Salivary Biomarkers in Periodontitis Post Scaling and Root Planing
Abstract
1. Introduction
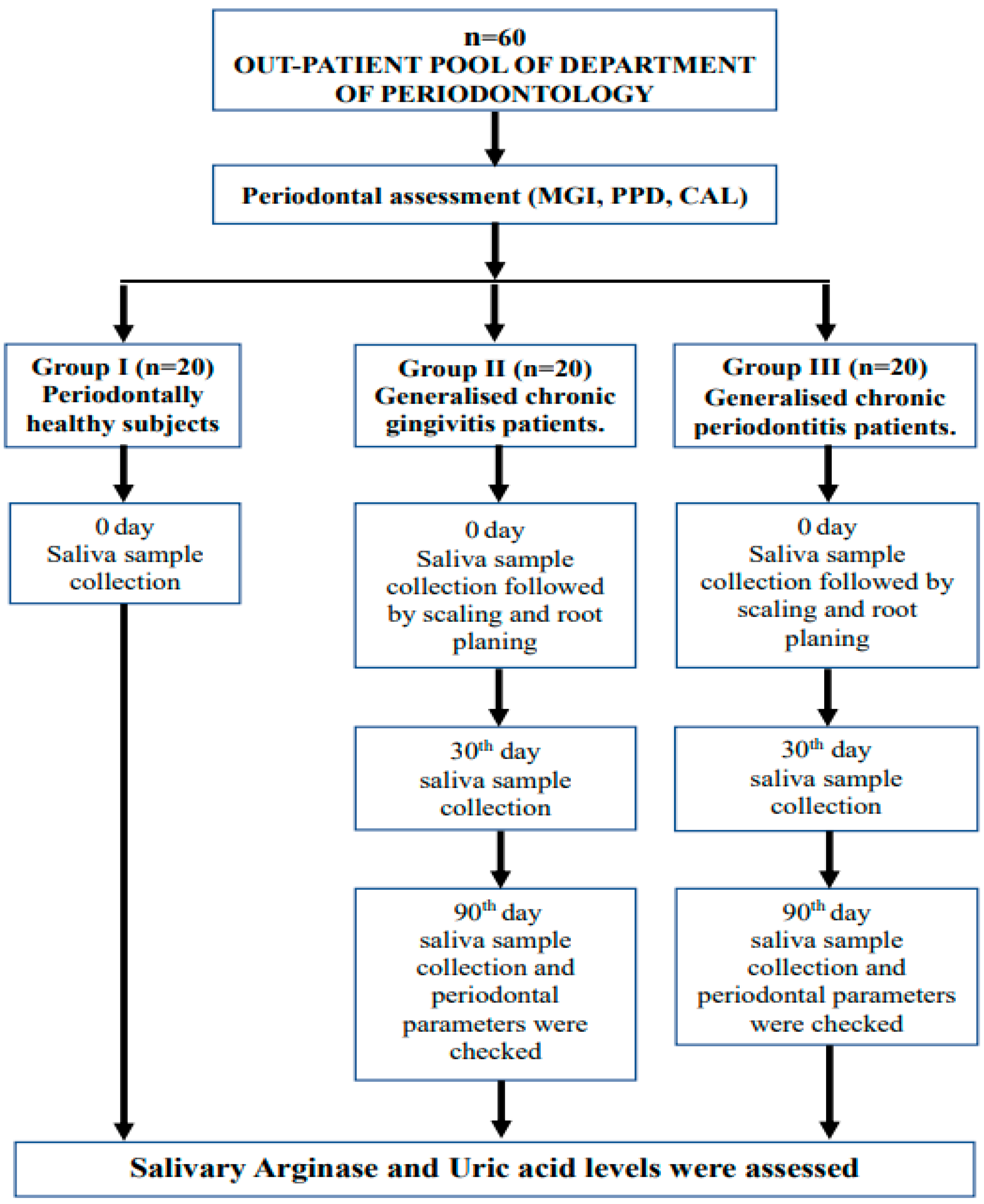
2. Materials and Methods
| Calculation for power analysis: |
| t-tests—Means: Difference between two independent means (two groups) Analysis: A priori: Compute required sample size Input: Tail(s) = Two Effect size d = 1.2041300 α err prob = 0.05 Power (1-β err prob) = 0.95 Allocation ratio N2/N1 = 1 Output: Noncentrality parameter δ = 3.7113779 Critical t = 2.0280940 Df = 36 Sample size group 1 = 19 Sample size group 2 = 19 Total sample size = 38 Actual power = 0.9506005 |
2.1. Non-Surgical Periodontal Therapy
2.2. Parameters Assessed
2.3. Collection of Unstimulated Saliva Samples
2.4. Estimation of Salivary Arginase and Uric Acid Levels
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Arigbede, A.; Babatope, B.; Bamidele, M. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Bhusari, B.M.; Mahajan, R.; Rajbhoj, S.; Shah, S. Reactive Oxygen Species& Its Role in Periodontal Disease. J. Dent. Sci. 2014, 13, 52–59. [Google Scholar]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.L.; Nalini, E.H.; Kumar, A.P.; Devi, R. Salivary biomarkers of periodontal disease-the ultimate diagnostic tool. Int. J. Recent. Sci. Res. 2018, 9, 25927–25932. [Google Scholar] [CrossRef]
- Huang, L.; Shao, D.; Wang, Y.; Cui, X.; Li, Y.; Chen, Q.; Cui, J. Human body-fluid proteome: Quantitative profiling and computational prediction. Brief. Bioinform. 2021, 22, 315–333. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y. Point-of-care diagnosis of periodontitis using saliva: Technically feasible but still a challenge. Front. Cell. Infect. Microbiol. 2015, 5, 65. [Google Scholar] [CrossRef]
- Tanwar, J.; Hungund, S.; Dodani, K. Nonsurgical periodontal therapy: A review. J. Oral Res. Rev. 2016, 8, 39. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in non-surgical periodontal therapy: Clinical and microbiological aspects in a 6-month follow-up domiciliary protocol for oral hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Martí i Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Rizal, M.I.; Vega, S. Level of Salivary Uric Acid in Gingivitis and Periodontitis Patients. Sci. Dent. J. 2017, 1, 7. [Google Scholar] [CrossRef]
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An Evidence-Based Update on the Molecular Mechanisms Underlying Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.J.; Byrd, K.M.; Kim, S.A. The Chairside Periodontal Diagnostic Toolkit: Past, Present, and Future. Diagnostics 2021, 11, 932. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Kalburgi, N.B.; Koregal, A.C.S.N. Arginase: An Emerging Key Player in Periodontal Disease and Diabetes Mellitus. Int. J. Dent. Sci. Innov. Res. 2019, 2, 153–159. [Google Scholar]
