Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD
Abstract
1. Introduction
2. Methods
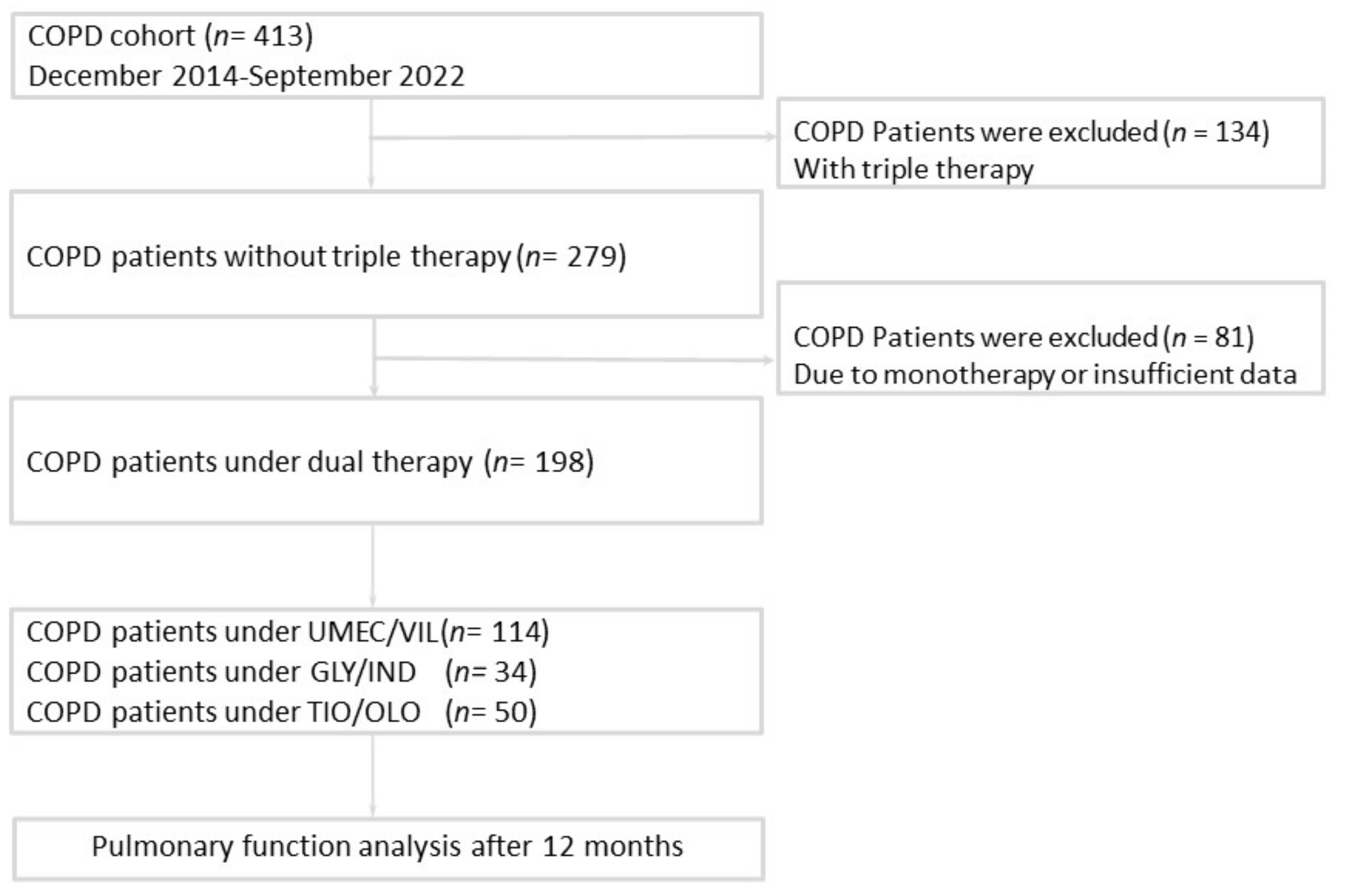
2.1. Study Patients
2.2. Clinical Data Collection and Treatment Assessment
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
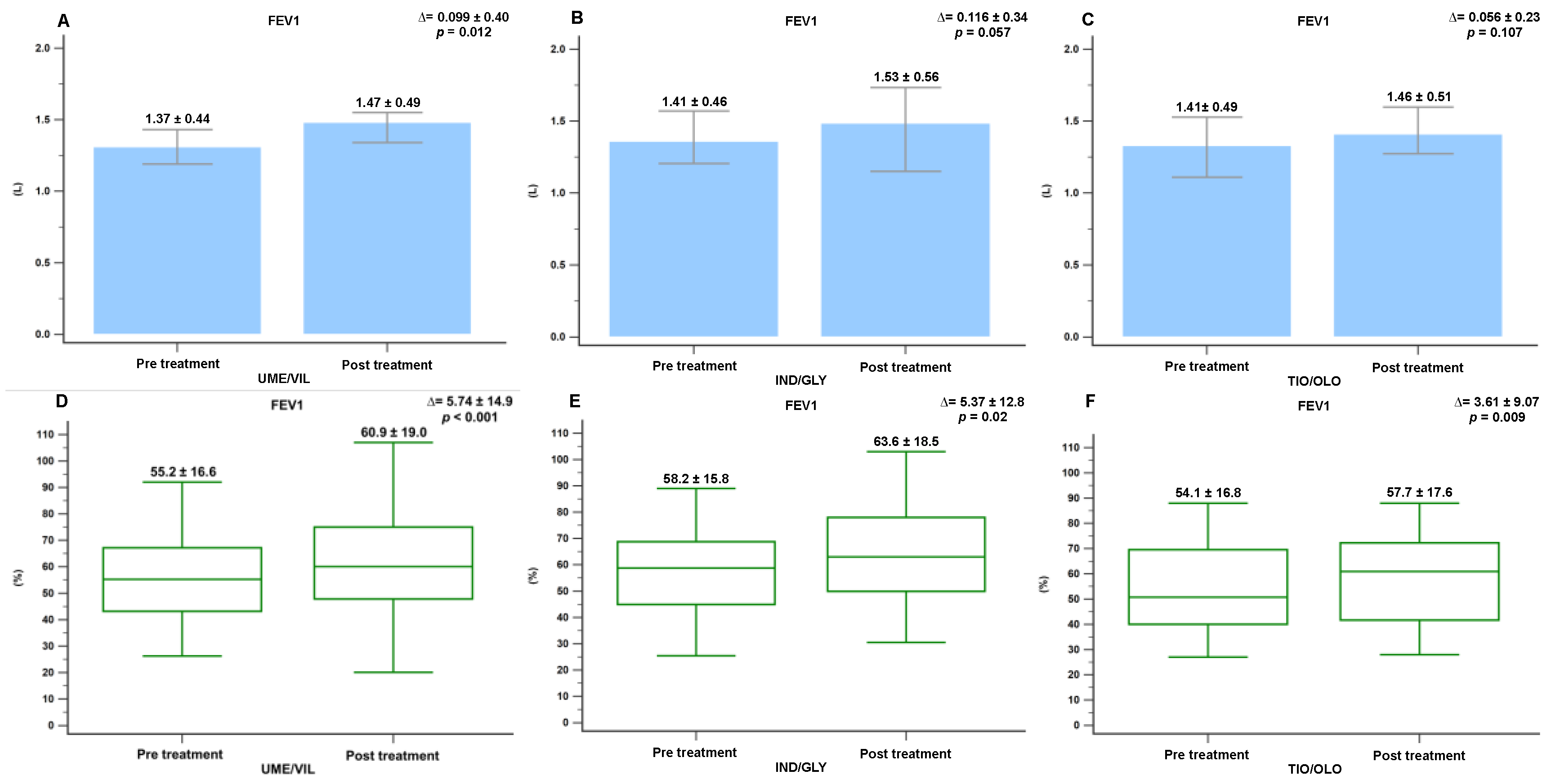
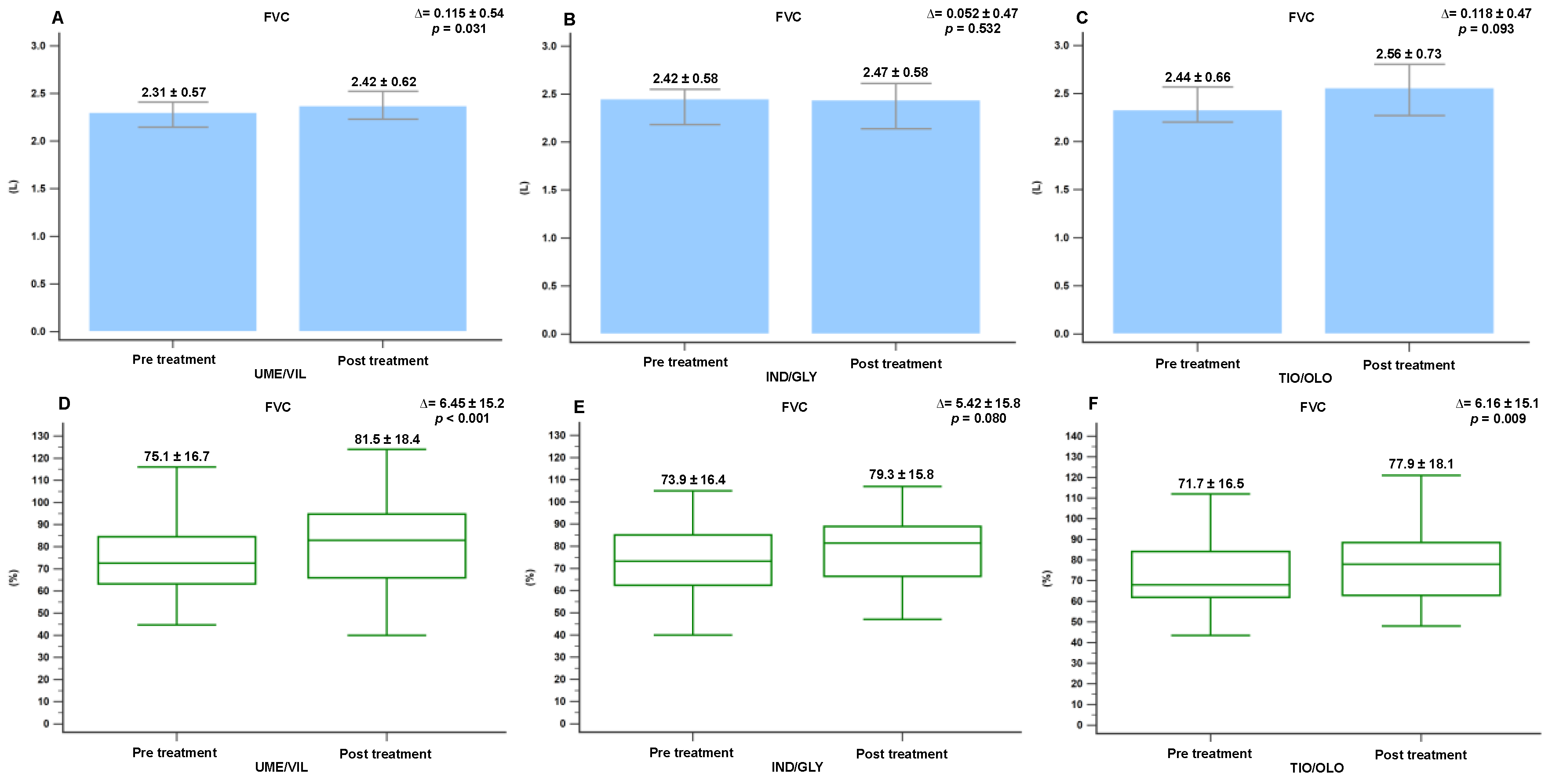
3.2. Pulmonary Function Changes Following Treatment
3.3. Minimal Clinically Important Differences in FEV1, FVC, and CAT Score Following Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Decramer, M.; Ficker, J.H.; Niewoehner, D.E.; Sandström, T.; Taylor, A.F.; D’Andrea, P.; Arrasate, C.; Chen, H.; Banerji, D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): A randomised, double-blind, parallel-group study. Lancet Respir. Med. 2013, 1, 199–209. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Bateman, E.D.; Pallante, J.; Alagappan, V.K.; D’Andrea, P.; Chen, H.; Banerji, D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): A randomised, double-blind, parallel group study. Lancet Respir. Med. 2013, 1, 51–60. [Google Scholar] [CrossRef]
- Celli, B.; Crater, G.; Kilbride, S.; Mehta, R.; Tabberer, M.; Kalberg, C.J.; Church, A. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: A randomized, controlled study. Chest 2014, 145, 981–991. [Google Scholar] [CrossRef]
- Ferguson, G.T.; Karpel, J.; Bennett, N.; Clerisme-Beaty, E.; Grönke, L.; Voß, F.; Buhl, R. Effect of tiotropium and olodaterol on symptoms and patient-reported outcomes in patients with COPD: Results from four randomised, double-blind studies. NPJ Prim. Care Respir. Med. 2017, 27, 7. [Google Scholar] [CrossRef][Green Version]
- Anzueto, A.R.; Vogelmeier, C.F.; Kostikas, K.; Mezzi, K.; Fucile, S.; Bader, G.; Shen, S.; Banerji, D.; Fogel, R. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1325–1337. [Google Scholar] [CrossRef][Green Version]
