Machine Learning Model Development and Validation for Predicting Outcome in Stage 4 Solid Cancer Patients with Septic Shock Visiting the Emergency Department: A Multi-Center, Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics
2.3. Outcome and Data Collection
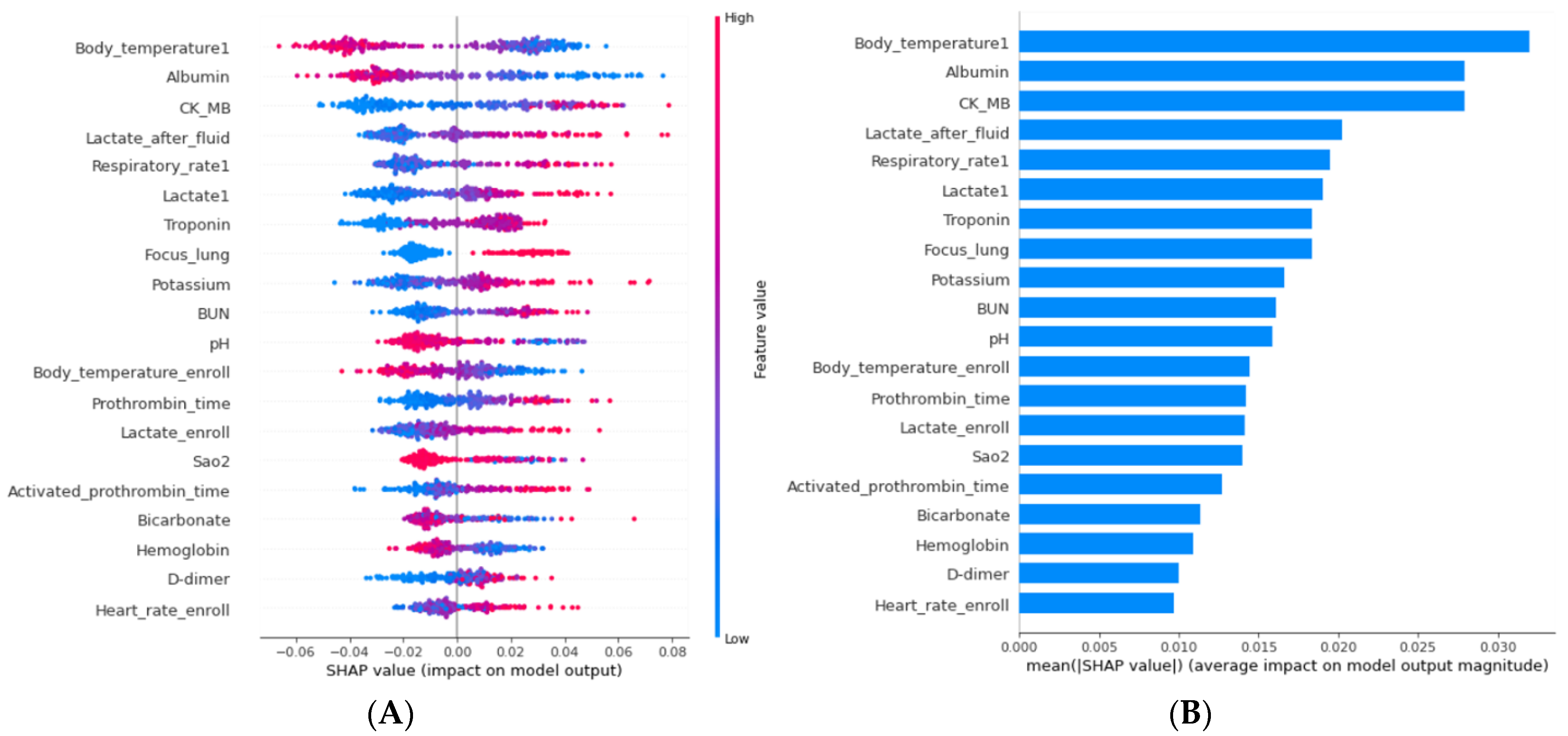
2.4. Machine Learning Model Development and Feature Analysis
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. ML Model Development
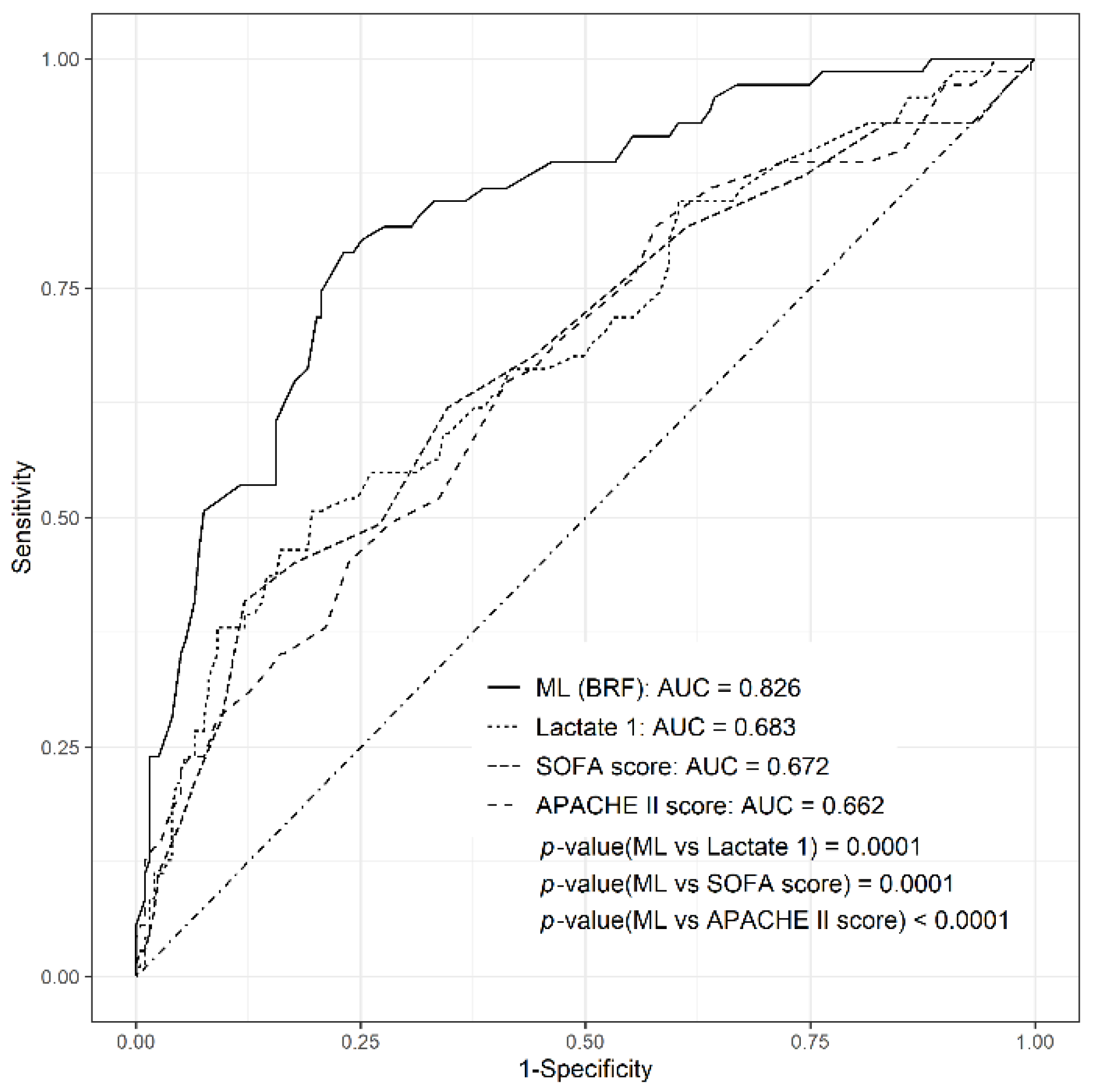
3.3. Comparison of Diagnostic Performance of ML Model with Other Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Caruso, P.; Silva, E.; Teles, J.M.M.; Lobo, S.M.A.; Friedman, G.; Dal Pizzol, F.; Mello, P.V.C.; Bozza, F.A.; Silva, U.V.A. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit. Care Med. 2010, 38, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Risk of critical illness among patients with solid cancers: A population-based observational study. JAMA Oncol. 2015, 1, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Danai, P.A.; Moss, M.; Mannino, D.M.; Martin, G.S. The epidemiology of sepsis in patients with malignancy. Chest 2006, 129, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Shapiro, M.F. Association between intensive care unit utilization during hospitalization and costs, use of invasive procedures, and mortality. JAMA Intern. Med. 2016, 176, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Dacosta, D.; Shapiro, M.F. Priority levels in medical intensive care at an academic public hospital. JAMA Intern. Med. 2017, 177, 280–281. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Moll, M.; Qiao, D.; Regan, E.A.; Hunninghake, G.M.; Make, B.J.; Tal-Singer, R.; McGeachie, M.J.; Castaldi, P.J.; Estepar, R.S.J.; Washko, G.R. Machine learning and prediction of all-cause mortality in COPD. Chest 2020, 158, 952–964. [Google Scholar] [CrossRef]
- Shung, D.L.; Au, B.; Taylor, R.A.; Tay, J.K.; Laursen, S.B.; Stanley, A.J.; Dalton, H.R.; Ngu, J.; Schultz, M.; Laine, L. Validation of a machine learning model that outperforms clinical risk scoring systems for upper gastrointestinal bleeding. Gastroenterology 2020, 158, 160–167. [Google Scholar] [CrossRef]
- Nemati, S.; Holder, A.; Razmi, F.; Stanley, M.D.; Clifford, G.D.; Buchman, T.G. An interpretable machine learning model for accurate prediction of sepsis in the ICU. Crit. Care Med. 2018, 46, 547. [Google Scholar] [CrossRef]
