.; Wielopolski, P.A.; De Bruijne, M.; Van der Ploeg, A.T.; et al. Chest MRI to diagnose early diaphragmatic weakness in Pompe disease. Orphanet. J. Rare Dis. 2021, 16, 21. [Google Scholar] [CrossRef]
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, L.; Ciet, P.; Van Tulder, G.; Brusse, E.; Timmermans, R.G.; Janssen, W.G.; De Bruijne, M.; Van der Ploeg, A.T.; Tiddens, H.A.; van Doorn, P.A.; et al. Diaphragmatic dysfunction in neuromuscular disease, an MRI study. Neuromuscul. Disord. 2022, 32, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pennati, F.; Arrigoni, F.; LoMauro, A.; Gandossini, S.; Russo, A.; D’Angelo, M.G.; Aliverti, A. Diaphragm Involvement in Duchenne Muscular Dystrophy (DMD): An MRI Study. J. Magn. Reson. Imaging 2020, 51, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Dubé, B.-P.; Dres, M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J. Clin. Med. 2016, 5, 113. [Google Scholar] [CrossRef]
- Takazakura, R.; Takahashi, M.; Nitta, N.; Murata, K. Diaphragmatic motion in the sitting and supine positions: Healthy subject study using a vertically open magnetic resonance system. J. Magn. Reson. Imaging 2004, 19, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Sehl, M.E.; Yates, F.E. Kinetics of Human Aging: I. Rates of Senescence between Ages 30 and 70 Years in Healthy People. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, B198–B208. [Google Scholar] [CrossRef]
- Kondo, T.; Kobayashi, I.; Taguchi, Y.; Hayama, N.; Tajiri, S.; Yanagimachi, N. An analysis of the chest wall motions using the dynamic MRI in healthy elder subjects. Tokai J. Exp. Clin. Med. 2005, 30, 15–20. [Google Scholar]
- Kantarci, F.; Mihmanli, I.; Demirel, M.K.; Harmanci, K.; Akman, C.; Aydogan, F.; Mihmanli, A.; Uysal, O. Normal diaphragmatic motion and the effects of body composition: Determination with M-mode sonography. J. Ultrasound Med. 2004, 23, 255–260. [Google Scholar] [CrossRef]
- Kabil, A.E.; Sobh, E.; Elsaeed, M.; Hassanin, H.E.; Yousef, I.H.; Eltrawy, H.H.; Ewis, A.M.; Aboseif, A.; Albalsha, A.M.; Elsawy, S.; et al. Diaphragmatic excursion by ultrasound: Reference values for the normal population; a cross-sectional study in Egypt. Multidiscip. Respir. Med. 2022, 17, 842. [Google Scholar] [CrossRef]
- Larsson, L.; Örlander, J. Skeletal muscle morphology, metabolism and function in smokers and non-smokers. A study on smoking-discordant monozygous twins. Acta Physiol. Scand. 1984, 120, 343–352. [Google Scholar] [CrossRef]
- Edwards, R. The problem of tobacco smoking. BMJ 2004, 328, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Dischereit, G.; Seimetz, M.; Wilhelm, J.; Weissmann, N.; Mooren, F.C. Time course of cigarette smoke-induced changes of systemic inflammation and muscle structure. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L119–L128. [Google Scholar] [CrossRef] [PubMed]