- Singh, D.; Maleki-Yazdi, M.R.; Tombs, L.; Iqbal, A.; Fahy, W.; Naya, I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1413–1424. [Google Scholar] [CrossRef]
- Singh, D.; D’Urzo, A.D.; Chuecos, F.; Muñoz, A.; Gil, E.G. Reduction in clinically important deterioration in chronic obstructive pulmonary disease with aclidinium/formoterol. Respir. Res. 2017, 18, 106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A Systematic Review with Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Sion, K.Y.J.; Huisman, E.L.; Punekar, Y.S.; Naya, I.; Ismaila, A.S. A Network Meta-Analysis of Long-Acting Muscarinic Antagonist (LAMA) and Long-Acting β2-Agonist (LABA) Combinations in COPD. Pulm. Ther. 2017, 3, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, M.; Gonzalez-Rojas, N.; Baldwin, M.; Groenke, L.; Voss, F.; Reason, T. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting β2-agonists: A systematic review and network meta-analysis. Ther. Adv. Respir. Dis. 2016, 10, 89–104. [Google Scholar] [CrossRef]
- Feldman, G.J.; Sousa, A.R.; Lipson, D.A.; Tombs, L.; Barnes, N.; Riley, J.H.; Patel, S.; Naya, I.; Compton, C.; Navarrete, B.A. Comparative Efficacy of Once-Daily Umeclidinium/Vilanterol and Tiotropium/Olodaterol Therapy in Symptomatic Chronic Obstructive Pulmonary Disease: A Randomized Study. Adv. Ther. 2017, 34, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Alcazar Navarrete, B.; Boucot, I.; Naya, I.; Tombs, L.; Lipson, D.A.; Compton, C.; Sousa, A.R.; Feldman, G. Umeclidinium/Vilanterol Versus Tiotropium/Olodaterol in Maintenance-Naive Patients with Moderate Symptomatic Chronic Obstructive Pulmonary Disease: A Post Hoc Analysis. Pulm. Ther. 2018, 4, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, E.; Ferguson, G.T.; Sanjar, S.; Goodin, T.; Yadao, A.; Fogel, R.; Maitra, S.; Sen, B.; Ayers, T.; Banerji, D. Dual Bronchodilation with Indacaterol Maleate/Glycopyrronium Bromide Compared with Umeclidinium Bromide/Vilanterol in Patients with