- González-Hernández, J.M.; Franco, L.; Colomer-Poveda, D.; Martinez-Subiela, S.; Cugat, R.; Cerón, J.J.; Márquez, G.; Martínez-Aranda, L.M.; Jimenez-Reyes, P.; Tvarijonaviciute, A. Influence of sampling conditions, salivary flow, and total protein content in uric acid measurements in saliva. Antioxidants 2019, 8, 389. [Google Scholar] [CrossRef]
- Schwartz MNeiers, F.; Feron, G.; Canon, F. The relationship between salivary redox, diet, and food flavo perception. Front. Nutr. 2021, 7, 612735. [Google Scholar] [CrossRef]
- Lobao, W.; Carvalho, R.C.; Leite, S.A.; Rodrigues, V.P.; Batista, J.E.; Gomes-Filho, I.S.; Pereira, A.L. Relationship between periodontal outcomes and serum biomarkers changes after non-surgical periodontal therapy. An. Da Acad. Bras. De Ciências 2019, 91, e20170652. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.N. Changes in Inflammatory Cytokines in Saliva after Non-Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 194. [Google Scholar] [CrossRef]
- Van der Wenden, G.A.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients. A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef]
- MeselI, S.E.; Bahar, K.U.; Leyla, K.U. Relationships between initial probing depth and changes in the clinical parameters following non-surgical periodontal treatment in chronic periodontitis. J. Istanb. Univ. Fac. Dent. 2017, 51, 11–17. [Google Scholar] [CrossRef]
- Shah, H.K.; Sharma, S.; Goel, K.; Shrestha, S.; Niraula, S.R. Probing Pocket Depth and Clinical Attachment Level between Non-Surgical and Surgical Periodontal Therapy in Chronic Periodontitis Patients: A Randomised Controlled Clinical Trial. J. Nepal. Soc. Periodontol. Oral Implantol. 2018, 2, 40–44.22. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Hess, J.V.; Stölzel, P.; Haubitz, I.; Jockel-Schneider, Y. Impact of a two-stage subgingival instrumentation scheme involving air polishing on attachment gain after active periodontal therapy. J. Periodontol. 2022, 93, 1500–1509. [Google Scholar] [CrossRef]
- De Castro, M.V.M.; Cortelli, S.C.; Rodrigues, E.; de Moraes, A.; Costa, F.O.; de Maximo, P.M.; Cortelli, J.R. Salivary arginase activity after mechanical-chemical therapy. Rev. Odontol. Da UNESP 2018, 47, 261–266. [Google Scholar] [CrossRef][Green Version]
- Haririan, H.; Andrukhov, O.; Laky, M.; Rausch-Fan, X. Saliva as a Source of Biomarkers for Periodontitis and Periimplantitis. Front. Dent. Med. 2021, 2, 687638. [Google Scholar] [CrossRef]
- Pattanshetti, J.; Kataria, N.; Kalburgi, N.B.; Athanasiadis, L.; Ioannidis, P.; Efrosini, K. Serum uric acid: Exploring the link between tobacco and periodontitis-Current Issue. Int. J. Sci. Res. 2017, 6, 420–423. [Google Scholar] [CrossRef]
