Utilization of Two- and Three-Dimensional Transesophageal Echocardiography in Successfully Guiding Transcatheter Mitral Valve in Bioprosthetic Mitral Valve/Mitral Ring Implantation without Complications in Patients with Thrombus in Left Atrium/Left Atrial Appendage
Abstract
1. Introduction
2. Materials and Methods (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5, Videos S1 and S2)

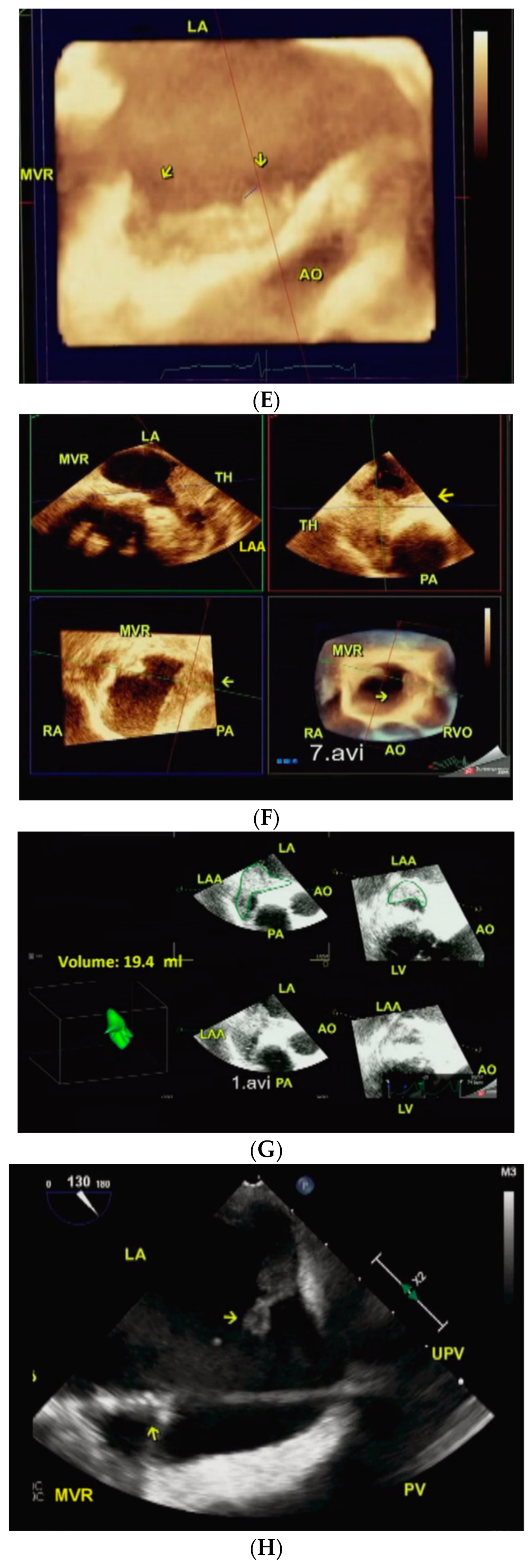

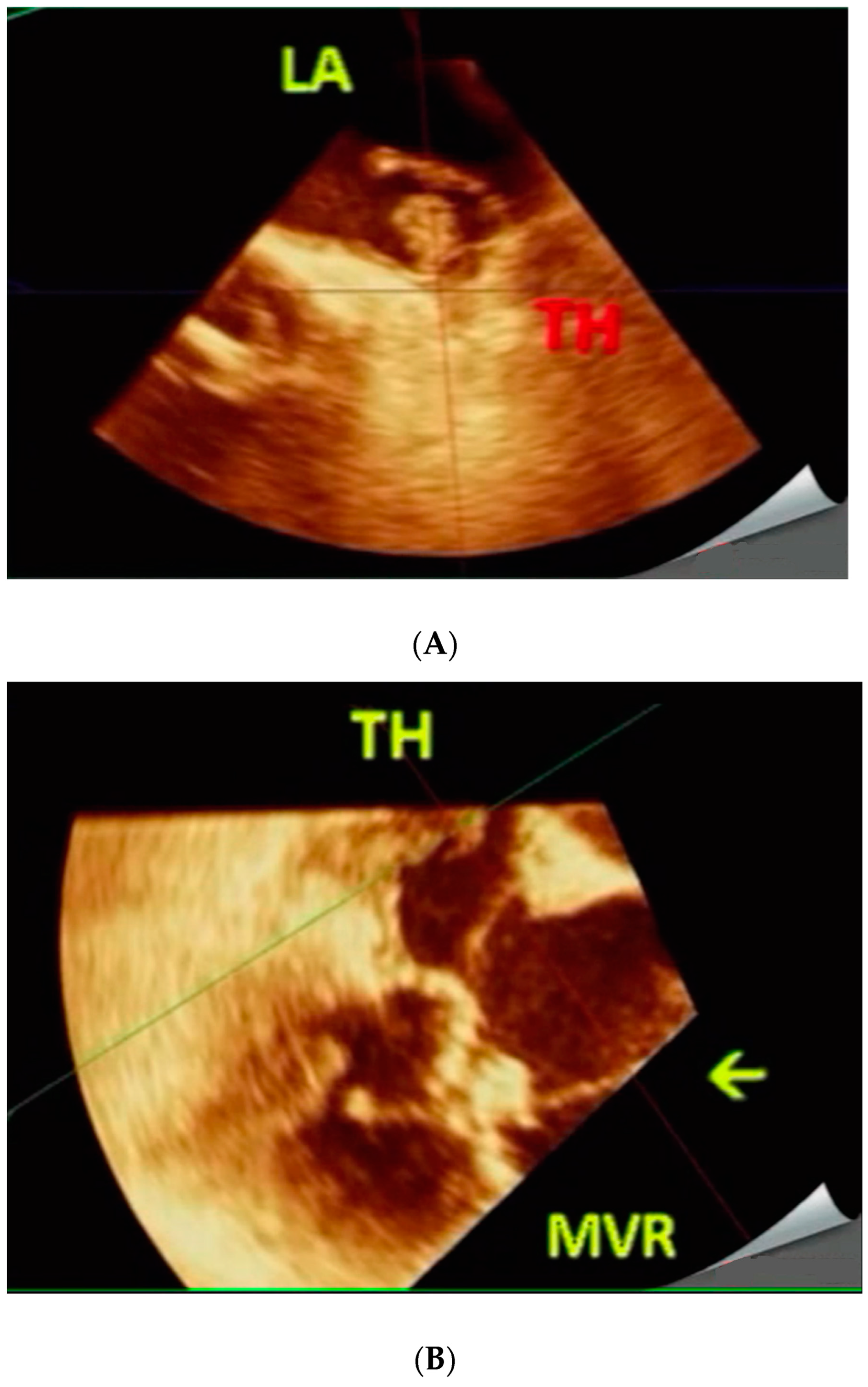
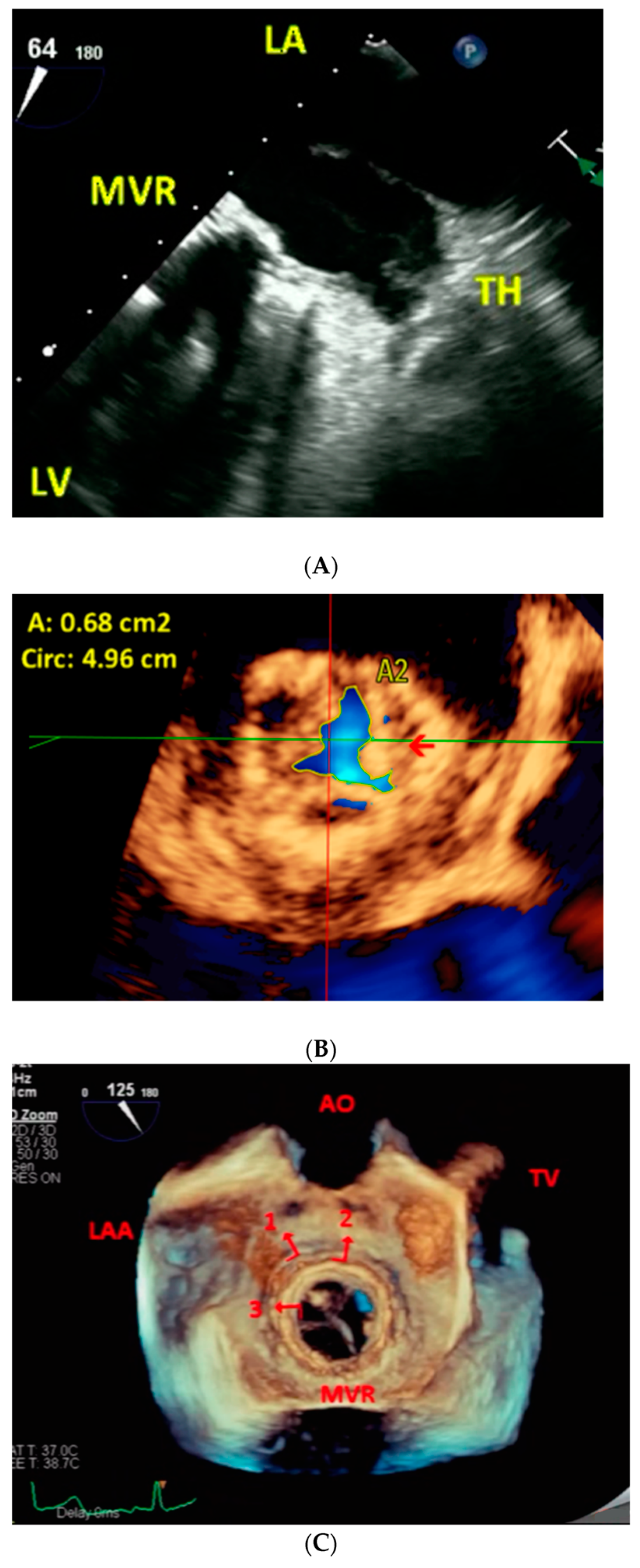


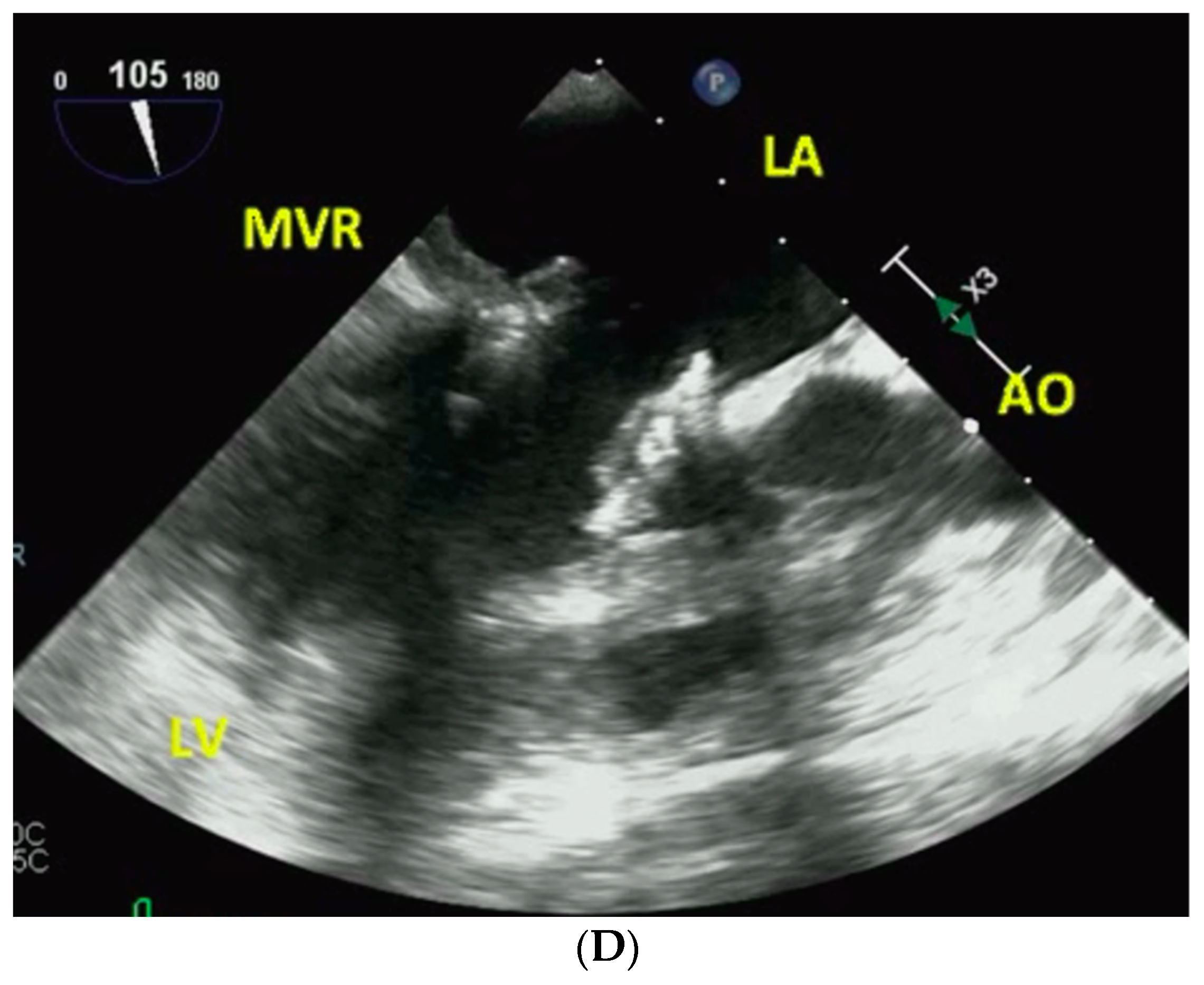
3. Procedure and Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.; et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, I.; Simm, A.; Kötting, J.; Thölen, F.; Fischer, B.; Silber, R.-E. Cardiac surgery in the elderly patient. Dtsch Arztebl Int. 2009, 106, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Urena, M.; Himbert, D.; Brochet, E.; Carrasco, J.L.; Iung, B.; Nataf, P.; Vahanian, A. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves. J. Am. Coll. Cardiol. Interv. 2017, 10, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, L.; Brooks, M.; Ihlemann, N.; Jonsson, A.; Holme, S.; Tang, M.; Terp, K.; Quadri, A. Transcatheter mitral valve implantation via transapical approach: An early experience. Eur. J. Cardiothorac. Surg. 2015, 48, 873–877, discussion 877–878. [Google Scholar] [CrossRef] [PubMed]
- Vohra, H.A.; Whistance, R.N.; Roubelakis, A.; Burton, A.; Barlow, C.W.; Tsang, G.M.; Livesey, S.A.; Ohri, S.K. Outcome after redo-mitral valve replacement in adult patients: A 10-year single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Dvir, D.; Demertzis, S.; Pedrazzini, G.; Berdajs, D.; Ferrari, E. Transcatheter