Moderate-to-Severe COPD: Results from Two Randomized, Controlled, Cross-over Studies. Lung 2017, 195, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-L. Comparison of Effectiveness Using Different Dual Bronchodilator Agents in Chronic Obstructive Pulmonary Disease Treatment. J. Clin. Med. 2021, 10, 2649. [Google Scholar] [CrossRef]
- Muraki, M.; Kunita, Y.; Shirahase, K.; Yamazaki, R.; Hanada, S.; Sawaguchi, H.; Tohda, Y. A randomized controlled trial of long-acting muscarinic antagonist and long-acting β2 agonist fixed-dose combinations in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2021, 21, 26. [Google Scholar] [CrossRef]
- Moretz, C.; Bengtson, L.G.; Sharpsten, L.; Koep, E.; Le, L.; Tong, J.; Stanford, R.H.; Hahn, B.; Ray, R. Evaluation of rescue medication use and medication adherence receiving umeclidinium/vilanterol versus tiotropium bromide/olodaterol. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2047–2060. [Google Scholar] [CrossRef]
- Moretz, C.; Cole, A.L.; Mu, G.; Wu, B.; Guisinger, A.; Liu, Y.; Hahn, B.; Baylis, L. Evaluation of Medication Adherence and Rescue Medication Use in Non-Exacerbating Patients with COPD Receiving Umeclidinium/Vilanterol or Budesonide/Formoterol as Initial Maintenance Therapy. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2207–2215. [Google Scholar] [CrossRef]
- Mäkelä, M.J.; Backer, V.; Hedegaard, M.; Larsson, K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir. Med. 2013, 107, 1481–1490. [Google Scholar] [CrossRef]
- Lange, P.; Halpin, D.M.; O’Donnell, D.E.; MacNee, W. Diagnosis, assessment, and phenotyping of COPD: Beyond FEV(1). Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3–12. [Google Scholar] [CrossRef]
