Preventing Growth Stagnation and Premature LH Surge Are the Keys to Obtaining a Viable Embryo in Monofollicular IVF Cycles: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Selection and Eligibility Criteria
2.2. Ovarian Stimulation and Oocyte Retrieval
2.3. Insemination and Embryo Culture
2.4. Endometrial Preparation, Embryo Transfer and Pregnancy Outcome
2.5. Statistical Analysis
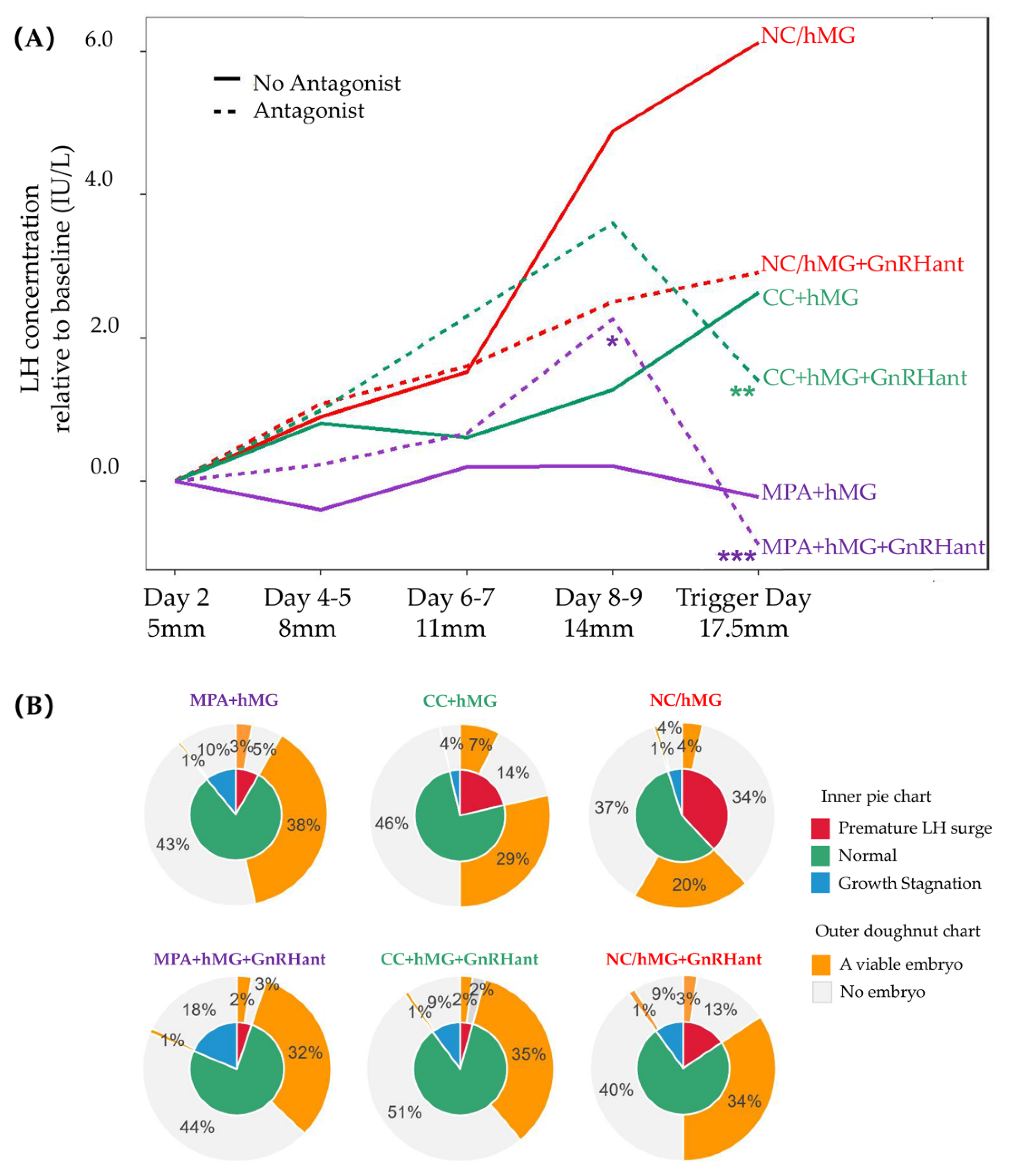
3. Results
3.1. Patient and Cycle Characteristics
3.2. IVF Outcome
3.3. LH Profile and the Effect of Growth Stagnation and LH Surge
3.4. Pregnancy Outcomes of Embryo Transfer Cycles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Li, X.; Yang, X.; Cai, S.; Lu, G.; Lin, G.; Humaidan, P.; Gong, F. Cumulative Live Birth Rates in Low Prognosis Patients According to the POSEIDON Criteria: An Analysis of 26,697 Cycles of in vitro Fertilization/Intracytoplasmic Sperm Injection. Front. Endocrinol. 2019, 10, 642. [Google Scholar] [CrossRef]
- Poseidon, G.; Alviggi, C.; Andersen, C.Y.; Buehler, K.; Conforti, A.; De Placido, G.; Esteves, S.C.; Fischer, R.; Galliano, D.; Polyzos, N.P.; et al. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef]
- Jaswa, E.G.; McCulloch, C.E.; Simbulan, R.; Cedars, M.I.; Rosen, M.P. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: Evidence for concomitant reduction in oocyte quality with quantity. Fertil. Steril. 2021, 115, 966–973. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Hua, Y.Z.; Santi, A.; Ocon, E.; Weiss, B. Follicle flushing in monofollicular in vitro fertilization almost doubles the number of transferable embryos. Acta Obstet. Gynecol. Scand. 2013, 92, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Ertas, S.; Urman, B.; Yakin, K. Does Oocyte Retrieval Performance in Mono-follicular Cycles Differ by Physician Experience? Reprod. Sci. 2022, 29, 2995–2999. [Google Scholar] [CrossRef] [PubMed]
- Kohl Schwartz, A.S.; Calzaferri, I.; Roumet, M.; Limacher, A.; Fink, A.; Wueest, A.; Weidlinger, S.; Mitter, V.R.; Leeners, B.; Von Wolff, M. Follicular flushing leads to higher oocyte yield in monofollicular IVF: A randomized controlled trial. Hum. Reprod. 2020, 35, 2253–2261. [Google Scholar] [CrossRef]
- Quinquin, M.; Mialon, O.; Isnard, V.; Massin, N.; Parinaud, J.; Delotte, J.; Bongain, A. In vitro fertilization versus conversion to intrauterine insemination in Bologna-criteria poor responders: How to decide which option? Fertil. Steril. 2014, 102, 1596–1601. [Google Scholar] [CrossRef]
