Combined Airway and Bariatric Surgery (CABS) for Obstructive Sleep Apnea Patients with Morbid Obesity: A Comprehensive Alternative Preliminary Study
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Population
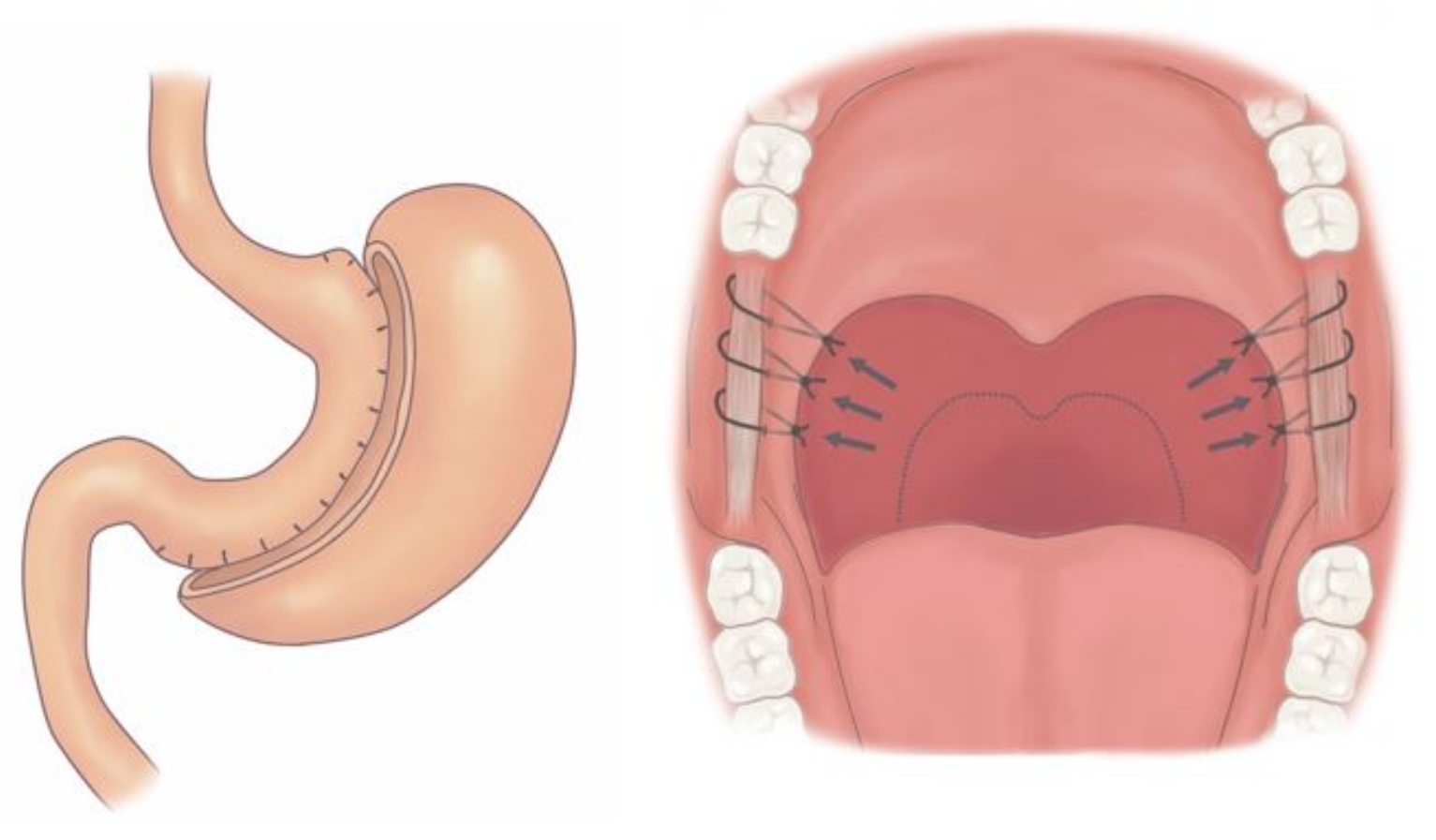
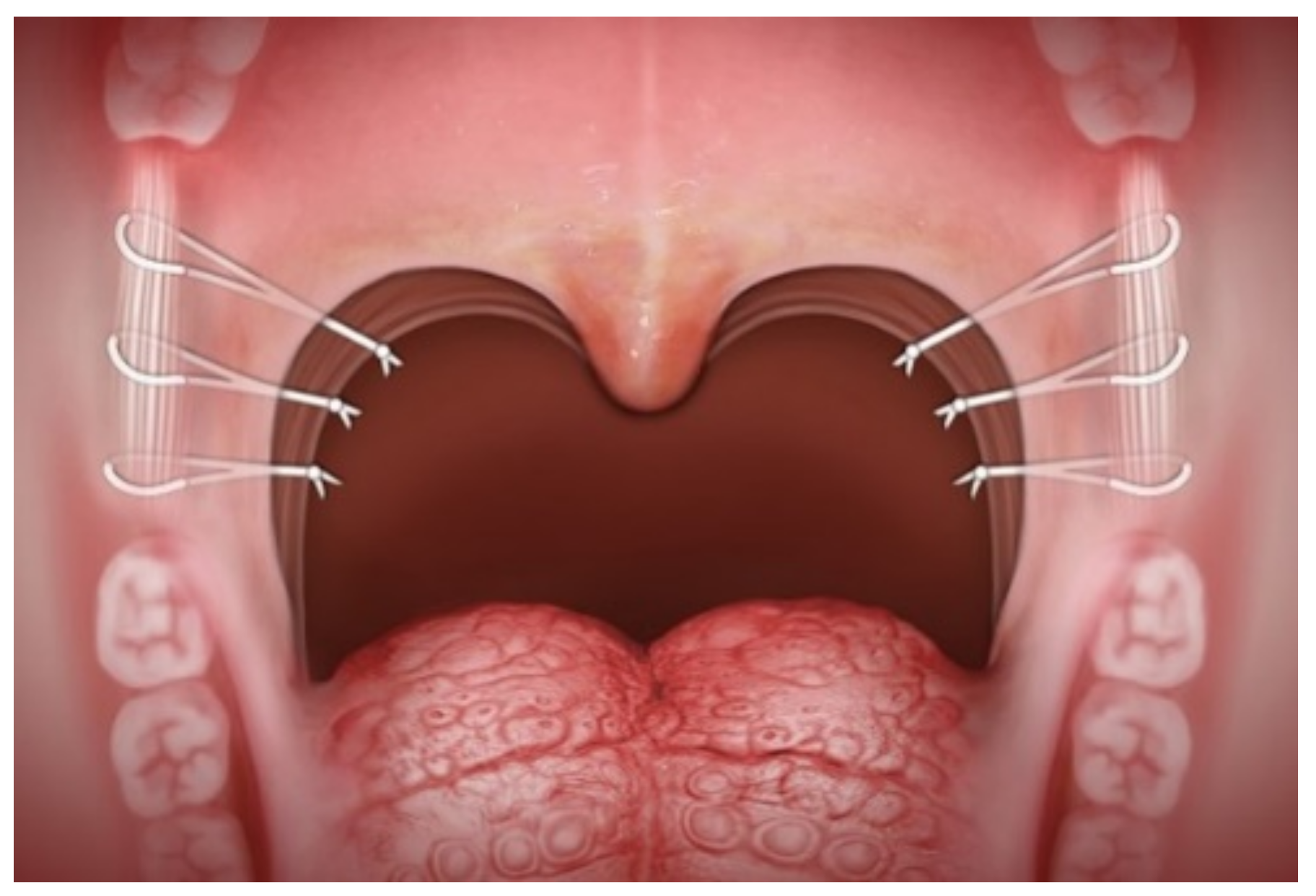
2.3. Surgical Technique
2.4. Home Sleep Apnea Test
2.5. Statistical Analysis
3. Results
Demographics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strollo, P.J.; Roger, R.M. Obstructive sleep apnea. N. Eng. J. Med. 1996, 334, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Lee, L.-A.; Hsin, L.-J.; Fang, T.-J.; Lin, W.-N.; Chen, H.-C.; Lu, Y.-A.; Lee, Y.-C.; Tsai, M.-S.; Tsai, Y.-T. Intrapharyngeal surgery with integrated treatment for obstructive sleep apnea. Biomed. J. 2019, 42, 84–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Young, T.; Peppard, P.E.; Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and sleep disturbance: The chicken or the egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Kreitinger, K.Y.; Lui, M.M.S.; Owens, R.L.; Schmickl, C.N.; Grunvald, E.; Horgan, S.; Raphelson, J.R.; Malhotra, A. Screening for obstructive sleep apnea in a diverse bariatric surgery population. Obesity 2020, 28, 2028–2034. [Google Scholar] [CrossRef]
- Palla, A.; Digiorgio, M.; Carpenè, N.; Rossi, G.; D’Amico, I.; Santini, F.; Pinchera, A. Sleep apnea in morbidly obese patients: Prevalence and clinical predictivity. Respiration 2009, 78, 134–140. [Google Scholar] [CrossRef]
- Drager, L.F.; Brunoni, A.R.; Jenner, R.; Lorenzi-Filho, G.; Benseñor, I.M.; Lotufo, P.A. Effects of CPAP on body weight in patients with obstructive sleep apnea: A meta-analysis of randomized trials. Thorax 2015, 70, 258–264. [Google Scholar] [CrossRef]
- Ming, X.; Yang, M.; Chen, X. Metabolic bariatric surgery as a treatment for obstructive sleep apnea hypopnea syndrome: Review of the literature and potential mechanisms. Surg. Obes. Relat. Dis. 2021, 17, 215–220. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Eliasson, A.H.; Greenburg, D.L. Persistence of obstructive sleep apnea after surgical weight loss. J. Clin. Sleep Med. 2008, 4, 333–338. [Google Scholar] [CrossRef]