- Kang, S.Y.; Cha, W.C.; Yoo, J.; Kim, T.; Park, J.H.; Yoon, H.; Hwang, S.Y.; Sim, M.S.; Jo, I.J.; Shin, T.G. Predicting 30-day mortality of patients with pneumonia in an emergency department setting using machine-learning models. Clin. Exp. Emerg. Med. 2020, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.; Woo, S.H.; Park, J.T.; Jeong, S.; Kim, J.; Hong, S. Artificial neural network approach for acute poisoning mortality prediction in emergency departments. Clin. Exp. Emerg. Med. 2021, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Cha, W.C. Artificial intelligence decision points in an emergency department. Clin. Exp. Emerg. Med. 2022, 9, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.G.; Hwang, S.Y.; Kang, G.H.; Kim, W.Y.; Ryoo, S.M.; Kim, K.; Jo, Y.H.; Chung, S.P.; Joo, Y.S.; Beom, J.H. Korean Shock Society septic shock registry: A preliminary report. Clin. Exp. Emerg. Med. 2017, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef]
- Investigators, P. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [PubMed]
- Berrar, D. Cross-Validation. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Motwani, M.; Dey, D.; Berman, D.S.; Germano, G.; Achenbach, S.; Al-Mallah, M.H.; Andreini, D.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: A 5-year multicentre prospective registry analysis. Eur. Heart J. 2017, 38, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.D.; Ulloa, A.; Wehner, G.J.; Jing, L.; Hartzel, D.; Good, C.W.; Williams, B.A.; Haggerty, C.M.; Fornwalt, B.K. Predicting survival from large echocardiography and electronic health record datasets: Optimization with machine learning. JACC Cardiovasc. Imaging 2019, 12, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.; Yella, S.; Dougherty, M.; Fleyeh, H. Machine learning algorithms in heavy process manufacturing. Am. J. Intell. Syst. 2016, 6, 1–13. [Google Scholar]
- Seiffert, C.; Khoshgoftaar, T.M.; Van Hulse, J.; Napolitano, A. RUSBoost: A hybrid approach to alleviating class imbalance. IEEE Trans. Syst. Man Cybern. A Syst. Hum. 2009, 40, 185–197. [Google Scholar] [CrossRef]
- Hido, S.; Kashima, H.; Takahashi, Y. Roughly balanced bagging for imbalanced data. Stat. Anal. Data Min. ASA Data Sci. J. 2009, 2, 412–426. [Google Scholar] [CrossRef]