- Polyzos, N.P.; Blockeel, C.; Verpoest, W.; De Vos, M.; Stoop, D.; Vloeberghs, V.; Camus, M.; Devroey, P.; Tournaye, H. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum. Reprod. 2012, 27, 3481–3486. [Google Scholar] [CrossRef]
- Pelinck, M.J.; Hoek, A.; Simons, A.H.; Heineman, M.J. Efficacy of natural cycle IVF: A review of the literature. Hum. Reprod. Update 2002, 8, 129–139. [Google Scholar] [CrossRef]
- Kolibianakis, E.; Zikopoulos, K.; Camus, M.; Tournaye, H.; Van Steirteghem, A.; Devroey, P. Modified natural cycle for IVF does not offer a realistic chance of parenthood in poor responders with high day 3 FSH levels, as a last resort prior to oocyte donation. Hum. Reprod. 2004, 19, 2545–2549. [Google Scholar] [CrossRef][Green Version]
- Ho, J.R.; Paulson, R.J. Modified natural cycle in in vitro fertilization. Fertil Steril 2017, 108, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, P.; Romito, A.; Errázuriz, J.; Santos-Ribeiro, S.; Popovic-Todorovic, B.; Racca, A.; Tournaye, H.; De Vos, M.; Blockeel, C. Modified natural cycle IVF versus conventional stimulation in advanced-age Bologna poor responders. Reprod. Biomed. Online 2019, 39, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Labarta, E.; Marin, D.; Remohí, J.; Bosch, E. Conventional versus minimal ovarian stimulation: An intra-patient comparison of ovarian response in poor-responder women according to Bologna Criteria. Reprod. Biomed. Online 2018, 37, 434–441. [Google Scholar] [CrossRef]
- Moffat, R.; Hansali, C.; Schoetzau, A.; Ahler, A.; Gobrecht, U.; Beutler, S.; Raggi, A.; Sartorius, G.; De Geyter, C. Randomised controlled trial on the effect of clomiphene citrate and gonadotropin dose on ovarian response markers and IVF outcomes in poor responders. Hum. Reprod. 2021, 36, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Sun, L.; Zhang, S.; Chai, W.; Hong, Q.; Long, H.; Wang, L.; Lyu, Q.; Kuang, Y. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod. Biol. Endocrinol. 2017, 15, 71. [Google Scholar] [CrossRef]
- Zhang, J.; Du, M.; Li, Z.; Liu, W.; Ren, B.; Zhang, Y.; Guan, Y. Comparison of Dydrogesterone and Medroxyprogesterone in the Progestin-Primed Ovarian Stimulation Protocol for Patients with Poor Ovarian Response. Front. Endocrinol. 2021, 12, 708704. [Google Scholar] [CrossRef]
- Ata, B.; Capuzzo, M.; Turkgeldi, E.; Yildiz, S.; La Marca, A. Progestins for pituitary suppression during ovarian stimulation for art: A comprehensive and systematic review including meta-analyses. Hum Reprod Update 2021, 27, 48–66. [Google Scholar] [CrossRef]