- Nastałek, P.; Polok, K.; Celejewska-Wójcik, N.; Kania, A.; Sładek, K.; Małczak, P.; Major, P. Impact of bariatric surgery on obstructive sleep apnea severity and continuous positive airway pressure therapy compliance—Prospective observational study. Sci. Rep. 2021, 11, 5003. [Google Scholar] [CrossRef]
- Yousef, G.T.; Abdalgalil, D.A.; Ibrahim, T.H. Orotracheal intubation of morbidly obese patients, comparison of GlideScope(®) video laryngoscope and the LMA CTrach™ with direct laryngoscopy. Anesth Essays Res. 2012, 6, 174–179. [Google Scholar] [CrossRef]
- Lee, W.J.; Wang, W. Bariatric surgery: Asia-Pacific perspective. Obes. Surg. 2005, 15, 751–757. [Google Scholar] [CrossRef]
- Johns, M.W. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993, 103, 30–36. [Google Scholar] [CrossRef]
- Chen, H.; Lowe, A.A.; Bai, Y.; Hamilton, P.; Fleetham, J.A.; Almeida, F.R. Evaluation of a portable recording device (ApneaLink™) for case selection of obstructive sleep apnea. Sleep Breath 2009, 13, 213–219. [Google Scholar] [CrossRef]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef]
- Moy, J.; Pomp, A.; Dakin, G.; Parikh, M.; Gagner, M. Laparoscopic sleeve gastrectomy for morbid obesity. Am. J. Surg. 2008, 196, e56–e59. [Google Scholar] [CrossRef]
- Schauer, P.R.; Ikramuddin, S.; Gourash, W.; Ramanathan, R.; Luketich, J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann. Surg. 2000, 232, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Lee, L.A.; Kezirian, E.J.; Nakayama, M. Suspension palatoplasty for obstructive sleep apnea- A preliminary study. Sci. Rep. 2018, 8, 4224. [Google Scholar] [CrossRef]
- Lu, Y.A.; Wang, C.J.; Chiang, Y.T.; Li, H.Y. Volumetric changes after coblation ablation tongue (CAT) in obstructive sleep apnea patients. J. Clin. Med. 2022, 11, 4186. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.; Barnes, H.N.; Joosten, S.A.; Landry, S.A.; Dabscheck, E.; Mansfield, D.R.; Dharmage, S.C.; Senaratna, C.V.; Edwards, B.A.; Hamilton, G.S. The effect of surgical weight loss on obstructive sleep apnoea: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 42, 85–99. [Google Scholar] [CrossRef]