- Kakavas, S.; Kotsiou, O.S.; Perlikos, F.; Mermiri, M.; Mavrovounis, G.; Gourgoulianis, K.; Pantazopoulos, I. Pulmonary function testing in COPD: Looking beyond the curtain of FEV1. NPJ Prim. Care Respir. Med. 2021, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Ritondo, B.L.; De Guido, I.; Puxeddu, E.; Cazzola, M.; Calzetta, L. Efficacy and cardiovascular safety profile of dual bronchodilation therapy in chronic obstructive pulmonary disease: A bidimensional comparative analysis across fixed-dose combinations. Pulm. Pharmacol. Ther. 2019, 59, 101841. [Google Scholar] [CrossRef]
- Duarte-De-Araújo, A.; Teixeira, P.; Hespanhol, V.; Correia-De-Sousa, J. COPD: Understanding patients’ adherence to inhaled medications. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2767–2773. [Google Scholar] [CrossRef]
- Ramadan, W.H.; Sarkis, A.T. Patterns of use of dry powder inhalers versus pressurized metered-dose inhalers devices in adult patients with chronic obstructive pulmonary disease or asthma: An observational comparative study. Chronic Respir. Dis. 2017, 14, 309–320. [Google Scholar] [CrossRef]
- Van der Palen, J.; Thomas, M.; Chrystyn, H.; Sharma, R.K.; van der Valk, P.D.; Goosens, M.; Wilkinson, T.; Stonham, C.; Chauhan, A.J.; Imber, V.; et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: Comparison of ELLIPTA with other inhaler devices. NPJ Prim. Care Respir. Med. 2017, 27, 17001. [Google Scholar] [CrossRef]
- Pisi, R.; Aiello, M.; Zanini, A.; Tzani, P.; Paleari, D.; Marangio, E.; Spanevello, A.; Nicolini, G.; Chetta, A. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1191–1197. [Google Scholar] [CrossRef]
- Lavorini, F.; Pedersen, S.; Usmani, O.S.; Barnes, P.; Corbetta, L.; Corrigan, C.; Chawes, B.; Dekhuijzen, P.; Hausen, T.; Levy, M.; et al. Dilemmas, Confusion, and Misconceptions Related to Small Airways Directed Therapy. Chest 2017, 151, 1345–1355. [Google Scholar] [CrossRef]
- Ciciliani, A.M.; Langguth, P.; Wachtel, H. In vitro dose comparison of Respimat((R)) inhaler with dry powder inhalers for COPD maintenance therapy. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Small Airway Disease in Patients with Chronic Obstructive Pulmonary Disease. Tuberc. Respir. Dis. 2017, 80, 317–324. [Google Scholar] [CrossRef]