valve-in-valve implantation for degenerated bioprosthetic aortic and mitral valves. Expert Rev. Med. Devices 2016, 13, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Nanda, N.C.; Khanna, D.; Dod, H.S.; Vengala, S.; Mehmood, F.; Agrawal, G.; Upendram, S. Morphological assessment of left ventricular thrombus by live three-dimensional transthoracic echocardiography. Echocardiography 2004, 21, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Thind, M.; Ahmed, M.I.; Gök, G.; Joson, M.; Elsayed, M.; Tuck, B.C.; Townsley, M.M.; Klas, B.; McGiffin, D.C.; Nanda, N.C. Incremental value of three-dimensional transesophageal echocardiography over the two-dimensional technique in the assessment of a thrombus in transit through a patent foramen ovale. Echocardiography 2015, 32, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Ge, Y.; Don, C.W.; Keraliya, A.; Aghayev, A.; Morgan, R.; Galper, B.; Bhatt, D.L.; Kaneko, T.; Di Carli, M.; et al. Use of cardiac computerized tomography to predict neo-left ventricular outflow tract obstruction before transcatheter mitral valve replacement. J. Am. Heart Assoc. 2017, 6, e007353. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, M.-C.; Yin, W.-H.; Lee, Y.-T.; Tsao, T.-P.; Lee, K.-C.; Huang, K.-C.; Chen, P.-E.; Chiang, W.-H.; Tung, T.-H.; Wei, J. Effects of transapical transcatheter mitral valve implantation. Front. Cardiovasc. Med. 2021, 8, 633369. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral embolic protection during transcatheter aortic valve replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Shivaraju, A.; Michel, J.; Frangieh, A.H.; Ott, I.; Thilo, C.; Schunkert, H.; Kastrati, A.; Leon, M.B.; Dvir, D.; Kodali, S.; et al. Transcatheter aortic and mitral valve-in-valve implantation using the Edwards Sapien 3 heart valve. J. Am. Heart Assoc. 2018, 7, e007767. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.C.; Rahman, S.M.A.-E.; Khatri, G.; Agrawal, G.; El-Sayed, A.A.; Hassanian, H.A.S.; Kamran, M.; Kirklin, J.; McGIFFIN, D.C.; Holman, W.L.; et al. Incremental value of three-dimensional echocardiography over transesophageal multiplane two-dimensional echocardiography in qualitative and quantitative assessment of cardiac masses and defects. Echocardiography 1995, 12, 619–628. [Google Scholar] [CrossRef]
- Anwar, A.M.; Nosir, Y.F.M.; Ajam, A.; Chamsi-Pasha, H. Central role of real-time three-dimensional echocardiography in the assessment of intracardiac thrombi. Int. J. Cardiovasc. Imaging 2010, 26, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Faza, N.N.; Little, S.H. Role of 3-dimensional transesophageal echocardiography in guiding transcatheter mitral valve replacement. Echocardiography 2020, 37, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by color Doppler 3D echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
| Case #. Age/Sex | Transcatheter MVR/Date/Other Interventions | Associated Comorbidities | Pre Procedure Surgical MVR/Date/Other Interventions |
|---|---|---|---|
| Case 1. 74/F | Boston Lotus, 27 mm/1/6/2016 | CKD, DM, HTN, PAD, CAD, chronic AF | Tissue MVR for severe MR, TVA for severe TR/2011 |
| Case 2. 56/F | Boston Lotus, 27 mm/1/11/2016 | ESRD, chronic hepatitis B with acute exacerbation, CAD, HTN | Tissue MVR for severe MR, 31 mm/2003 |