- Ravesloot, M.J.; Hilgevoord, A.A.; van Wagensveld, B.A.; de Vries, N. Assessment of the effect of bariatric surgery on obstructive sleep apnea at two postoperative intervals. Obes. Surg. 2014, 24, 22–31. [Google Scholar] [CrossRef]
- Peromaa-Haavisto, P.; Tuomilehto, H.; Kössi, J.; Virtanen, J.; Luostarinen, M.; Pihlajamäki, J.; Käkelä, P.; Victorzon, M. Obstructive sleep apnea: The effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017, 35, 85–90. [Google Scholar] [CrossRef]
- Fritscher, L.G.; Canani, S.; Mottin, C.C.; Fritscher, C.C.; Berleze, D.; Chapman, K.; Chatkin, J.M. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Joosten, S.A.; Edwards, B.; Wellman, A.; Turton, A.; Skuza, E.M.; Berger, P.J.; Hamilton, G. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep 2015, 38, 1469–1478. [Google Scholar] [CrossRef]
- Joosten, S.A.; Khoo, J.K.; Edwards, B.A.; Landry, S.A.; Naughton, M.T.; Dixon, J.B.; Hamilton, G.S. Improvement in obstructive sleep apnea with weight loss is dependent on body position during sleep. Sleep 2017, 40, zsx047. [Google Scholar] [CrossRef]
- Turnbull, C.D.; Wang, S.H.; Manuel, A.R.; Keenan, B.T.; McIntyre, A.G.; Schwab, R.J.; Stradling, J.R. Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath 2018, 22, 673–681. [Google Scholar] [CrossRef]
- Wang, S.H.; Keenan, B.T.; Wiemken, A.; Zang, Y.; Staley, B.; Sarwer, D.B.; Torigian, D.A.; Williams, N.; Pack, A.I.; Schwab, R.J. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am. J. Respir. Crit. Care Med. 2020, 201, 718–727. [Google Scholar] [CrossRef]
- Eckert, D.J.; Jordan, A.S.; Merchia, P.; Malhotra, A. Central sleep apnea: Pathophysiology and treatment. Chest 2007, 131, 595–607. [Google Scholar] [CrossRef]
- Dixon, J.B.; Schachter, L.M.; O’Brien, P.E.; Jones, K.; Grima, M.; Lambert, G.; Brown, W.; Bailey, M.; Naughton, M.T. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: A randomized controlled trial. JAMA 2012, 308, 1142–1149. [Google Scholar] [CrossRef]