- Dean, J.; Kolsum, U.; Hitchen, P.; Gupta, V.; Singh, D. Clinical characteristics of COPD patients with tidal expiratory flow limitation. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.; Mafort, T.T. Correlations between Small Airway Function, Ventilation Distribution, and Functional Exercise Capacity in COPD Patients. Lung 2014, 192, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballeira, M.; Heredia, J.; Rue, M.; Quintana, S.; Almagro, P. The bronchodilator test in chronic obstructive pulmonary disease: Interpretation methods. Respir. Med. 2007, 101, 34–42. [Google Scholar] [CrossRef][Green Version]
- Hanania, N.A.; Celli, B.R.; Donohue, J.F.; Martin, U.J. Bronchodilator Reversibility in COPD. Chest 2011, 140, 1055–1063. [Google Scholar] [CrossRef]
- Calverley, P.M.; Albert, P.; Walker, P.P. Bronchodilator reversibility in chronic obstructive pulmonary disease: Use and limitations. Lancet Respir. Med. 2013, 1, 564–573. [Google Scholar] [CrossRef]
- Ben Saad, H.; Prefaut, C.; Tabka, Z.; Zbidi, A.; Hayot, M. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm. Pharmacol. Ther. 2008, 21, 767–773. [Google Scholar] [CrossRef]
- Newton, M.F.; O’Donnell, D.E.; Forkert, L. Response of Lung Volumes to Inhaled Salbutamol in a Large Population of Patients with Severe Hyperinflation. Chest 2002, 121, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Boni, E.; Corda, L.; Franchini, D.; Chiroli, P.; Damiani, G.P.; Pini, L.; Grassi, V.; Tantucci, C. Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at rest. Thorax 2002, 57, 528–532. [Google Scholar] [CrossRef]
