Association between Parkinson’s Disease Medication and the Risk of Lower Urinary Tract Infection (LUTI): A Retrospective Cohort Study
Abstract
:1. Introduction
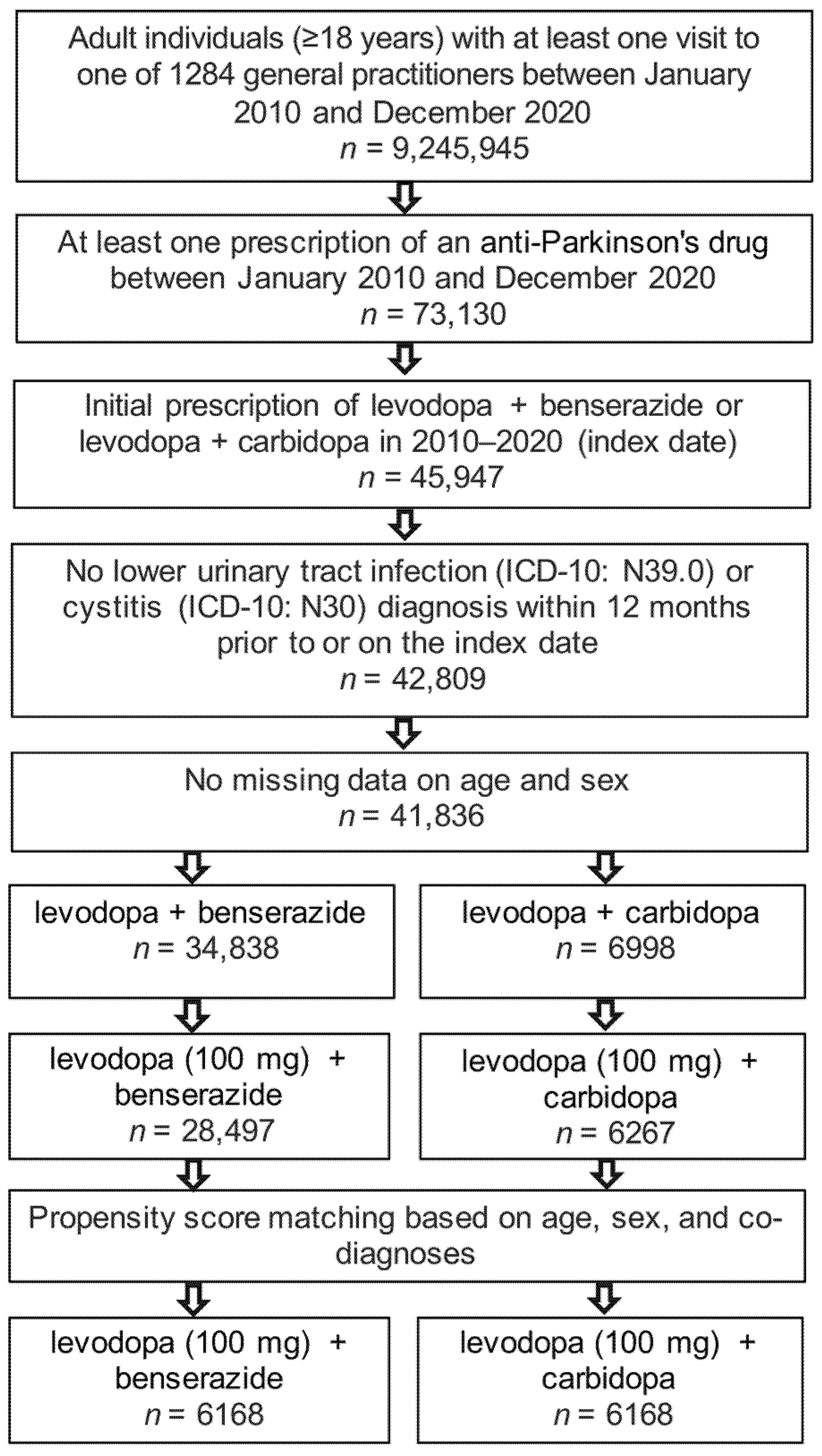
2. Materials and Methods
2.1. Database
2.2. Study Population
2.3. Study Outcomes
2.4. Statistical Analyses
3. Results
3.1. Basic Characteristics of the Study Sample
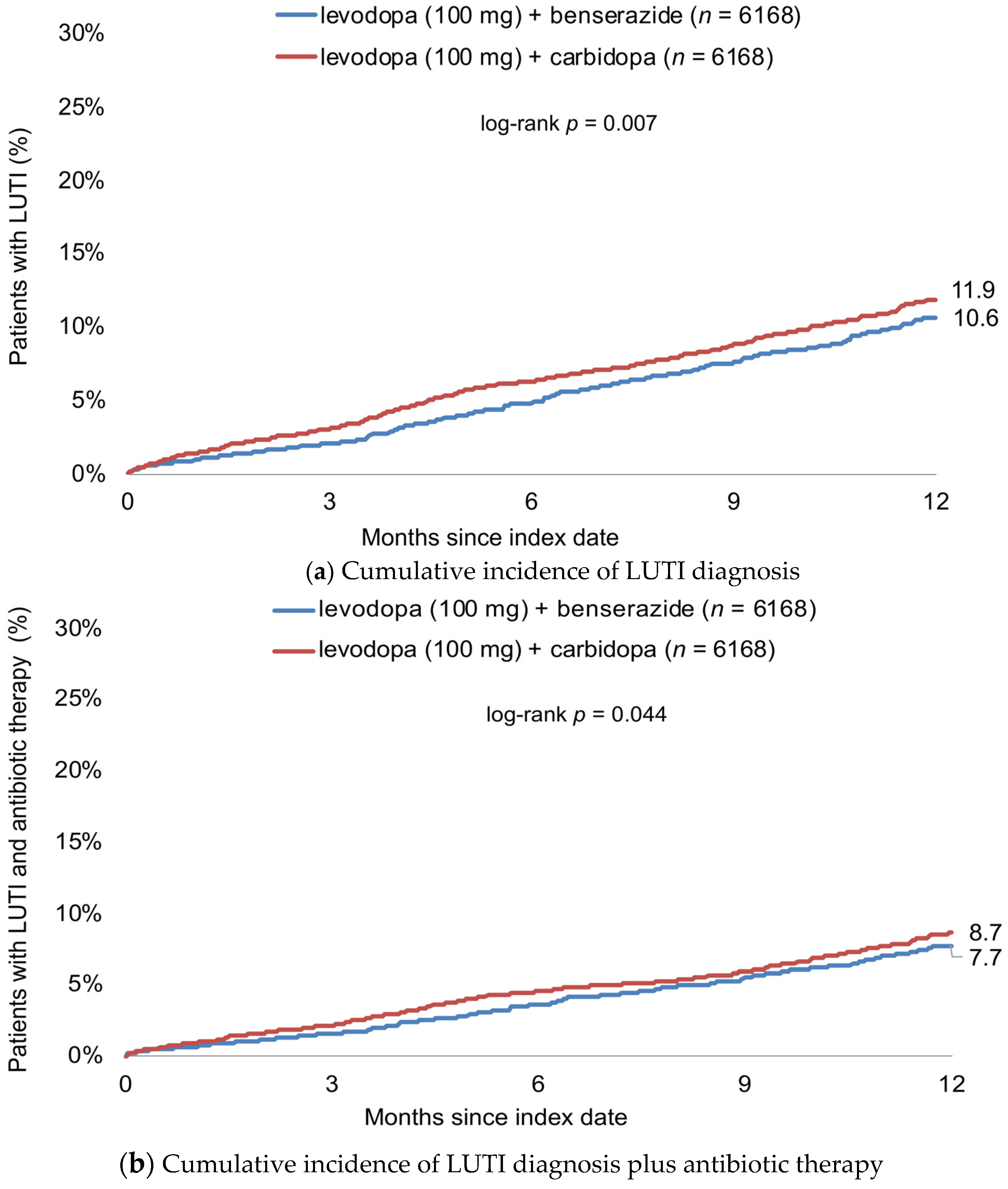
3.2. Cumulative Incidence of LUTI Diagnosis
3.3. Association between Levodopa-Based Therapy and Incidence of LUTI
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Austrilia, 2018. [Google Scholar]
- Group, P.D.M.C.; Gray, R.; Ives, N.; Rick, C.; Patel, S.; Gray, A.; Jenkinson, C.; McIntosh, E.; Wheatley, K.; Williams, A.; et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): A large, open-label, pragmatic randomised trial. Lancet 2014, 384, 1196–1205. [Google Scholar] [CrossRef]
- Manconi, M.; Garcia-Borreguero, D.; Schormair, B.; Videnovic, A.; Berger, K.; Ferri, R.; Dauvilliers, Y. Restless legs syndrome. Nat. Rev. Dis. Prim. 2021, 7, 80. [Google Scholar] [CrossRef]
- Greenacre, J.K.; Coxon, A.; Petrie, A.; Reid, J.L. Comparison of levodopa with carbidopa or benserazide in parkinsonism. Lancet 1976, 2, 381–384. [Google Scholar] [CrossRef]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef]
- Hustad, E.; Aasly, J.O. Clinical and Imaging Markers of Prodromal Parkinson’s Disease. Front. Neurol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Winge, K.; Fowler, C.J. Bladder dysfunction in Parkinsonism: Mechanisms, prevalence, symptoms, and management. Mov. Disord. 2006, 21, 737–745. [Google Scholar] [CrossRef]
- Wang, J.; Cao, R.; Huang, T.; Liu, C.; Fan, Y. Urinary Dysfunction Is Associated with Nigrostriatal Dopaminergic Degeneration in Early and Untreated Patients with Parkinson’s Disease. Park. Dis. 2020, 2020, 4981647. [Google Scholar] [CrossRef] [PubMed]