- Uppin, R.B.; Varghese, S.S. Estimation of serum, salivary, and gingival crevicular uric acid of individuals with and without periodontal disease: A systematic review and meta-analysis. J. Int. Soc. Prev. Community Dent. 2022, 12, 393. [Google Scholar] [CrossRef]
- Fatima, G.; Uppin, R.B.; Kasagani, S.; Tapshetty, R.; Rao, A. Comparison of Salivary Uric Acid Level among Healthy Individuals without Periodontitis with that of Smokers and Non-smokers with Periodontitis. J. Adv. Oral Res. 2016, 7, 24–28. [Google Scholar] [CrossRef]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef]
- Sayar, F.; Ahmadi, R.S.; Montazeri, M. Effect of nonsurgical periodontal therapy on the level of salivary antioxidants in patients with generalized moderate-to-severe chronic periodontitis. J. Adv. Periodontol. Implant. Dent. 2019, 11, 21–27. [Google Scholar] [CrossRef]
- Baz, E.L.; Mohamed, K.; Abd El Gwad, A.; Awadallah, H.I.; Mahallawy, O.S. The use of antioxidants in treatment of patients with gingivitis & chronic periodontitis-intervention study. J. Environ. Sci. Ain Shams 2021, 50, 217–237. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 122. [Google Scholar] [CrossRef]
| Periodontal Parameters | Time Interval (Days) | Group I (Mean ± SD) | Group II (Mean ± SD) | Group III (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| Modified gingival index | 0 | 0.42 ± 0.20 | 1.17 ± 0.20 | 2.52 ± 0.21 | 0.000 * |
| 90 | - | 0.30 ± 0.13 | 1.66 ± 0.28 | 0.000 * | |
| Mean change 0 to 90 | - | 0.86 ± 0.20 | 0.86 ± 0.27 | 0.984 NS | |
| p-value | - | 0.000 * | 0.000 * | ||
| Probing pocket depth (mm) | 0 | 1.36 ± 0.27 | 1.48 ± 0.14 | 5.10 ± 0.26 | 0.000 * |
| 90 | - | 1.18 ± 0.07 | 2.29 ± 0.48 | 0.000 * | |
| Mean change 0 to 90 | - | 0.29 ± 0.15 | 2.80 ± 0.52 | 0.000 * | |
| p-value | - | 0.000 * | 0.000 * | ||
| Clinical attachment level (mm) | 0 | 1.35 ± 0.27 | 1.48 ± 0.14 | 5.47 ± 0.23 | 0.000 * |
| 90 | - | 1.18 ± 0.07 | 2.41 ± 0.62 | 0.000 * | |
| Mean change 0 to 90 | - | 0.29 ± 0.15 | 3.05 ± 0.60 | 0.000 * | |
| p-value | - | 0.000 * | 0.000 * |
| Salivary Parameters | Time Intervals (Days) | Group I | Group II | Group III | p-Value |
|---|---|---|---|---|---|
| Salivary arginase levels Units/L | 0 | 3.21 ± 4.15 | 9.20 ± 5.93 | 14.31 ± 6.02 | 0.000 * |
| 30 | - | 7.74 ± 1.30 | 13.70 ± 1.37 | 0.003 * | |
| 90 | - | 5.43 ± 1.17 | 11.18 ± 1.46 | 0.004 * | |
| p-value | - | 0.076 NS | 0.404 NS | - | |
| Mean change 0 to 30th day | - | 1.46 ± 8.32 | 0.60 ± 7.23 | 0.731 NS | |
| p-value | - | 1.000 NS | 1.00 0 NS | ||
| Mean change 0 to 90th day | - | 3.74 ± 6.75 | 3.13 ± 10.43 | 0.818 NS | |
| p-value | - | 0.066 NS | 0.586 NS | ||
| Mean change 30th to 90th day | - | 2.31 ± 8.32 | 2.52 ± 8.71 | 0.938 NS | |
| p-value | 0.687 NS | 0.632 NS | |||