- Fortis, S.; Comellas, A.; Make, B.J.; Hersh, C.P.; Bodduluri, S.; Georgopoulos, D.; Kim, V.; Criner, G.J.; Dransfield, M.T.; Bhatt, S.P.; et al. Combined Forced Expiratory Volume in 1 Second and Forced Vital Capacity Bronchodilator Response, Exacerbations, and Mortality in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2019, 16, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-J.; Chen, N.-H.; Cheng, S.-L.; Tao, C.-W.; Wei, Y.-F.; Wu, Y.-K.; Chan, M.-C.; Liu, S.-F.; Hsu, W.-H.; Yang, T.-M.; et al. Comparing Clinical Outcomes of Tiotropium/Olodaterol, Umeclidinium/Vilanterol, and Indacaterol/Glycopyrronium Fixed-Dose Combination Therapy in Patients with Chronic Obstructive Pulmonary Disease in Taiwan: A Multicenter Cohort Study. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 967–976. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 198) | UMEC/VIL (n = 114) | IND/GLY (n = 34) | TIO/OLO (n = 50) | p-Value | |
|---|---|---|---|---|---|
| Age, years (SD) | 70.4 (10.4) | 70.6 (10.9) | 72.4 (7.9) | 68.5 (10.3) | 0.229 |
| Male (%) | 186 (93.9) | 109 (95.6) | 32 (94.1) | 45 (90.0) | 0.381 |
| Body Height, cm (SD) | 163.1 (6.6) | 163.1 (7.0) | 162.3 (6.9) | 163.6 (5.7) | 0.651 |
| Body Weight, kg (SD) | 64.2 (11.6) | 63.9 (11.3) | 65.5 (11.7) | 63.7 (12.2) | 0.74 |
| BMI, kg/m2 (SD) | 24.2 (4.1) | 24.1 (4.1) | 24.8 (3.8) | 23.7 (4.3) | 0.483 |
| Smoking, packs/year (SD) | 40.2 (28.8) | 40.3 (29.2) | 48.2 (31.5) | 34.5 (25.1) | 0.100 |
| ACO (%) | 14 (7.1) | 7 (6.1) | 4 (11.8) | 3 (6.0) | 0.502 |
| CAD (%) | 39 (19.7) | 22 (19.3) | 7 (20.6) | 10 (20.0) | 0.984 |
| CHF (%) | 41 (20.7) | 24 (21.1) | 6 (17.6) | 11 (22.0) | 0.881 |
| Cancers (%) | 39 (19.7) | 22 (19.3) | 8 (23.5) | 9 (18.0) | 0.811 |
| Bronchiectasis (%) | 15 (7.6) | 8 (7.0) | 3 (8.8) | 4 (8.0) | 0.932 |
| FEV1/FVC, % (SD) | 59.1 (9.9) | 59.4 (9.7) | 59.3 (8.1) | 58.1 (11.3) | 0.74 |
| FEV1, L (SD) | 1.38 (0.45) | 1.36 (0.43) | 1.41 (0.46) | 1.41 (0.48) | 0.788 |
| FEV1, % (SD) | 55.7 (16.2) | 55.3 (16.3) | 58.2 (15.7) | 54.8 (16.7) | 0.610 |
| FVC, L (SD) | 2.35 (0.59) | 2.29 (0.56) | 2.38 (0.61) | 2.46 (0.65) | 0.214 |