| Case 3. 55/M | Edwards SAPIEN, 26 mm/3/14/2016 | Pulmonary hypertension, chronic AF | Tissue MVR for severe MR, TVA for severe TR/09/20/2010 |
| Case 4. 67/M | Edwards SAPIEN XT, 29 mm and redo AVR for severe AS/11/14/2016 | CAD, chronic AF | Tissue MVR for severe MR, tissue AVR for severe AR/2009 |
| Case 2. 58/F | Edwards SAPIEN XT, 29 mm/9/21/2018 | Cardiogenic shock, ESRD, HTN, CAD, chronic hepatitis B with acute exacerbation | Tissue MVR for severe MR, 31 mm/2003, transcatheter MVR for severe MS/1/11/2016 |
| Case 5. 61/M | Edwards SAPIEN XT, 29 mm/11/4/2018 | HBV carrier, chronic AF, CAD | Tissue MVR for severe MR/11/13/2009 |
| Case 6. 64/F | Edwards SAPIEN, 26 mm/4/29/2020 | COPD, antithrombin III deficiency, obstructive sleep apnea | Tissue MVR for severe MR, TVA for moderate TR/11/04/1998 |
| Case 7. 73/M | Edwards SAPIEN, 39 mm/5/12/202 | CKD, hepatocellular carcinoma, COPD, CAD, idiopathic pulmonary fibrosis, polyangiitis, chronic AF | Tissue MVR for severe MS, TVA for severe TR/05/18/2009 |
| Case 8. 58/F | Edwards SAPIEN, 26 mm/10/27/2020 | Pulmonary hypertension, chronic AF | MVA with Sorin ring for severe MS, TVA for severe TR/2016 |
| Case 9. 68/F | Edwards SAPIEN, 29 mm/12/29/2020 | DM, ESRD, HTN, CAD, severe pulmonary hypertension, liver cirrhosis | Tissue MVR for severe MR, TVA for severe TR/09/30/2011 |
| Case 10. 78/F | Edwards SAPIEN, 26 mm/5/10/2021 | CAD, chronic AF, adenocarcinoma of lung | Tissue MVR for severe MS/2011 |
| Case 11. 67/F | Edwards SAPIEN, 29 mm/11/7/2016 | Stroke, chronic AF | Tissue MVR for severe MR, TVA for severe TR/03/13/2007 |