- Hogg, E.; Frank, S.; Oft, J.; Benway, B.; Rashid, M.H.; Lahiri, S. Urinary Tract Infection in Parkinson’s Disease. J. Park. Dis. 2022, 12, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Pederzoli, M.; Antonini, A.; Beretta, F.; Crespi, V. Reasons for hospitalization in Parkinson’s disease: A case-control study. Park. Relat. Disord. 2014, 20, 488–492. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.E.; Ryu, D.W.; Oh, Y.S.; Lee, K.S.; Hong, S.H.; Kim, J.S. Urinary Dysfunctions and Post-Void Residual Urine in Typical and Atypical Parkinson Diseases. J. Park. Dis. 2018, 8, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, ahe.13.38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.F.; Cui, Y.S.; Yan, R.; Cao, S.S.; Feng, T. Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 977572. [Google Scholar] [CrossRef]
- Sakakibara, R.; Tateno, F.; Nagao, T.; Yamamoto, T.; Uchiyama, T.; Yamanishi, T.; Yano, M.; Kishi, M.; Tsuyusaki, Y.; Aiba, Y. Bladder function of patients with Parkinson’s disease. Int. J. Urol. 2014, 21, 638–646. [Google Scholar] [CrossRef]
- Gremke, N.; Printz, M.; Moller, L.; Ehrenberg, C.; Kostev, K.; Kalder, M. Association between anti-seizure medication and the risk of lower urinary tract infection in patients with epilepsy. Epilepsy Behav. 2022, 135, 108910. [Google Scholar] [CrossRef]
- Gremke, N.; Kostev, K.; Kalder, M. Association between antihypertensive medication and the risk of urinary tract infection (UTI) of outpatients: A retrospective cohort study. Infection 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Su, C.M.; Kung, C.T.; Chen, F.C.; Cheng, H.H.; Hsiao, S.Y.; Lai, Y.R.; Huang, C.C.; Tsai, N.W.; Lu, C.H. Manifestations and Outcomes of Patients with Parkinson’s Disease and Serious Infection in the Emergency Department. Biomed. Res. Int. 2018, 2018, 6014896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peach, B.C.; Garvan, G.J.; Garvan, C.S.; Cimiotti, J.P. Risk Factors for Urosepsis in Older Adults: A Systematic Review. Gerontol. Geriatr. Med. 2016, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hufschmidt, A.; Shabarin, V.; Rauer, S.; Zimmer, T. Neurological symptoms accompanying urinary tract infections. Eur. Neurol. 