- Chen, C.; Liaw, A.; Breiman, L. Using Random Forest to Learn Imbalanced Data; University of California: Berkeley, CA, USA, 2004; Volume 110, p. 24. [Google Scholar]
- Kotsiantis, S.; Kanellopoulos, D.; Pintelas, P. Handling imbalanced datasets: A review. GESTS Int. Trans. Comput. Sci. Eng. 2006, 30, 25–36. [Google Scholar]
- Johnson, J.M.; Khoshgoftaar, T.M. Survey on deep learning with class imbalance. J. Big Data 2019, 6, 27. [Google Scholar] [CrossRef]
- Hamad, R.A.; Kimura, M.; Lundström, J. Efficacy of imbalanced data handling methods on deep learning for smart homes environments. SN Comput. Sci. 2020, 1, 204. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Taccone, F.S.; Artigas, A.A.; Sprung, C.L.; Moreno, R.; Sakr, Y.; Vincent, J.-L. Characteristics and outcomes of cancer patients in European ICUs. Crit. Care 2009, 13, R15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kang, J.; Kim, M.-J.; Ryoo, S.M.; Kang, G.H.; Shin, T.G.; Park, Y.S.; Choi, S.-H.; Kwon, W.Y.; Chung, S.P. Development and validation of the VitaL CLASS score to predict mortality in stage IV solid cancer patients with septic shock in the emergency department: A multi-center, prospective cohort study. BMC Med. 2020, 18, 390. [Google Scholar] [CrossRef]
- Costa, R.T.; Nassar Jr, A.P.; Caruso, P. Accuracy of SOFA, qSOFA, and SIRS scores for mortality in cancer patients admitted to an intensive care unit with suspected infection. J. Crit. Care 2018, 45, 52–57. [Google Scholar] [CrossRef]
- Macdonald, S.P.J.; Arendts, G.; Fatovich, D.M.; Brown, S.G.A. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad. Emerg. Med. 2014, 21, 1257–1263. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Li, C.-S. Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: A cohort study. Crit. Care 2014, 18, R74. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Van Ginkel, C.; Batech, M.; Banta, J.; Corbett, S.W. Comparison of Predisposition, Insult/Infection, Response, and Organ dysfunction, Acute Physiology And Chronic Health Evaluation II, and Mortality in Emergency Department Sepsis in patients meeting criteria for early goal-directed therapy and the severe se. J. Crit. Care 2012, 27, 362–369. [Google Scholar] [CrossRef]