- Du, M.; Zhang, J.; Li, Z.; Liu, X.; Li, J.; Liu, W.; Guan, Y. Comparison of the Cumulative Live Birth Rates of Progestin-Primed Ovarian Stimulation and Flexible GnRH Antagonist Protocols in Patients With Low Prognosis. Front. Endocrinol. 2021, 12, 705264. [Google Scholar] [CrossRef]
- Tu, X.; You, B.; Jing, M.; Lin, C.; Zhang, R. Progestin-Primed Ovarian Stimulation Versus Mild Stimulation Protocol in Advanced Age Women with Diminished Ovarian Reserve Undergoing Their First In Vitro Fertilization Cycle: A Retrospective Cohort Study. Front. Endocrinol. 2022, 12, 801026. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, M.; Xu, W.; Zhang, L.; Qi, M.; Shu, J. Confrontment and solution to gonadotropin resistance and low oocyte retrieval in in vitro fertilization for type I BPES: A case series with review of literature. J. Ovarian Res. 2021, 14, 143. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive, M.; Embryology, E.S.I.G.o. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, A.; D’Agostino, R.B., Sr. Multiplicity Considerations in Clinical Trials. N. Engl. J. Med. 2018, 378, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J.; Fabregues, F. LH in the follicular phase: Neither too high nor too low. Reprod. Biomed. Online 2006, 12, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.L. The luteinized unruptured follicle syndrome. Baillieres Clin. Obstet. Gynaecol. 1993, 7, 363–387. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Duan, J.; Xu, P.; Cheng, X.; Mao, C.; Croll, T.; He, X.; Shi, J.; Luan, X.; Yin, W.; You, E.; et al. Structures of full-length glycoprotein hormone receptor signalling complexes. Nature 2021, 598, 688–692. [Google Scholar] [CrossRef]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Casarini, L.; Riccetti, L.; De Pascali, F.; Gilioli, L.; Marino, M.; Vecchi, E.; Morini, D.; Nicoli, A.; La Sala, G.B.; Simoni, M. Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro. Int. J. Mol. Sci. 2017, 18, 926. [Google Scholar] [CrossRef]