| FVC, % (SD) | 73.8 (16.5) | 74.8 (17.1) | 73.9 (16.3) | 72.3 (15.9) | 0.713 |
| RV, % (SD) | 171.1 (63.1) | 170.3 (57.5) | 173.4 (68.5) | 171.2 (74.1) | 0.972 |
| MMEF25-75, % (SD) | 27.9 (12.1) | 29.1 (11.2) | 27.2 (9.7) | 26.5 (14.8) | 0.551 |
| GOLD stage, % | 0.448 | ||||
| I | 15 (7.7) | 9 (8.0) | 2 (5.9) | 4 (8.0) | |
| II | 110 (56.1) | 65 (58.0) | 23 (67.6) | 22 (44.0) | |
| III | 61 (31.1) | 33 (29.5) | 7 (20.6) | 21 (42.0) | |
| IV | 10 (5.1) | 5 (4.5) | 2 (5.9) | 3 (6.0) | |
| CAT (IQR) | 6.5 (6.5–7) | 6.0 (4–8.75) | 6.5 (4–10.0) | 7.5 (5.0–13.0) | 0.058 |
| AE in last year (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0.270 |
| UMEC/VIL (n = 114) | IND/GLY (n = 34) | TIO/OLO (n = 50) | p-Value | |
|---|---|---|---|---|
| FEV1, MCID (%) | 49 (43.5) | 14 (41.2) | 19 (38.3) | 0.868 |
| FVC, MCID (%) | 55 (48.1) | 11 (33.3) | 27 (46.8) | 0.316 |
| CAT, MCID (%) | 30 (28.0) | 10 (30.3) | 14 (28.5) | 0.873 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Chen, C.-Y.; Liao, W.-C.; Wu, B.-R.; Chen, W.-C.; Tu, C.-Y.; Chen, C.-H.; Cheng, W.-C. Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD. J. Clin. Med. 2022, 11, 7165. https://doi.org/10.3390/jcm11237165
Huang W-C, Chen C-Y, Liao W-C, Wu B-R, Chen W-C, Tu C-Y, Chen C-H, Cheng W-C. Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD. Journal of Clinical Medicine. 2022; 11(23):7165. https://doi.org/10.3390/jcm11237165
Chicago/Turabian StyleHuang, Wei-Chun, Chih-Yu Chen, Wei-Chih Liao, Biing-Ru Wu, Wei-Chun Chen, Chih-Yen Tu, Chia-Hung Chen, and Wen-Chien Cheng. 2022. "Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD" Journal of Clinical Medicine 11, no. 23: 7165. https://doi.org/10.3390/jcm11237165
APA StyleHuang, W.-C., Chen, C.-Y., Liao, W.-C., Wu, B.-R., Chen, W.-C., Tu, C.-Y., Chen, C.-H., & Cheng, W.-C. (2022). Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD. Journal of Clinical Medicine, 11(23), 7165. https://doi.org/10.3390/jcm11237165