- Thome, T.; Miguez, K.; Willms, A.J.; Burke, S.K.; Chandran, V.; de Souza, A.R.; Fitzgerald, L.F.; Baglole, C.; Anagnostou, M.; Bourbeau, J.; et al. Chronic aryl hydrocarbon receptor activity phenocopies smoking-induced skeletal muscle impairment. J. Cachex-Sarcopenia Muscle 2022, 13, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Choi, J.; Chae, K.J.; Shin, K.M.; Lee, C.-H.; Guo, J.; Lin, C.-L.; Hoffman, E.A.; Lee, C. CT-derived 3D-diaphragm motion in emphysema and IPF compared to normal subjects. Sci. Rep. 2021, 11, 14923. [Google Scholar] [CrossRef]

| Subjects | Clinical Characteristics |
|---|---|
| Number | 74 |
| Gender | Male |
| Mean age (years) (range) | 37 ± 11 (22–67) |
| Height (cm) | 171 ± 5.7 |
| Weight (kg) | 70.4 ± 7.7 |
| BMI (kg/m2) | 24.2 ± 2.9 |
| Smoker, n (%) | 24 (32.4%) |
| Smoking habits (pack/years) | 4–50 |
| Duration of smoking (years) | 2–35 |
| Pulmonary Function Tests | |
| FEV1% predicted | 98.1 ± 11.0 |
| FVC % predicted | 102.6 ± 10.7 |
| FEV1/FVC % predicted | 82.9 ± 8.7 |
| TLC % predicted | 97.1 ± 8.3 |
| DLCO % predicted | 100.2 ± 14.3 |
| Ratios | Quiet Breathing | Deep Breathing | ||||||
|---|---|---|---|---|---|---|---|---|
| Age < 30 Years Old (n = 28) | 31–50 Years Old (n = 26) | Age > 50 Years Old (n = 20) | p | Age < 30 Years Old (n = 28) | 31–50 Years Old (n = 26) | Age > 50 Years Old (n = 20) | p | |
| The arcus aortae level | ||||||||
| Left AP | 1.02 ± 0.03 | 1.02 ± 0.03 | 1.02 ± 0.03 | 0.560 | 1.20 ± 0.14 | 1.21 ± 0.11 | 1.22 ± 0.13 | 0.720 |
| Right AP | 1.01 ± 0.02 | 1.02 ± 0.03 | 1.01 ± 0.03 | 0.504 | 1.22 ± 0.17 | 1.19 ± 0.10 | 1.18 ± 0.06 | 0.844 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 1.01 ± 0.02 | 0.124 | 1.07 ± 0.04 | 1.07 ± 0.03 | 1.08 ± 0.04 | 0.687 |
| Thoracic Area | 1.07 ± 0.05 | 1.06 ± 0.03 | 1.07 ± 0.02 | 0.330 | 1.44 ± 0.23 | 1.50 ± 0.22 | 1.51 ± 0.22 | 0.384 |
| The tracheal carina level | ||||||||
| Left AP | 1.02 ± 0.02 | 1.02 ± 0.03 | 1.02 ± 0.03 | 0.672 | 1.15 ± 0.08 | 1.15 ± 0.07 | 1.15 ± 0.07 | 0.869 |
| Right AP | 1.02 ± 0.02 | 1.01 ± 0.01 | 1.01 ± 0.02 | 0.072 | 1.14 ± 0.08 | 1.15 ± 0.07 | 1.14 ± 0.07 | 0.990 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.211 | 1.04 ± 0.03 | 1.04 ± 0.02 | 1.05 ± 0.03 | 0.919 |
| Thoracic Area | 1.08 ± 0.04 | 1.07 ± 0.06 | 1.08 ± 0.05 | 0.347 | 1.43 ± 0.23 | 1.38 ± 0.12 | 1.36 ± 0.10 | 0.676 |
| The liver apex level | ||||||||
| Left AP | 1.01 ± 0.01 | 1.02 ± 0.03 | 1.02 ± 0.02 | 0.247 | 1.12 ± 0.05 | 1.13 ± 0.06 | 1.11 ± 0.03 | 0.753 |
| Right AP | 1.02 ± 0.01 | 1.01 ± 0.01 | 1.01 ± 0.02 | 0.133 | 1.12 ± 0.05 | 1.11 ± 0.05 | 1.10 ± 0.03 | 0.294 |
| LR | 1.01 ± 0.01 | 1.00 ± 0.01 | 1.01 ± 0.02 | 0.546 | 1.05 ± 0.04 | 1.04 ± 0.17 | 1.04 ± 0.02 | 0.805 |
| Thoracic Area | 1.02 ± 0.02 | 1.01 ± 0.03 | 1.05 ± 0.06 | 0.001 * | 1.18 ± 0.10 | 1.20 ± 0.11 | 1.20 ± 0.09 | 0.775 |
| The coronal plane | ||||||||
| Left CC | 1.10 ± 0.05 | 1.09 ± 0.05 | 1.10 ± 0.05 | 0.200 | 1.33 ± 0.08 | 1.39 ± 0.12 | 1.27 ± 0.15 | 0.081 |
| Right CC | 1.10 ± 0.05 | 1.11 ± 0.07 | 1.12 ± 0.07 | 0.750 | 1.41 ± 0.11 | 1.47 ± 0.13 | 1.32 ± 0.16 | 0.053 |