- Choi, H.; Roh, J. LH-induced Transcriptional Regulation of Klf4 Expression in Granulosa Cells Occurs via the cAMP/PKA Pathway and Requires a Putative Sp1 Binding Site. Int. J. Mol. Sci. 2020, 21, 7385. [Google Scholar] [CrossRef]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Granulosa Cell Apoptosis in the Ovarian Follicle-A Changing View. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Paradiso, E.; Lazzaretti, C.; D’Alessandro, S.; Roy, N.; Mascolo, E.; Zaręba, K.; García-Gasca, A.; Simoni, M. Regulation of antral follicular growth by an interplay between gonadotropins and their receptors. J. Assist. Reprod. Genet. 2022, 39, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Y.; Chai, W.R.; Hong, Q.Q.; Wang, N.L.; Sun, L.H.; Long, H.; Wang, L.; Tian, H.; Lyu, Q.F.; et al. The pregnancy outcome of progestin-primed ovarian stimulation using 4 versus 10 mg of medroxyprogesterone acetate per day in infertile women undergoing in vitro fertilisation: A randomised controlled trial. Bjog 2017, 124, 1048–1055. [Google Scholar] [CrossRef]
- Kuang, Y.P.; Chen, Q.J.; Fu, Y.L.; Wang, Y.; Hong, Q.Q.; Lyu, Q.F.; Ai, A.; Shoham, Z. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 2015, 104, 62–70.e3. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Yu, W.; Yu, S.; Yin, M.; Wu, L.; Chen, Q.; Cai, R.; Suo, L.; Wang, L.; Lyu, Q.; et al. Progesterone affects clinic oocyte yields by coordinating with follicle stimulating hormone via PI3K/AKT and MAPK pathways. J. Adv. Res. 2021, 33, 189–199. [Google Scholar] [CrossRef]
- Goldstein, S.R.; Siddhanti, S.; Ciaccia, A.V.; Plouffe, L., Jr. A pharmacological review of selective oestrogen receptor modulators. Hum. Reprod. Update 2000, 6, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Mikkelson, T.J.; Kroboth, P.D.; Cameron, W.J.; Dittert, L.W.; Chungi, V.; Manberg, P.J. Single-dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil. Steril. 1986, 46, 392–396. [Google Scholar] [CrossRef]
- Teramoto, S.; Kato, O. Minimal ovarian stimulation with clomiphene citrate: A large-scale retrospective study. Reprod. Biomed. Online 2007, 15, 134–148. [Google Scholar] [CrossRef]
- von Wolff, M.; Nitzschke, M.; Stute, P.; Bitterlich, N.; Rohner, S. Low-dosage clomiphene reduces premature ovulation rates and increases transfer rates in natural-cycle IVF. Reprod. Biomed. Online 2014, 29, 209–215. [Google Scholar] [CrossRef]
- Al-Inany, H.; Azab, H.; El-Khayat, W.; Nada, A.; El-Khattan, E.; Abou-Setta, A.M. The effectiveness of clomiphene citrate in LH surge suppression in women undergoing IUI: A randomized controlled trial. Fertil. Steril. 2010, 94, 2167–2171. [Google Scholar] [CrossRef]
