Risk Factors for Rapid Recurrence of Hyperkalemia following Cessation of Sodium Zirconium Cyclosilicate
Abstract
1. Background
2. Methods
2.1. Patient Selection and Study Design
2.2. SZC Therapy
2.3. Primary Outcome
2.4. Data Collection
2.5. Statistics
3. Results
3.1. Baseline Characteristics
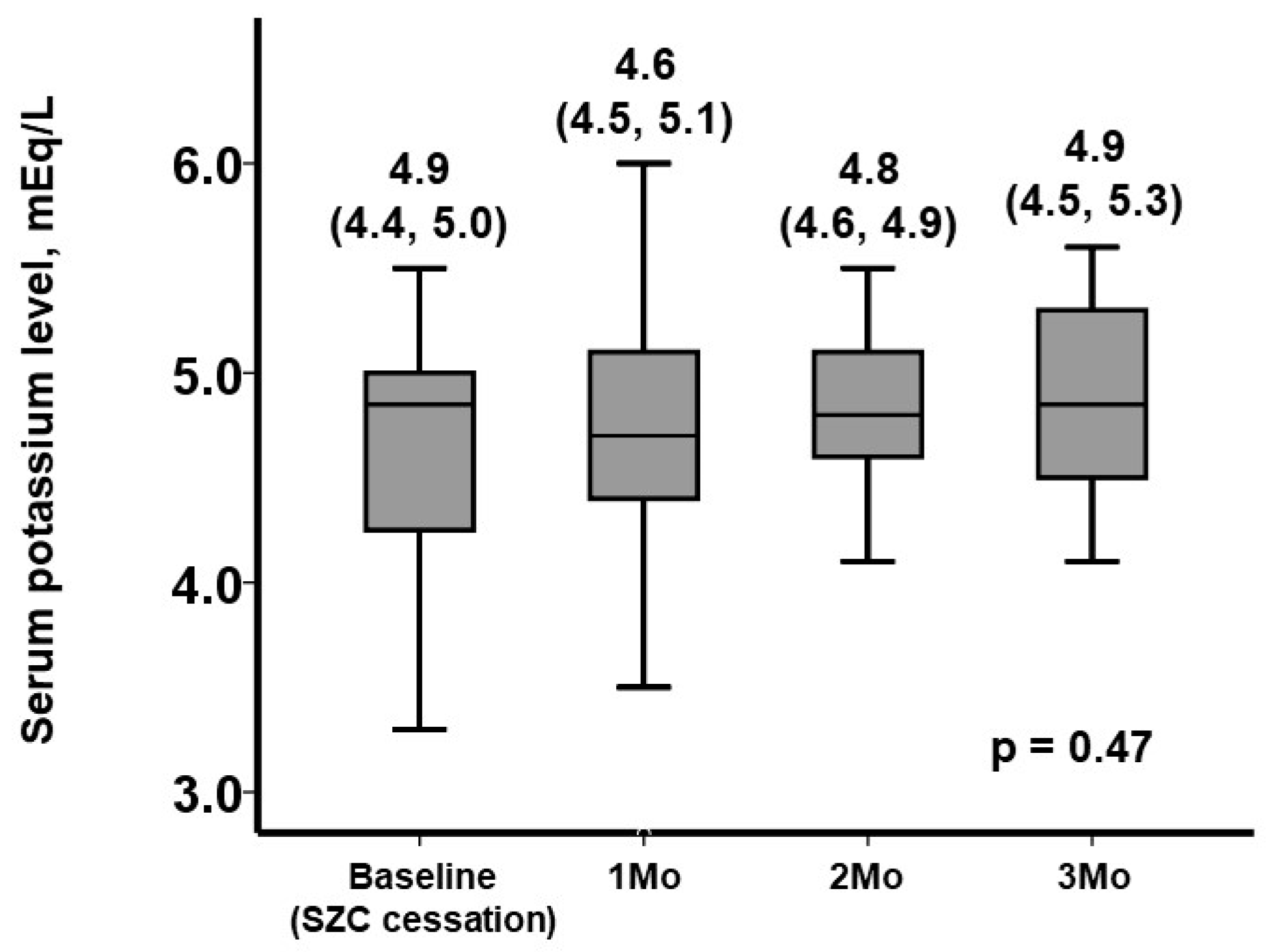
3.2. The Trend in Serum Potassium Levels following SZC Cessation
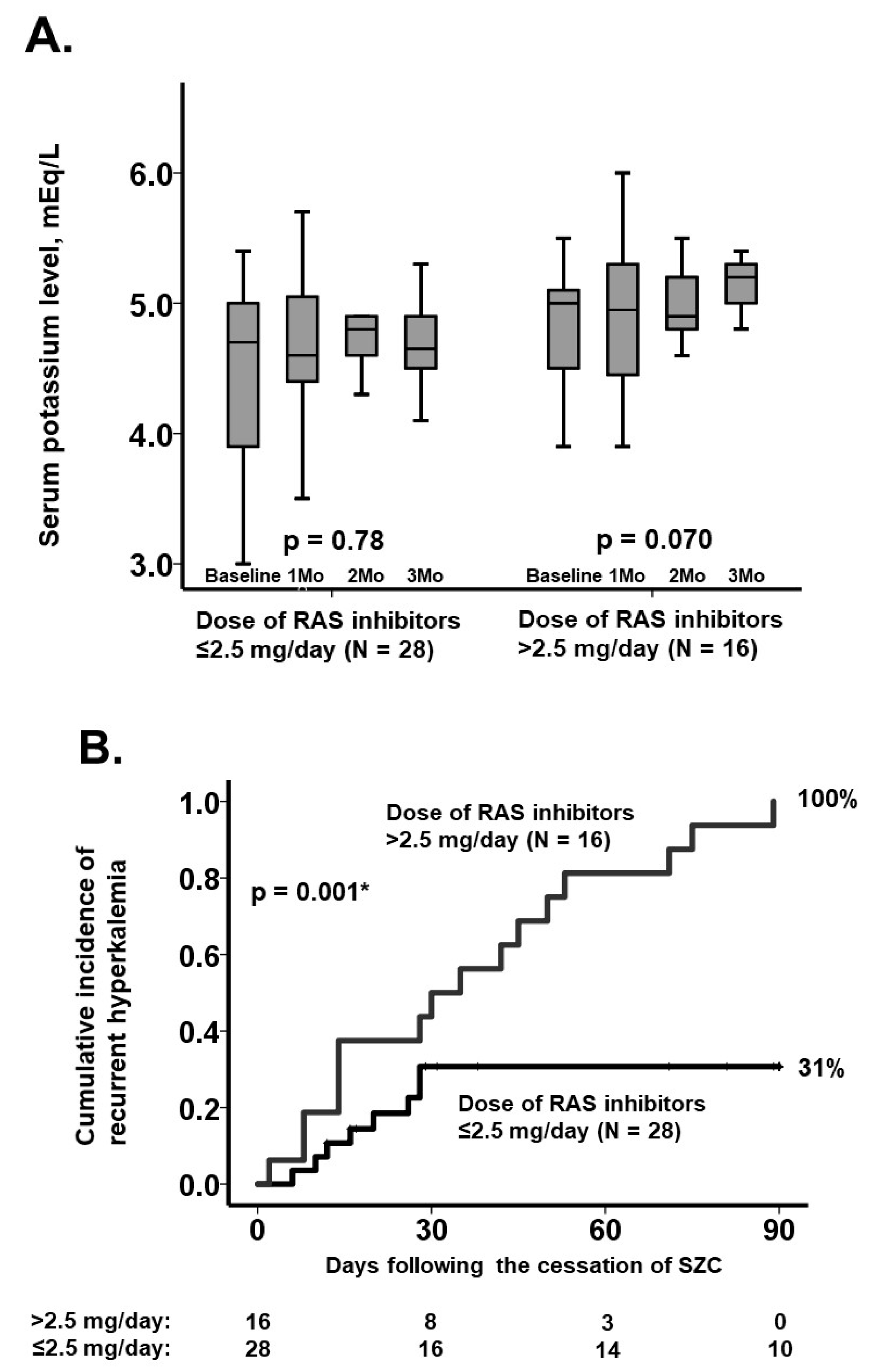
3.3. Rapid Recurrence of Hyperkalemia
3.4. Stratification Using the Dose of RAS Inhibitors
4. Discussion
4.1. Discussion of Our Findings
4.2. Limitations
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoy, S.M. Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia. Drugs 2018, 78, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Kohsaka, S.; Kanda, E.; Okami, S.; Yajima, T. Hyperkalemia in Real-World Patients Under Continuous Medical Care in Japan. Kidney Int. Rep. 2019, 4, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Narang, N.; Kinugawa, K. Impact of Sodium Zirconium Cyclosilicate Therapy Cessation in Patients with Systolic Heart Failure. J. Clin. Med. 2022, 11, 5330. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Kim, J.; Mellstrom, C.; Ford, K.L.; Jenkins, N.C.; Tsang, C.; Evans, M.; McEwan, P. Serum potassium variability as a predictor of clinical outcomes in patients with cardiorenal disease or diabetes: A retrospective UK database study. Clin. Kidney J. 2022, 15, 758–770. [Google Scholar] [CrossRef]