2010, 63, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.; Braconier, J.; Andreasson, A.; Svanborg, C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 1999, 179, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Sparrow, N.A.; Anwar, F.; Guidry, G.; Covarrubias, A.E.; Pang, H.; Bogguri, C.; Karumanchi, S.A.; Lahiri, S. Interleukin-6 mediates delirium-like phenotypes in a murine model of urinary tract infection. J. Neuroinflamm. 2021, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nguyen, L.T.; Burlak, C.; Chegini, F.; Guo, F.; Chataway, T.; Ju, S.; Fisher, O.S.; Miller, D.W.; Datta, D.; et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein alpha-synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 9587–9592. [Google Scholar] [CrossRef] [Green Version]
- Cocoros, N.M.; Svensson, E.; Szepligeti, S.K.; Vestergaard, S.V.; Szentkuti, P.; Thomsen, R.W.; Borghammer, P.; Sorensen, H.T.; Henderson, V.W. Long-term Risk of Parkinson Disease Following Influenza and Other Infections. JAMA Neurol. 2021, 78, 1461–1470. [Google Scholar] [CrossRef]
- Admani, A.K.; Verma, S.; Cordingley, G.J.; Harris, R.I. Patient benefits of l-dopa and a decarboxylase inhibitor in the treatment of Parkinson’s disease in elderly patients. Pharmatherapeutica 1985, 4, 132–140. [Google Scholar]
- Iwaki, H.; Nishikawa, N.; Nagai, M.; Tsujii, T.; Yabe, H.; Kubo, M.; Ieiri, I.; Nomoto, M. Pharmacokinetics of levodopa/benserazide versus levodopa/carbidopa in healthy subjects and patients with Parkinson’s disease. Neurol. Clin. Neurosci. 2015, 3, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Lemos, H.; Bhatt, B.; Islam, B.N.; Singh, A.; Gurav, A.; Huang, L.; Browning, D.D.; Mellor, A.; Fulzele, S.; et al. Carbidopa, a drug in use for management of Parkinson disease inhibits T cell activation and autoimmunity. PLoS ONE 2017, 12, e0183484. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.; Albo, M.; Brubaker, L. Recurrent Urinary Tract Infections in Adult Women. JAMA 2020, 323, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Erdal, Y.; Akdogan, O.; Nalbantoglu, M.; Kavasoglu, G.; Emre, U. Autonomic dysfunction in restless legs syndrome. Sleep Breath 2020, 24, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; Van Hilten, J.J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord. 2004, 19, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Nagayama, H.; Ota, T.; Nishiyama, Y.; Mishina, M.; Ueda, M. Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin. Neuropharmacol. 2014, 37, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, P.; Contin, M.; Scaglione, C.; Riva, R.; Albani, F.; Baruzzi, A. Levodopa pharmacokinetics anddyskinesias: Are there sex-related differences? Neurol. Sci. 2003, 24, 192–193. [Google Scholar] [CrossRef]
- Russillo, M.C.; Andreozzi, V.; Erro, R.; Picillo, M.; Amboni, M.; Cuoco, S.; Barone, P.; Pellecchia, M.T. Sex Differences in Parkinson’s Disease: From Bench to Bedside. Brain Sci. 2022, 12, 917. [Google Scholar] [CrossRef]
- Conti, V.; Izzo, V.; Russillo, M.C.; Picillo, M.; Amboni, M.; Scaglione, C.L.M.; Nicoletti, A.; Cani, I.; Cicero, C.E.; De Bellis, E.; et al. Gender Differences in Levodopa Pharmacokinetics in Levodopa-Naive Patients with Parkinson’s Disease. Front. Med. 2022, 9, 909936. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef]
- Becher, H.; Kostev, K.; Schröder-Bernhardi, D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int. J. Clin. Pharmacol. Ther. 2009, 47, 617–626. [Google Scholar] [CrossRef]
| Variable | Proportion among Patients Treated with Levodopa (100 mg) + Benserazide (%) | Proportion among Patients Treated with Levodopa (100 mg) + Carbidopa (%) | p-Value |
|---|---|---|---|
| n | 6168 | 6168 | |
| Age (Mean, SD) | 76.1 (11.4) | 76.1 (11.4) | 0.925 |
| Age ≤ 70 | 23.7 | 23.9 | 0.982 |
| Age 71–80 | 34.7 | 35.0 | |
| Age > 80 | 41.6 | 41.2 | |
| Female | 54.4 | 54.2 | 0.828 |
| Male | 45.6 | 45.8 | |
| Diabetes | 28.2 | 28.2 | 0.968 |
| Parkinson’s disease | 52.6 | 52.6 | 0.971 |
| RLS | 23.4 | 23.3 | 0.932 |
| Benign prostate hyperplasia | 11.5 | 11.6 | 0.822 |
| Urinary incontinency | 8.1 | 8.4 | 0.577 |