- Feigel-Guiller, B.; Drui, D.; Dimet, J.; Zair, Y.; Le Bras, M.; Fuertes-Zamorano, N.; Cariou, B.; Letessier, E.; Nobécourt-Dupuy, E.; Krempf, M. Laparoscopic gastric banding in obese patients with sleep apnea: A 3-year controlled study and follow-up after 10 years. Obes. Surg. 2015, 25, 1886–1892. [Google Scholar] [CrossRef]
- Turnbull, C.D. Intermittent hypoxia, cardiovascular disease and obstructive sleep apnoea. J. Thorac. Dis. 2018, 10 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef]
- Lee, L.A.; Chen, N.H.; Huang, C.G.; Lin, S.W.; Fang, T.J.; Li, H.Y. Patients with severe obstructive sleep apnea syndrome and elevated high-sensitivity C-reactive protein need priority treatment. Otolaryngol. Head Neck Surg. 2010, 143, 72–77. [Google Scholar] [CrossRef]
- Wali, S.O.; Abaalkhail, B.; AlQassas, I.; Alhejaili, F.; Spence, D.W.; Pandi-Perumal, S.R. The correlation between oxygen saturation indices and the standard obstructive sleep apnea severity. Ann. Thorac. Med. 2020, 15, 70–75. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Ostrowski, M.; Moussavi, Z.; Hanly, P.; Younes, M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep 2013, 36, 881–889. [Google Scholar] [CrossRef]
- Guilleminault, C.; Connolly, S.; Winkle, R.; Melvin, K.; Tilkian, A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984, 1, 126–131. [Google Scholar] [CrossRef]
- Kawano, Y.; Tamura, A.; Watanabe, T.; Kadota, J. Influence of the severity of obstructive sleep apnea on heart rate. J. Cardio 2010, 56, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Tkacova, R.; Hall, M.J.; Ando, S.; Floras, J.S. Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin. Sci. 2003, 104, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; Mark, A.L.; Zavala, D.C.; Abboud, F.M. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J. Appl. Physiol. 1989, 67, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part II: Central sleep apnea. Circulation 2003, 107, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Bourassa, M.G.; Guertin, M.C.; Tardif, J.C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur. Heart J. 2005, 26, 967–974. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, X.; Qi, X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ 2016, 188, e53–e63. [Google Scholar] [CrossRef]