- Kashihara, N.; Yamasaki, Y.; Osonoi, T.; Harada, H.; Shibagaki, Y.; Zhao, J.; Kim, H.; Yajima, T.; Sarai, N. A phase 3 multicenter open-label maintenance study to investigate the long-term safety of sodium zirconium cyclosilicate in Japanese subjects with hyperkalemia. Clin. Exp. Nephrol. 2021, 25, 140–149. [Google Scholar] [CrossRef]
- Senni, M.; McMurray, J.J.V.; Wachter, R.; McIntyre, H.F.; Anand, I.S.; Duino, V.; Sarkar, A.; Shi, V.; Charney, A. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: Insights from the TITRATION study. Eur. J. Heart Fail. 2018, 20, 491–500. [Google Scholar] [CrossRef]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of Serum Potassium with All-Cause Mortality in Patients with and without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef]
- Linde, C.; Bakhai, A.; Furuland, H.; Evans, M.; McEwan, P.; Ayoubkhani, D.; Qin, L. Real-World Associations of Renin-Angiotensin-Aldosterone System Inhibitor Dose, Hyperkalemia, and Adverse Clinical Outcomes in a Cohort of Patients with New-Onset Chronic Kidney Disease or Heart Failure in the United Kingdom. J. Am. Heart Assoc. 2019, 8, e012655. [Google Scholar] [CrossRef]
- Takkar, C.; Nassar, T.; Qunibi, W. An evaluation of sodium zirconium cyclosilicate as a treatment option for hyperkalemia. Expert Opin. Pharmacother. 2021, 22, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Ide, T.; Ito, H.; Kihara, Y.; Kinugawa, K.; Kinugawa, S.; Makaya, M.; Murohara, T.; Node, K.; Saito, Y.; et al. JCS/JHFS 2021 Guideline Focused Update on Diagnosis and Treatment of Acute and Chronic Heart Failure. J. Card. Fail. 2021, 27, 1404–1444. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Pedro Ferreira, J.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef] [PubMed]