| Salivary uric acid levels mg/dL | 0 | 21.49 ± 10.01 | 9.73 ± 7.56 | 5.64 ± 4.32 | 0.000 * |
| 30 | - | 10.21 ± 11.19 | 16.30 ± 13.91 | 0.136 NS | |
| 90 | - | 16.35 ± 12.10 | 18.54 ± 10.01 | 0.537 NS | |
| p-value | - | 0.127 NS | 0.000 * | - | |
| Mean change 0 to 30th day | - | 0.48 ± 12.27 | 10.66 ± 13.68 | 0.018 * | |
| p-value | - | 1.000 NS | 0.007 * | ||
| Mean change 0 to 90th day | - | −6.62 ± 13.50 | −12.90 ± 10.22 | 0.106 NS | |
| p-value | - | 0.123 NS | 0.000 * | ||
| Mean change 30th to 90th day | - | −6.13 ± 16.09 | −2.23 ± 16.34 | 0.452 NS | |
| p-value | - | 0.31 NS | 1.000 NS |
| Periodontal Parameters | Salivary Arginase (units/L) (N = 60) | Salivary Uric Acid (mg/dL) (N = 60) | |
|---|---|---|---|
| Modified gingival index | CORRELATION | 0.625 | −0.568 |
| p-value | 0.000 * | 0.000 * | |
| Probing pocket depth (mm) | CORRELATION | 0.556 | −0.458 |
| p-value | 0.000 * | 0.000 * | |
| Clinical attachment level (mm) | CORRELATION | 0.555 | −0.461 |
| p-value | 0.000 * | 0.000 * |
| Periodontal Parameters | Salivary Arginase (units/L) (n = 40) | Salivary Uric Acid (mg/dL) (n = 40) | |
|---|---|---|---|
| Modified gingival index | CORRELATION | 0.426 | 0.124 |
| p-value | 0.006 * | 0.447 NS | |
| Probing pocket depth (mm) | CORRELATION | 0.343 | 0.100 |
| p-value | 0.03 * | 0.538 NS | |
| Clinical attachment level (mm) | CORRELATION | 0.316 | 0.090 |
| p-value | 0.047 * | 0.583 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priya, K.L.; Mahendra, J.; Mahendra, L.; Kanakamedala, A.; Alsharif, K.F.; Mugri, M.H.; Varadarajan, S.; Alamoudi, A.; Hassan, A.A.-H.A.-A.; Alnfiai, M.M.; et al. Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. J. Clin. Med. 2022, 11, 7142. https://doi.org/10.3390/jcm11237142
Priya KL, Mahendra J, Mahendra L, Kanakamedala A, Alsharif KF, Mugri MH, Varadarajan S, Alamoudi A, Hassan AA-HA-A, Alnfiai MM, et al. Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. Journal of Clinical Medicine. 2022; 11(23):7142. https://doi.org/10.3390/jcm11237142
Chicago/Turabian StylePriya, K. Lakshmi, Jaideep Mahendra, Little Mahendra, Anilkumar Kanakamedala, Khalaf F. Alsharif, Maryam H. Mugri, Saranya Varadarajan, Ahmed Alamoudi, Ali Abdel-Halim Abdel-Azim Hassan, Mrim M. Alnfiai, and et al. 2022. "Salivary Biomarkers in Periodontitis Post Scaling and Root Planing" Journal of Clinical Medicine 11, no. 23: 7142. https://doi.org/10.3390/jcm11237142
APA StylePriya, K. L., Mahendra, J., Mahendra, L., Kanakamedala, A., Alsharif, K. F., Mugri, M. H., Varadarajan, S., Alamoudi, A., Hassan, A. A.-H. A.-A., Alnfiai, M. M., Alzahrani, K. J., Bahammam, M. A., Baeshen, H. A., Balaji, T. M., & Bhandi, S. (2022). Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. Journal of Clinical Medicine, 11(23), 7142. https://doi.org/10.3390/jcm11237142