- Sonmezer, M.; Pelin Cil, A.; Atabekoglu, C.; Ozkavukcu, S.; Ozmen, B. Does premature luteinization or early surge of LH impair cycle outcome? Report of two successful outcomes. J. Assist. Reprod. Genet. 2009, 26, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Meseguer, M.; Garrido, N.; Bosch, E.; Pellicer, A.; Remohi, J. The significance of premature luteinization in an oocyte-donation programme. Hum. Reprod. 2006, 21, 1503–1507. [Google Scholar] [CrossRef]
- Shen, X.; Long, H.; Guo, W.; Xie, Y.; Gao, H.; Zhang, J.; Wang, Y.; Lyu, Q.; Kuang, Y.; Wang, L. The ovulation trigger-OPU time interval of different ovarian protocols in ART: A retrospective study. Arch. Gynecol. Obstet. 2020, 302, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Long, H.; Guo, W.; Gao, H.; Cai, R.; Jin, W.; Yan, Z.; Zhang, S.; Wang, Y.; Lyu, Q.; et al. Optimal Ovulation Trigger-Oocyte Pickup Interval in Progestin-Primed Ovarian Stimulation Protocol: A Retrospective Study Using Propensity Score Matching. Front. Endocrinol. (Lausanne) 2019, 10, 694. [Google Scholar] [CrossRef] [PubMed]
| Group 1 MPA | Group 2 CC | Group 3 NC | p Value | ||||
|---|---|---|---|---|---|---|---|
| 1a | 1b | 2a | 2b | 3a | 3b | ||
| MPA + hMG | MPA + hMG + GnRHant | CC + hMG | CC + hMG + GnRHant | NC/hMG | NC/hMG + GnRHant | ||
| n = 204 | n = 118 | n = 28 | n = 139 | n = 166 | n = 160 | ||
| Age, year | 38.3 ± 6.2 | 39.1 ± 6.0 | 38.9 ± 6.2 | 38.7 ± 6.0 | 39.1 ± 6.7 | 37.8 ± 6.9 | 0.430 |
| BMI, kg/m2 | 21.9 ± 2.8 | 21.9 ± 2.8 | 22.3 ± 2.6 | 22.4 ± 2.8 | 22.2 ± 3.0 | 21.8 ± 2.7 | 0.461 |
| AMH, ng/mL | 0.39 ± 0.33 | 0.42 ± 0.35 | 0.65 ± 0.48 *,^ | 0.54 ± 0.41 *,^ | 0.38 ± 0.34 | 0.47 ± 0.45 | <0.001 |
| Primary Infertility, n (%) | 69 (33.8%) | 36 (30.5%) | 9 (32.1%) | 48 (34.5%) | 51 (30.7%) | 54 (33.8%) | 0.967 |
| Infertility duration, year | 4.1 ± 3.8 | 4.8 ± 4.3 | 4.2 ± 3.3 | 4.0 ± 3.7 | 4.5 ± 4.5 | 4.1 ± 3.7 | 0.595 |
| Previous IVF failures, n | 2.9 ± 3.3 | 2.9 ± 3.7 | 2.6 ± 2.9 | 2.5 ± 3.3 | 3.0 ± 3.1 | 2.8 ± 3.0 | 0.788 |
| Infertility causes | |||||||
| Tubal factor, n (%) | 87 (42.6%) | 57 (48.3%) | 9 (32.1%) | 53 (38.1%) | 67 (40.4%) | 61 (38.1%) | 0.132 |
| Endometriosis, n (%) | 25 (12.3%) | 14 (11.9%) | 3 (10.7%) | 17 (12.2%) | 24 (14.5%) | 29 (18.1%) | 0.086 |
| Male factor, n (%) | 142 (69.6%) | 89 (75.4%) | 18 (64.3%) | 101 (72.7%) | 108 (65.1%) | 113 (70.6%) | 0.116 |
| Unexplained, n (%) | 26 (12.7%) | 17 (14.4%) | 8 (28.6%) | 18 (12.9%) | 25 (15.1%) | 20 (12.5%) | 0.152 |
| Day 2 FSH, IU/L | 12.3(8.6) | 13.5 (7.3) | 8.4 (4.5) | 9.0 (5.0) *,#,^ | 13.5 (9.4) | 10.5 (5.1) | <0.001 |