- Kushimoto, S.; Gando, S.; Saitoh, D.; Mayumi, T.; Ogura, H.; Fujishima, S.; Araki, T.; Ikeda, H.; Kotani, J.; Miki, Y. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: An analysis from a multicenter, prospective survey of severe sepsis. Crit. Care 2013, 17, R271. [Google Scholar] [CrossRef]
- Kushimoto, S.; Abe, T.; Ogura, H.; Shiraishi, A.; Saitoh, D.; Fujishima, S.; Mayumi, T.; Hifumi, T.; Shiino, Y.; Nakada, T. Impact of body temperature abnormalities on the implementation of sepsis bundles and outcomes in patients with severe sepsis: A retrospective sub-analysis of the focused outcome research on emergency care for acute respiratory distress syndrome, sepsis an. Crit. Care Med. 2019, 47, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Sundén-Cullberg, J.; Rylance, R.; Svefors, J.; Norrby-Teglund, A.; Björk, J.; Inghammar, M. Fever in the emergency department predicts survival of patients with severe sepsis and septic shock admitted to the ICU. Crit. Care Med. 2017, 45, 591–599. [Google Scholar] [CrossRef]
- Laupland, K.B.; Shahpori, R.; Kirkpatrick, A.W.; Ross, T.; Gregson, D.B.; Stelfox, H.T. Occurrence and outcome of fever in critically ill adults. Crit. Care Med. 2008, 36, 1531–1535. [Google Scholar] [CrossRef]
- Arnau-Barrés, I.; Güerri-Fernández, R.; Luque, S.; Sorli, L.; Vázquez, O.; Miralles, R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Kendall, H.; Abreu, E.; Cheng, A.-L. Serum albumin trend is a predictor of mortality in ICU patients with sepsis. Biol. Res. Nurs. 2019, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, R.; Kabata, D.; Shimizu, K.; Hisano, S.; Ogura, H.; Shintani, A.; Shimazu, T. Serum albumin as a risk factor for death in patients with prolonged sepsis: An observational study. J. Crit. Care 2019, 51, 139–144. [Google Scholar] [CrossRef]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The septic heart: Current understanding of molecular mechanisms and clinical implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Fanizza, C.; Carrer, S.; Caricato, A.; Fassini, P.; Vago, T.; Romero, M.; Tognoni, G.; Gattinoni, L. Sequential N-terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock. Crit. Care Med. 2016, 44, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bessière, F.; Khenifer, S.; Dubourg, J.; Durieu, I.; Lega, J.