| Case 12. 74/F | Edwards SAPIEN XT, 29 mm/7/24/2018 | Right breast cancer, hepatitis C, chronic AF | Tissue MVR for severe MR, TVA for severe TR/08/14/1996 |
| Case #. Date of MV in V | Severe MVR Stenosis/MPG (mmHg) or Severe MVR Regurgitation by 2D Echo | MVA by 3D Echo Planimetry (cm2) | Thrombus Location by 2D Echo and 3D Echo | Largest Thrombus Area by 2D Echo Planimetry(cm2) | Largest Thrombus Area by 3D Echo Planimetry (cm2) | Thrombus Mobility by 2D Echo and 3D Echo | Largest Echolucency in Thrombus by 2D Echo (cm2) | Largest Echolucency in Thrombus by 3D Echo (cm2) | Thrombus Volume by 3D Echo (mL) | Associated Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | LAA | LA Body | Total | LAA | LA BODY | |||||||||
| Case 1. 1/6/2016 | Stenosis/11 | 1.01 | LAA + LA body | 1.5 | 1.1 | 0.4 | 1.68 | 1.13 | 0.55 | No | 0.18 | 0.48 | 1.0 | Mild MR, mild TR, mild AR, mild PR, normal LV/RV function |
| Case 2. 1/11/2016 | Stenosis/19 | 1.09 | LAA + LA body | 2.0 | 1.2 | 0.8 | 2.68 | 1.86 | 0.82 | Yes | 0.07 | 0.53 | 3.7 | Mild MR, moderate TR, normal LV/RV function |
| Case 3. 3/14/2016 | Stenosis/12 | 1.07 | LAA + LA body | 2.4 | 2.1 | 0.3 | 2.85 | 2.24 | 0.61 | Yes | 0.12 | 0.47 | 2.0 | Mild MR, mild TR, mild AR, normal LV/RV function |
| Case 4. 11/14/2016 | Stenosis/12 | 0.84 | LAA + LA body | 4.7 | 3.0 | 1.7 | 6.38 | 4.44 | 1.94 | No | 0.33 | 0.60 | 6.6 | Mild MR, mild TR, mild AS with peak/mean gradient 37/17.5 mmHg, normal LV/RV function |
| Case 2. 9/21/2018 | Stenosis/17 | 0.68 | LAA | 0.85 | 0.85 | 0.0 | 1.39 | 1.39 | 0.0 | No | 0.16 | 0.34 | 3.8 | Mild MR, mild to moderate TR, normal LV function, severely reduced RV function |