| Day 2 LH, IU/L | 4.5 (4.3) | 5.1 (3.4) | 3.2 (1.4) | 3.7 (2.4)^ | 6.1 (4.5) | 4.9 (4.5) | 0.003 |
| hMG dose, IU | 856.62 ^ (487.82) | 959.04 ^ (404.69) | 668.06 (609.30) | 824.32 ^ (620.97) | 507.95 (484.92) | 725.94 (567.52) | <0.001 |
| hMG duration, day | 7.61 (2.48) | 8.17 (2.06) | 5.52(3.64) *,$ | 6.61(2.80) *,$,^ | 4.99(3.46) *,$ | 6.05 (2.96) *,$ | <0.001 |
| Group 1 MPA | Group 2 CC | Group 3 NC | p Value | ||||
|---|---|---|---|---|---|---|---|
| 1a | 1b | 2a | 2b | 3a | 3b | ||
| MPA + hMG | MPA + hMG + GnRHant | CC + hMG | CC + hMG + GnRHant | NC/hMG | NC/hMG + GnRHant | ||
| n = 204 | n = 118 | n = 28 | n = 139 | n = 166 | n = 160 | ||
| Premature LH surge, n (%) | 18 (8.8%) ^,$ | 6 (5.1%) ^,$ | 6 (21.4%) | 7 (5.0%) ^,$ | 67 (40.4%) | 32 (20%) ^ | <0.001 |
| Growth stagnation, n (%) | 22 (10.8%) | 22 (18.6%) ^ | 1 (3.6%) | 14 (10.1%) | 8 (4.8%) | 16 (10.0%) | 0.007 |
| Premature ovulation, n (%) | 11 (5.4%) ^ | 6 (5.1%) ^ | 0 (0%) | 4 (2.9%) ^ | 13 (7.8%) | 9 (5.6%) ^ | 0.371 |
| Emergency retrieval, n (%) | 16 (7.8) | 5 (4.2%) | 2 (7.1%) | 7 (5.0%) | 41 (24.7%) | 19 (11.9%) | <0.001 |
| Oocytes retrieved, n (%) | 153 (75.0%) ^ | 73 (61.9%) | 20 (71.4%) | 109 (78.4%) ^,# | 99 (59.6%) | 119 (74.4%) ^ | 0.001 |
| Abnormal | 14 (6.9%) | 6 (5.1%) | 2 (7.1%) | 8 (5.8%) | 10 (6.0%) | 9 (5.6%) | 0.99 |
| GV/MI | 1 (0.5%) | 1 (0.8%) | 0 (0%) | 3 (2.2%) | 3 (1.8%) | 3 (1.9%) | 0.698 |
| MII | 138 (67.6%) ^ | 66 (55.9%) | 18 (64.3%) | 98 (70.5%) ^ | 86 (51.8%) | 107 (66.9%) | 0.003 |
| ICSI, n (%) | 47 (33%) | 25 (37.3%) | 8 (42.1%) | 55 (39.6%) | 47 (28.3%) | 34 (29.6%) | 0.145 |
| 2PN zygotes, n (%) | 103 (53.4%) | 53 (47.7%) | 14 (50.0%) | 74 (54.8%) | 60 (39.5%) | 80 (53.0%) | 0.237 |
| Cleaved embryos, n (%) | 97 (50.8%) | 50 (45.0%) | 13 (46.4%) | 72 (53.3%) ^ | 53 (35.1%) | 76 (50.3%) | 0.026 |
| Top quality embryos, n (%) | 74 (36.3%) | 29 (24.6%) | 8 (28.6%) | 39 (28.1%) | 25 (15.2%) | 46 (28.9%) | 0.001 |
| Viable embryos, n (%) | 85 (41.7%) ^ | 42 (35.6%) | 10 (35.7%) | 52 (37.4%) | 41 (24.7%) | 61 (38.1%) | 0.029 |
| Day 3 embryos | 76 (37.3%) ^ | 34 (28.8%) | 9 (32.1%) | 43 (30.9%) | 36 (21.7%) | 55 (34.4%) | 0.041 |
| Day 5/6 embryos | 9 (4.4%) | 8 (6.8%) | 1 (3.6%) | 9 (6.5%) | 5 (3.0%) | 6 (3.8%) | 0.603 |
| Group 1 MPA | Group 2 CC | Group 3 NC | p Value | ||||
|---|---|---|---|---|---|---|---|
| 1a | 1b | 2a | 2b | 1a | 1b | ||
| MPA + hMG | MPA + hMG + GnRHant | CC + hMG | CC + hMG + GnRHant | NC/hMG | NC/hMG + GnRHant | ||