-C. Prognostic value of troponins in sepsis: A meta-analysis. Intensive Care Med. 2013, 39, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, R.J.; Alvarez, J.; Flynn, L.M.; Sherwin, R.L.; Jones, S.S. Development and evaluation of a machine learning model for the early identification of patients at risk for sepsis. Ann. Emerg. Med. 2019, 73, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Schellongowski, P.; Darmon, M.; Bauer, P.R.; Benoit, D.; Depuydt, P.; Divatia, J.V.; Lemiale, V.; van Vliet, M.; Meert, A.-P. The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med. 2017, 43, 1366–1382. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Pène, F.; Darmon, M.; Lengliné, E.; Benoit, D.; Soares, M.; Vincent, F.; Bruneel, F.; Perez, P.; Lemiale, V. Managing critically ill hematology patients: Time to think differently. Blood Rev. 2015, 29, 359–367. [Google Scholar] [CrossRef]
- Laird, B.J.; Kaasa, S.; McMillan, D.C.; Fallon, M.T.; Hjermstad, M.J.; Fayers, P.; Klepstad, P. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin. Cancer Res. 2013, 19, 5456–5464. [Google Scholar] [CrossRef]
- Zimmermann, C.; Burman, D.; Bandukwala, S.; Seccareccia, D.; Kaya, E.; Bryson, J.; Rodin, G.; Lo, C. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support. Care Cancer 2010, 18, 609–616. [Google Scholar] [CrossRef]

| Total Patients | Survivors (n = 660) | Non-Survivors (n = 237) | p-Value | |
|---|---|---|---|---|
| Age, mean ± standard deviation, y | 66 ± 11.5 | 65.6 ± 11.2 | 66.9 ± 12.4 | 0.165 |
| male, no. (%) | 561 (62.5%) | 408 (61.8%) | 153 (64.6%) | 0.482 |
| Vital signs, mean ± standard deviation | ||||
| Initial systolic blood pressure, mmHg | 96.1 ± 27.1 | 94.9 ± 26.2 | 99.5 ± 26.1 | 0.033 |
| Initial diastolic blood pressure, mmHg | 59.5 ± 18.5 | 58.82 ± 17.6 | 61.2 ± 20.7 | 0.11 |
| Initial heart rate, beats per min | 114 ± 25.1 | 113.9 ± 24.9 | 114.3 ± 25.6 | 0.818 |
| Initial respiratory rate, breaths per min | 21.4 ± 4.9 | 20.8 ± 4.1 | 23.1 ± 6.4 | <0.001 |
| Initial body temperature, °C | 37.8 ± 1.3 | 38 ± 1.3 | 37.2 ± 1.2 | <0.001 |
| Laboratory findings, median (interquartile range) | ||||
| White blood cells, 103/mm3 | 8.1 (3.0–15.4) | 7.9 (3.1–15) | 8.9 (2.5–18) | 0.165 |
| Hb, g/dL | 10.2 (8.6–11.7) | 10.2 (8.7–11.6) | 9.9 (8.5–11.8) | 0.604 |
| Platelets, 103/mm3 | 143 (71–232) | 147 (78–238) | 129 (61–226) | 0.043 |
| Albumin, g/dL | 2.8 (2.3–3.2) | 2.8 (2.5–3.3) | 2.5 (2.1–3) | <0.001 |