| Case 5. 11/4/2018 | Stenosis/24 | 1.04 | LAA + LA body | 1.3 | 1.2 | 0.1 | 2.02 | 1.53 | 0.49 | No | 0.02 | 0.19 | 2.6 | Mild MR, mild TR, trivial AR, normal LV/RV function |
| Case 6. 4/29/2020 | Stenosis/17 | 0.86 | LAA + LA body | 2.0 | 1.9 | 0.1 | 4.05 | 3.43 | 0.66 | No | 0.0 | 0.30 | 2.8 | Mild MR, mild TR, trivial AR, normal LV/RV function |
| Case 7. 5/12/2020 | Stenosis/10 | 0.7 | LA body | 2.4 | 0.0 | 0.0 | 5.3 | 0.0 | 0.0 | No | 0.43 | 1.27 | 8.7 | Mild TR, normal LV/RV function |
| Case 8. 10/27/2020 | Stenosis/14 | 0.56 | LA body | 4.1 | 0.0 | 0.0 | 4.22 | 0.0 | 0.0 | No | 0.04 | 0.82 | 6.0 | Mild MR, mild TR, normal LV/RV function |
| Case 9. 12/29/2020 | Stenosis/15 | 0.76 | LAA + LA body | 6.3 | 5.9 | 0.4 | 7.77 | 6.92 | 0.85 | No | 1.2 | 2.46 | 14.1 | Mild MR, mild TR, normal LV function, severely reduced RV function |
| Case 10. 5/10/2021 | Stenosis/13 | 0.72 | LAA + LA body | 10.5 | 3.9 | 6.6 | 17.74 | 6.12 | 11.6 | Yes | 0.37 | 2.33 | 26 | Mild MR, moderate TR, mild AR, mild PR, normal LV/RV function |
| Case 11. 11/7/2016 | Regurgitation | * | LAA | 0.96 | 0.96 | 0.0 | 1.7 | 1.7 | 0.0 | No | 0.12 | 0.38 | 1.3 | Trivial MR, mild TR, normal LV/RV function |
| Case 12. 7/24/2018 | Regurgitation | * | LAA | 1.1 | 1.1 | 0.0 | 2.55 | 2.55 | 0.0 | No | 0.13 | 0.23 | 1.1 | Mild TR, normal LV/RV function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmarzouky, Z.M.; Hsiung, M.-C.; Darwish, A.; Dulal, S.; Maturi, B.; Yin, W.-H.; Lee, Y.-T.; Tsao, T.-P.; Wei, J.; Nanda, N.C. Utilization of Two- and Three-Dimensional Transesophageal Echocardiography in Successfully Guiding Transcatheter Mitral Valve in Bioprosthetic Mitral Valve/Mitral Ring Implantation without Complications in Patients with Thrombus in Left Atrium/Left Atrial Appendage. J. Clin. Med. 2022, 11, 7084. https://doi.org/10.3390/jcm11237084