| n = 44 | |
|---|---|
| Demographics | |
| Age, years | 81 (69, 87) |
| Men | 26 (59%) |
| Body mass index | 22.0 (20.2, 24.4) |
| Comorbidity | |
| Heart failure | 27 (61%) |
| Diabetes mellitus | 23 (52%) |
| Atrial fibrillation | 11 (25%) |
| History of coronary intervention | 18 (41%) |
| History of stroke | 8 (18%) |
| Hemodialysis | 5 (11%) |
| Laboratory | |
| Hemoglobin, g/dL | 10.2 (9.0, 11.6) |
| Serum sodium, mEq/L | 139 (137, 141) |
| Serum potassium, mEq/L | 4.9 (4.3, 5.0) |
| Estimated glomerular filtration ratio, mL/min/1.73 m2 | 23.6 (18.3, 38.9) |
| Plasma B-type natriuretic peptide, pg/mL | 154 (132, 205) |
| Echocardiography | |
| Left ventricular end-diastolic diameter, mm | 49 (42, 54) |
| Left ventricular ejection fraction, % | 49 (37, 65) |
| Left atrial diameter, mm | 41 (34, 45) |
| Medication | |
| Beta-blocker | 29 (66%) |
| Dose of beta-blocker, mg/day | 2.5 (0, 10.0) |
| Renin-angiotensin system inhibitor | 27 (61%) |
| Dose of renin-angiotensin system inhibitor, mg/day | 1.25 (0, 3.75) |
| Mineralocorticoid receptor antagonist | 10 (23%) |
| Dose of mineralocorticoid receptor antagonist, mg/day | 0 (0, 0) |
| Loop diuretics | 25 (57%) |
| Dose of diuretics, mg/day | 10 (0, 20) |
| Univariable Analysis | Multivariable Analysis | |||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age, years | 0.99 (0.96–1.02) | 0.51 | 0.97 (0.94–1.05) | 0.65 |