| Blood urea nitrogen, mg/dL | 25 (17–38) | 23 (16.4–34.5) | 31 (20.3–47.2) | <0.001 |
| Creatinine, mg/dL | 1.3 (0.9–1.9) | 1.2 (0.9–1.9) | 1.4 (0.9–2.2) | 0.023 |
| C-reactive protein, mg/dL | 12.8 (6.1–22.1) | 12.5 (5.5–21.6) | 13.6 (6.9–25.1) | 0.137 |
| Lactate, mmol/L | 3.6 (2–5.5) | 3.3 (1.9–5) | 5 (3.1–7.7) | <0.001 |
| Cancer type, no. (%) | ||||
| Stomach | 55 (6.1%) | 39 (5.9%) | 16 (6.8%) | 0.753 |
| Colorectal | 63 (7%) | 53 (8%) | 10 (4.2%) | 0.054 |
| Liver | 113 (12.6%) | 90 (13.6%) | 23 (9.7%) | 0.138 |
| Biliary | 85 (9.5%) | 67 (10.2%) | 18 (7.6%) | 0.301 |
| Pancreas | 88 (9.8%) | 70 (10.6%) | 18 (7.6%) | 0.204 |
| Lung | 151 (16.8%) | 81 (12.3%) | 70 (29.5%) | <0.001 |
| Gynecologic | 82 (9.1%) | 67 (10.2%) | 15 (6.3%) | 0.088 |
| Urologic | 78 (8.7%) | 65 (9.8%) | 13 (5.5%) | 0044 |
| Other | 182 (20.3%) | 128 (19.4%) | 54 (22.8%) | 0.3 |
| Infection focus, no. (%) | ||||
| Lung | 256 (28.5%) | 151 (22.9%) | 105 (44.3%) | <0.001 |
| Urinary tract | 146 (16.3%) | 113 (17.1%) | 33 (13.9%) | 0.262 |
| Gastrointestinal | 163 (18.2%) | 115 (17.4%) | 48 (20.3%) | 0.377 |
| Hepatobiliary | 257 (28.7%) | 210 (31.8%) | 47 (19.8%) | 0.001 |
| Bone soft tissue | 20 (2.2%) | 16 (2.4%) | 4 (1.7%) | 0.616 |
| Others | 35 (3.9%) | 32 (5.6%) | 3 (1.6%) | 0.026 |
| Comorbidities, no. (%) | ||||
| Hypertension | 281 (31.3%) | 207 (31.4%) | 74 (31.2%) | 1.000 |
| Diabetes mellitus | 207 (23.1%) | 152 (23%) | 55 (23.2%) | 1.000 |
| Cardiac disease | 77 (8.6%) | 47 (7.1%) | 30 (12.7%) | 0.011 |
| Cerebrovascular accident | 39 (4.3%) | 17 (3%) | 14 (7.3%) | 0.011 |
| Chronic lung disease | 60 (6.7%) | 37 (6.5%) | 17 (8.9%) | 0.328 |
| Chronic renal disease | 33 (3.7%) | 28 (4.2%) | 5 (2.1%) | 0.161 |
| Chronic liver disease | 78 (8.7%) | 62 (9.4%) | 16 (6.8%) | 0.23 |
| SOFA score, median (interquartile range) | 8 (6–10) | 7 (5–10) | 10 (7–13) | <0.001 |
| APACHE II score, median (interquartile range) | 20 (15–26) | 19 (14–24) | 24 (19–32) | <0.001 |
| Outcomes, no. (%) | ||||
| 28-day mortality | 237 (26.4%) | |||
| Time to death, day | 8 (2–7) | |||
| Vasopressor | 653 (72.8%) | 493 (86%) | 160 (83.8%) | 0.477 |
| Renal replacement treatment | 80 (8.9%) | 30 (4.5%) | 50 (21.1%) | <0.001 |
| Intensive care unit admission | 412 (45.9%) | 324 (49.1%) | 88 (37.1%) | 0.002 |