| Area | 1.19 ± 0.11 | 1.18 ± 0.17 | 1.21 ± 0.07 | 0.082 | 1.88 ± 0.25 | 1.92 ± 0.33 | 1.68 ± 0.33 | 0.150 |
| The right sagittal plane | ||||||||
| AOD | 1.04 ± 0.02 | 1.05 ± 0.04 | 1.05 ± 0.02 | 0.237 | 1.15 ± 0.08 | 1.14 ± 0.07 | 1.16 ± 0.14 | 0.852 |
| APD | 1.07 ± 0.02 | 1.09 ± 0.05 | 1.07 ± 0.09 | 0.140 | 1.29 ± 0.10 | 1.29 ± 0.08 | 1.30 ± 0.19 | 0.632 |
| POD | 1.09 ± 0.02 | 1.10 ± 0.05 | 1.09 ± 0.04 | 0.896 | 1.28 ± 0.14 | 1.33 ± 0.08 | 1.28 ± 0.13 | 0.281 |
| Thoracic Area | 1.12 ± 0.03 | 1.13 ± 0.06 | 1.14 ± 0.04 | 0.123 | 1.55 ± 0.19 | 1.61 ± 0.16 | 1.60 ± 0.27 | 0.562 |
| Ratios | Quiet Breathing | Deep Breathing | ||||
|---|---|---|---|---|---|---|
| BMI < 25 (kg/m2) (n = 44) | BMI ≥ 25 (kg/m2) (n = 30) | p | BMI < 25 (kg/m2) (n = 44) | BMI ≥ 25 (kg/m2) (n = 30) | p | |
| The arcus aortae level | ||||||
| Left AP | 1.02 ± 0.04 | 1.02 ± 0.02 | 0.692 | 1.17 ± 0.10 | 1.26 ± 0.14 | 0.086 |
| Right AP | 1.02 ± 0.03 | 1.01 ± 0.03 | 0.481 | 1.18 ± 0.11 | 1.23 ± 0.13 | 0.160 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.093 | 1.07 ± 0.04 | 1.08 ± 0.03 | 0.640 |
| Thoracic Area | 1.07 ± 0.04 | 1.06 ± 0.02 | 0.895 | 1.42 ± 0.18 | 1.58 ± 0.24 | 0.061 |
| The tracheal carina level | ||||||
| Left AP | 1.02 ± 0.03 | 1.02 ± 0.02 | 0.567 | 1.15 ± 0.68 | 1.16 ± 0.08 | 0.533 |
| Right AP | 1.02 ± 0.02 | 1.01 ± 0.01 | 0.271 | 1.14 ± 0.07 | 1.14 ± 0.07 | 0.435 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.202 | 1.05 ± 0.03 | 1.04 ± 0.02 | 0.763 |
| Thoracic Area | 1.08 ± 0.06 | 1.07 ± 0.02 | 0.271 | 1.39 ± 0.19 | 1.40 ± 0.13 | 0.876 |
| The liver apex level | ||||||
| Left AP | 1.02 ± 0.02 | 1.04 ± 0.04 | 0.137 | 1.12 ± 0.04 | 1.13 ± 0.07 | 0.160 |
| Right AP | 1.02 ± 0.02 | 1.01 ± 0.01 | 0.146 | 1.11 ± 0.04 | 1.11 ± 0.05 | 0.533 |
| LR | 1.01 ± 0.01 | 1.00 ± 0.01 | 0.134 | 1.04 ± 0.03 | 1.05 ± 0.03 | 0.435 |
| Thoracic Area | 1.01 ± 0.01 | 1.02 ± 0.03 | 0.792 | 1.19 ± 0.10 | 1.20 ± 0.11 | 0.349 |
| The coronal plane | ||||||
| Left CC | 1.10 ± 0.05 | 1.08 ± 0.04 | 0.567 | 1.32 ± 0.12 | 1.37 ± 0.13 | 0.275 |
| Right CC | 1.11 ± 0.06 | 1.11 ± 0.06 | 0.826 | 1.39 ± 0.13 | 1.46 ± 0.15 | 0.533 |
| Thoracic Area | 1.21 ± 0.15 | 1.18 ± 0.08 | 0.660 | 1.79 ± 0.29 | 1.93 ± 0.33 | 0.533 |
| The right sagittal plane | ||||||
| AOD | 1.04 ± 0.03 | 1.05 ± 0.03 | 0.378 | 1.15 ± 0.10 | 1.15 ± 0.08 | 0.896 |
| APD | 1.08 ± 0.03 | 1.07 ± 0.07 | 0.078 | 1.29 ± 0.12 | 1.30 ± 0.12 | 0.275 |
| POD | 1.09 ± 0.04 | 1.09 ± 0.03 | 0.758 | 1.28 ± 0.12 | 1.33 ± 0.12 | 0.435 |
| Thoracic Area | 1.12 ± 0.05 | 1.13 ± 0.04 | 0.053 | 1.56 ± 0.18 | 1.61 ± 0.22 | 0.876 |
| Ratios | Quiet Breathing | Deep Breathing | ||||
|---|---|---|---|---|---|---|
| Non-Smokers (n = 24) | Smokers (n = 50) | p | Non-Smokers (n = 24) | Smokers (n = 50) | p | |
| The arcus aortae level | ||||||
| Left AP | 1.02 ± 0.02 | 1.02 ± 0.03 | 0.474 | 1.23 ± 0.13 | 1.18 ± 0.10 | 0.072 |