| Male sex | 1.67 (0.69–4.05) | 0.25 | 1.76 (0.64–4.09) | 0.34 |
| Heart failure | 0.81 (0.36–1.82) | 0.61 | ||
| Hemodialysis | 0.99 (0.30–3.34) | 0.99 | ||
| eGFR, mL/min/1.73 m2 | 0.99 (0.98–1.02) | 0.76 | ||
| Logarithm of plasma BNP, pg/mL | 13.3 (1.70–103) | 0.014 * | 4.68 (0.46–40.5) | 0.28 |
| Left ventricular ejection fraction, % | 1.01 (0.98–1.03) | 0.72 | ||
| Dose of RAS inhibitor, mg/day | 1.38 (1.11–1.72) | 0.004 * | 1.26 (1.02–1.69) | 0.045 * |
| Dose of MRA, mg/day | 1.03 (0.97–1.10) | 0.36 | ||
| Dose of diuretics, mg/day | 0.99 (0.97–1.03) | 0.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Narang, N.; Kinugawa, K. Risk Factors for Rapid Recurrence of Hyperkalemia following Cessation of Sodium Zirconium Cyclosilicate. J. Clin. Med. 2022, 11, 7096. https://doi.org/10.3390/jcm11237096
Imamura T, Narang N, Kinugawa K. Risk Factors for Rapid Recurrence of Hyperkalemia following Cessation of Sodium Zirconium Cyclosilicate. Journal of Clinical Medicine. 2022; 11(23):7096. https://doi.org/10.3390/jcm11237096
Chicago/Turabian StyleImamura, Teruhiko, Nikhil Narang, and Koichiro Kinugawa. 2022. "Risk Factors for Rapid Recurrence of Hyperkalemia following Cessation of Sodium Zirconium Cyclosilicate" Journal of Clinical Medicine 11, no. 23: 7096. https://doi.org/10.3390/jcm11237096
APA StyleImamura, T., Narang, N., & Kinugawa, K. (2022). Risk Factors for Rapid Recurrence of Hyperkalemia following Cessation of Sodium Zirconium Cyclosilicate. Journal of Clinical Medicine, 11(23), 7096. https://doi.org/10.3390/jcm11237096