| Mechanical ventilation | 192 (21.4%) | 88 (13.3%) | 104 (43.9%) | <0.001 |
| DNR in intensive care unit or general ward | 246 (27.4%) | 80 (12.1%) | 166 (70%) | <0.001 |
| AUC (95% CI) | F1 Score | |
|---|---|---|
| LR-bw | 0.763 (0.696–0.831) | 0.596 |
| XGB-bw | 0.779 (0.717–0.841) | 0.562 |
| RF-bw | 0.811 (0.755–0.868) | 0.292 |
| BBC | 0.796 (0.738–0.855) | 0.605 |
| BRF | 0.826 (0.77–0.881) | 0.64 |
| SOFA score | 0.672 (0.596–0.748) | 0.321 |
| APACHE II score | 0.662 (0.587–0.736) | 0.294 |
| Initial lactate | 0.683 (0.609–0.757) | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, B.S.; Jeon, S.; Son, D.; Choi, S.-H.; Shin, T.G.; Jo, Y.H.; Ryoo, S.M.; Kim, Y.-J.; Park, Y.S.; Kwon, W.Y.; et al. Machine Learning Model Development and Validation for Predicting Outcome in Stage 4 Solid Cancer Patients with Septic Shock Visiting the Emergency Department: A Multi-Center, Prospective Cohort Study. J. Clin. Med. 2022, 11, 7231. https://doi.org/10.3390/jcm11237231
Ko BS, Jeon S, Son D, Choi S-H, Shin TG, Jo YH, Ryoo SM, Kim Y-J, Park YS, Kwon WY, et al. Machine Learning Model Development and Validation for Predicting Outcome in Stage 4 Solid Cancer Patients with Septic Shock Visiting the Emergency Department: A Multi-Center, Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(23):7231. https://doi.org/10.3390/jcm11237231
Chicago/Turabian StyleKo, Byuk Sung, Sanghoon Jeon, Donghee Son, Sung-Hyuk Choi, Tae Gun Shin, You Hwan Jo, Seung Mok Ryoo, Youn-Jung Kim, Yoo Seok Park, Woon Yong Kwon, and et al. 2022. "Machine Learning Model Development and Validation for Predicting Outcome in Stage 4 Solid Cancer Patients with Septic Shock Visiting the Emergency Department: A Multi-Center, Prospective Cohort Study" Journal of Clinical Medicine 11, no. 23: 7231. https://doi.org/10.3390/jcm11237231
APA StyleKo, B. S., Jeon, S., Son, D., Choi, S.-H., Shin, T. G., Jo, Y. H., Ryoo, S. M., Kim, Y.-J., Park, Y. S., Kwon, W. Y., Suh, G. J., Lim, T. H., & Kim, W. Y., on behalf of the Korean Shock Society (KoSS) Investigators. (2022). Machine Learning Model Development and Validation for Predicting Outcome in Stage 4 Solid Cancer Patients with Septic Shock Visiting the Emergency Department: A Multi-Center, Prospective Cohort Study. Journal of Clinical Medicine, 11(23), 7231. https://doi.org/10.3390/jcm11237231