| Right AP | 1.01 ± 0.03 | 1.02 ± 0.03 | 0.853 | 1.22 ± 0.14 | 1.19 ± 0.05 | 0.083 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.152 | 1.08 ± 0.03 | 1.07 ± 0.04 | 0.212 |
| Thoracic Area | 1.06 ± 0.02 | 1.06 ± 0.04 | 0.460 | 1.51 ± 0.23 | 1.44 ± 0.18 | 0.152 |
| The tracheal carina level | ||||||
| Left AP | 1.01 ± 0.01 | 1.02 ± 0.03 | 0.124 | 1.16 ± 0.08 | 1.13 ± 0.05 | 0.065 |
| Right AP | 1.01 ± 0.01 | 1.02 ± 0.02 | 0.611 | 1.16 ± 0.07 | 1.13 ± 0.04 | 0.052 |
| LR | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.083 | 1.05 ± 0.02 | 1.04 ± 0.03 | 0.139 |
| Thoracic Area | 1.07 ± 0.02 | 1.08 ± 0.06 | 0.853 | 1.42 ± 0.18 | 1.33 ± 0.12 | 0.016 * |
| The liver apex level | ||||||
| Left AP | 1.04 ± 0.04 | 1.02 ± 0.03 | 0.024 * | 1.12 ± 0.06 | 1.12 ± 0.04 | 0.853 |
| Right AP | 1.01 ± 0.01 | 1.02 ± 0.02 | 0.042 * | 1.12 ± 0.05 | 1.11 ± 0.03 | 0.514 |
| LR | 1.00 ± 0.01 | 1.01 ± 0.01 | 0.127 | 1.05 ± 0.03 | 1.04 ± 0.02 | 0.432 |
| Thoracic Area | 1.03 ± 0.03 | 1.02 ± 0.05 | 0.034 * | 1.20 ± 0.11 | 1.19 ± 0.09 | 0.782 |
| The coronal plane | ||||||
| Left CC | 1.09 ± 0.03 | 1.10 ± 0.05 | 0.746 | 1.36 ± 0.11 | 1.30 ± 0.14 | 0.072 |
| Right CC | 1.12 ± 0.07 | 1.11 ± 0.06 | 0.248 | 1.38 ± 0.12 | 1.34 ± 0.17 | 0.098 |
| Thoracic Area | 1.20 ± 0.08 | 1.19 ± 0.15 | 0.180 | 1.93 ± 0.28 | 1.69 ± 0.31 | 0.003 * |
| The right sagittal plane | ||||||
| AOD | 1.04 ± 0.02 | 1.05 ± 0.03 | 0.926 | 1.15 ± 0.08 | 1.15 ± 0.12 | 0.817 |
| APD | 1.07 ± 0.08 | 1.08 ± 0.04 | 0.268 | 1.30 ± 0.09 | 1.29 ± 0.17 | 0.890 |
| POD | 1.10 ± 0.04 | 1.09 ± 0.04 | 0.432 | 1.31 ± 0.12 | 1.29 ± 0.12 | 0.548 |
| Thoracic Area | 1.13 ± 0.04 | 1.12 ± 0.05 | 0.160 | 1.59 ± 0.19 | 1.56 ± 0.23 | 0.488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sun, H.; Deng, M.; Chen, Y.; Li, C.; Yu, P.; Zhang, R.; Liu, M.; Dai, H.; Wang, C. Characteristics of Diaphragmatic and Chest Wall Motion in People with Normal Pulmonary Function: A Study with Free-Breathing Dynamic MRI. J. Clin. Med. 2022, 11, 7276. https://doi.org/10.3390/jcm11247276
Yang X, Sun H, Deng M, Chen Y, Li C, Yu P, Zhang R, Liu M, Dai H, Wang C. Characteristics of Diaphragmatic and Chest Wall Motion in People with Normal Pulmonary Function: A Study with Free-Breathing Dynamic MRI. Journal of Clinical Medicine. 2022; 11(24):7276. https://doi.org/10.3390/jcm11247276
Chicago/Turabian StyleYang, Xiaoyan, Haishuang Sun, Mei Deng, Yicong Chen, Chen Li, Pengxin Yu, Rongguo Zhang, Min Liu, Huaping Dai, and Chen Wang. 2022. "Characteristics of Diaphragmatic and Chest Wall Motion in People with Normal Pulmonary Function: A Study with Free-Breathing Dynamic MRI" Journal of Clinical Medicine 11, no. 24: 7276. https://doi.org/10.3390/jcm11247276
APA StyleYang, X., Sun, H., Deng, M., Chen, Y., Li, C., Yu, P., Zhang, R., Liu, M., Dai, H., & Wang, C. (2022). Characteristics of Diaphragmatic and Chest Wall Motion in People with Normal Pulmonary Function: A Study with Free-Breathing Dynamic MRI. Journal of Clinical Medicine, 11(24), 7276. https://doi.org/10.3390/jcm11247276