Elmarzouky ZM, Hsiung M-C, Darwish A, Dulal S, Maturi B, Yin W-H, Lee Y-T, Tsao T-P, Wei J, Nanda NC. Utilization of Two- and Three-Dimensional Transesophageal Echocardiography in Successfully Guiding Transcatheter Mitral Valve in Bioprosthetic Mitral Valve/Mitral Ring Implantation without Complications in Patients with Thrombus in Left Atrium/Left Atrial Appendage. Journal of Clinical Medicine. 2022; 11(23):7084. https://doi.org/10.3390/jcm11237084
Chicago/Turabian StyleElmarzouky, Zeyad M., Ming-Chon Hsiung, Amr Darwish, Subash Dulal, Bhanu Maturi, Wei-Hsian Yin, Yung-Tsai Lee, Tien-Ping Tsao, Jeng Wei, and Navin C. Nanda. 2022. "Utilization of Two- and Three-Dimensional Transesophageal Echocardiography in Successfully Guiding Transcatheter Mitral Valve in Bioprosthetic Mitral Valve/Mitral Ring Implantation without Complications in Patients with Thrombus in Left Atrium/Left Atrial Appendage" Journal of Clinical Medicine 11, no. 23: 7084. https://doi.org/10.3390/jcm11237084
APA StyleElmarzouky, Z. M., Hsiung, M.-C., Darwish, A., Dulal, S., Maturi, B., Yin, W.-H., Lee, Y.-T., Tsao, T.-P., Wei, J., & Nanda, N. C. (2022). Utilization of Two- and Three-Dimensional Transesophageal Echocardiography in Successfully Guiding Transcatheter Mitral Valve in Bioprosthetic Mitral Valve/Mitral Ring Implantation without Complications in Patients with Thrombus in Left Atrium/Left Atrial Appendage. Journal of Clinical Medicine, 11(23), 7084. https://doi.org/10.3390/jcm11237084