| Cohort | LUTI | LUTI + Antibiotic Therapy | ||
|---|---|---|---|---|
| HR (95% CI) for Levodopa (100 mg) + Benserazide Compared to Levodopa (100 MG) + Carbidopa | p-Value | HR (95% CI) for Levodopa (100 mg) + Benserazide Compared to Levodopa (100 mg) + Carbidopa | p-Value | |
| Total | 0.82 (0.71–0.95) | 0.007 | 0.84 (0.71–1.00) | 0.045 |
| Age ≤ 70 | 0.82 (0.57–1.16) | 0.261 | 0.92 (0.60–1.40) | 0.690 |
| Age 71–80 | 0.70 (0.54–0.90) | 0.006 | 0.79 (0.59–1.06) | 0.118 |
| Age > 80 | 0.90 (0.74–1.10) | 0.287 | 0.84 (0.67–1.07) | 0.156 |
| Women | 0.77 (0.65–0.92) | 0.004 | 0.76 (0.62–0.93) | 0.009 |
| Men | 0.93 (0.73–1.18) | 0.531 | 1.04 (0.78–1.40) | 0.792 |
| Parkinson’s disease | 0.82 (0.67–1.01) | 0.063 | 0.85 (0.67–1.08) | 0.179 |
| RLS | 0.65 (0.47–0.90) | 0.008 | 0.64 (0.44–0.93) | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gremke, N.; Griewing, S.; Printz, M.; Kostev, K.; Wagner, U.; Kalder, M. Association between Parkinson’s Disease Medication and the Risk of Lower Urinary Tract Infection (LUTI): A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7077. https://doi.org/10.3390/jcm11237077
Gremke N, Griewing S, Printz M, Kostev K, Wagner U, Kalder M. Association between Parkinson’s Disease Medication and the Risk of Lower Urinary Tract Infection (LUTI): A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(23):7077. https://doi.org/10.3390/jcm11237077
Chicago/Turabian StyleGremke, Niklas, Sebastian Griewing, Marcel Printz, Karel Kostev, Uwe Wagner, and Matthias Kalder. 2022. "Association between Parkinson’s Disease Medication and the Risk of Lower Urinary Tract Infection (LUTI): A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 23: 7077. https://doi.org/10.3390/jcm11237077
APA StyleGremke, N., Griewing, S., Printz, M., Kostev, K., Wagner, U., & Kalder, M. (2022). Association between Parkinson’s Disease Medication and the Risk of Lower Urinary Tract Infection (LUTI): A Retrospective Cohort Study. Journal of Clinical Medicine, 11(23), 7077. https://doi.org/10.3390/jcm11237077