| n = 33 | n = 14 | n = 6 | n = 15 | n = 21 | n = 30 | ||
| Endometrial thickness, mm | 9.20 ± 2.10 | 9.36 ± 2.03 | 9.12 ± 1.78 | 11.17 ± 2.50 | 9.47 ± 2.31 | 9.42 ± 2.50 | 0.124 |
| Frozen embryo transfer, n (%) | 33 (100%) | 14 (100%) | 6 (100%) | 14 (93.3%) | 19 (90.5%) | 27 (90%) | 0.671 |
| Endometrial preparation by hormone replacement, n (%) | 29 (87.9%) | 13 (92.9%) | 6 (100%) | 14 (93.3%) | 18 (85.7%) | 26 (86.7%) | 0.890 |
| Day 3 embryos, n (%) | 31 (93.9%) | 13 (92.9%) | 5 (83.3%) | 11 (73.3%) | 19 (90.5%) | 28 (93.3%) | 0.311 |
| Top quality embryos, n (%) | 30 (90.9%) | 8 (57.1%) | 5 (83.3%) | 11 (73.3%) | 13 (65.0%) | 22 (73.3%) | 0.142 |
| Clinical pregnancy, n (%) | 6 (18.2%) | 1 (7.1%) | 0 (0.0%) | 3 (20.0%) | 8 (38.1%) | 11 (36.7%) | 0.086 |
| Biochemical pregnancy, n (%) | 3 (9.1%) | 1 (7.1%) | 3 (50.0%) | 0 (0.0%) | 3 (14.3%) | 3 (10.0%) | 0.082 |
| Miscarriage, n (%) | 1 (3.0%) | 1 (7.1%) | 0 (0.0%) | 1 (6.7%) | 2 (9.5%) | 6 (20.0%) | 0.338 |
| Ectopic pregnancy, n (%) | 1 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.3%) | 1.000 |
| Ongoing pregnancy, n (%) | 1 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0.859 |
| Live birth, n (%) | 3 (9.1%) | 0 (0.0%) | 0 (0.0%) | 2 (13.4%) | 5 (23.8%) | 4 (13.3%) | 0.393 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhu, X.; Wu, X.; Yu, Y.; Zhang, L.; Shu, J. Preventing Growth Stagnation and Premature LH Surge Are the Keys to Obtaining a Viable Embryo in Monofollicular IVF Cycles: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7140. https://doi.org/10.3390/jcm11237140
Guo X, Zhu X, Wu X, Yu Y, Zhang L, Shu J. Preventing Growth Stagnation and Premature LH Surge Are the Keys to Obtaining a Viable Embryo in Monofollicular IVF Cycles: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(23):7140. https://doi.org/10.3390/jcm11237140
Chicago/Turabian StyleGuo, Xiaoyan, Xiaoping Zhu, Xiangli Wu, Yiqi Yu, Ling Zhang, and Jing Shu. 2022. "Preventing Growth Stagnation and Premature LH Surge Are the Keys to Obtaining a Viable Embryo in Monofollicular IVF Cycles: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 23: 7140. https://doi.org/10.3390/jcm11237140
APA StyleGuo, X., Zhu, X., Wu, X., Yu, Y., Zhang, L., & Shu, J. (2022). Preventing Growth Stagnation and Premature LH Surge Are the Keys to Obtaining a Viable Embryo in Monofollicular IVF Cycles: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(23), 7140. https://doi.org/10.3390/jcm11237140