- Sumi, K.; Chin, K.; Takahashi, K.; Nakamura, T.; Matsumoto, H.; Niimi, A.; Mishima, M. Effect of nCPAP therapy on heart rate in patients with obstructive sleep apnoea-hypopnoea. QJM 2006, 99, 545–553. [Google Scholar] [CrossRef]
- Dalmar, A.; Singh, M.; Pandey, B.; Stoming, C.; Heis, Z.; Ammar, K.A.; Jan, M.F.; Choudhuri, I.; Chua, T.Y.; Sra, J.; et al. The beneficial effect of weight reduction on adverse cardiovascular outcomes following bariatric surgery is attenuated in patients with obstructive sleep apnea. Sleep 2018, 41, zsy028. [Google Scholar] [CrossRef]
- Chen, C.K.; Shen, S.C.; Lee, L.A.; Sun, M.H.; Chen, N.H.; Chuang, L.P.; Li, H.Y. Idiopathic sudden sensorineural hearing loss in patients with obstructive sleep apnea. Nat. Sci. Sleep 2021, 13, 1877–1885. [Google Scholar] [CrossRef]
- Lee, G.S.; Lee, L.A.; Wang, C.Y.; Chen, N.H.; Fang, T.J.; Huang, C.G.; Cheng, W.N.; Li, H.Y. The frequency and energy of snoring sounds are associated with common carotid artery intima-media thickness in obstructive sleep apnea patients. Sci. Rep. 2016, 29, 30559. [Google Scholar] [CrossRef]
- Lu, C.T.; Lee, L.A.; Lee, G.S.; Li, H.Y. Obstructive sleep apnea and auditory dysfunction-Does snoring sound play a role? Diagnostics 2022, 12, 2374. [Google Scholar] [CrossRef]



| Variable | CABS (n = 10) | BS (n = 10) | p Value |
|---|---|---|---|
| Age (year) | 44 (34–47) | 36.5 (29–46.3) | 0.28 |
| Gender (Male/female) | 7/3 | 6/4 | 1 |
| BMI (Kg/m2) | 40.4 (34.1–42.4) | 41.3 (37.9–46.7) | 0.25 |
| AHI (event/hour) | 75.1 (30.9–105.1) | 44.7 (34.7–56.4) | 0.19 |
| Bariatric surgery (LSG/LRYGB) | 8/2 | 8/2 | 1 |
| Variables | Preoperative Median (Interquartile) | Postoperative Median (Interquartile) | p Value |
|---|---|---|---|
| Snore (VAS) | 10 (9–10) | 1 (0–2) | <0.0001 |
| ESS | 18 (13–20) | 2 (0–6) | 0.004 |
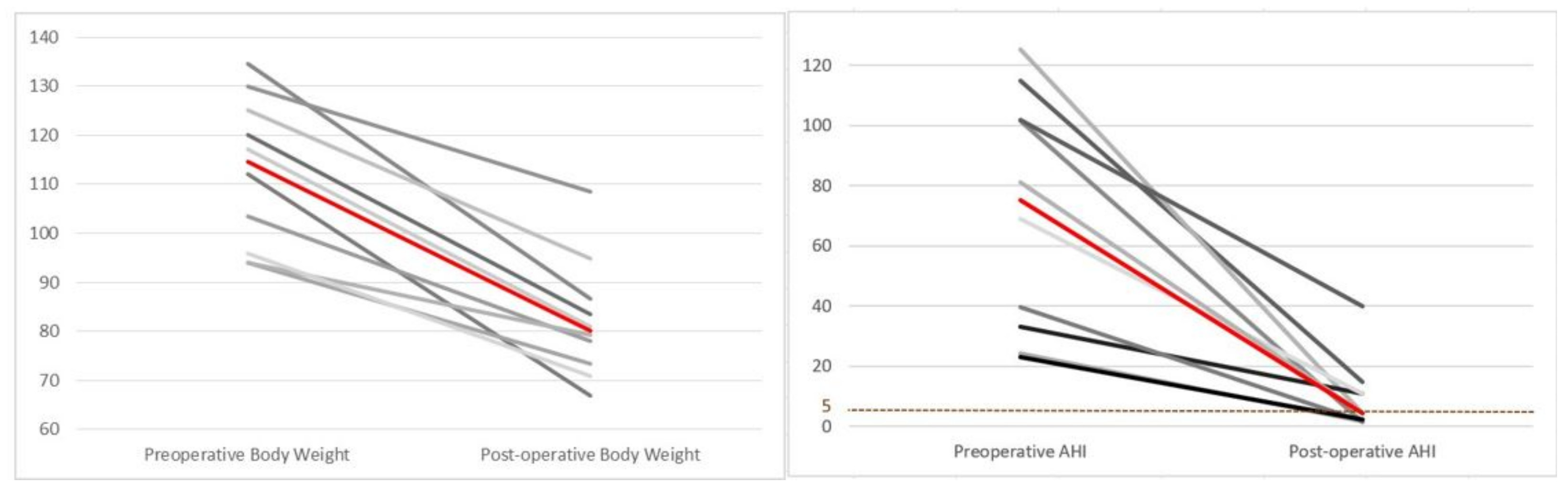
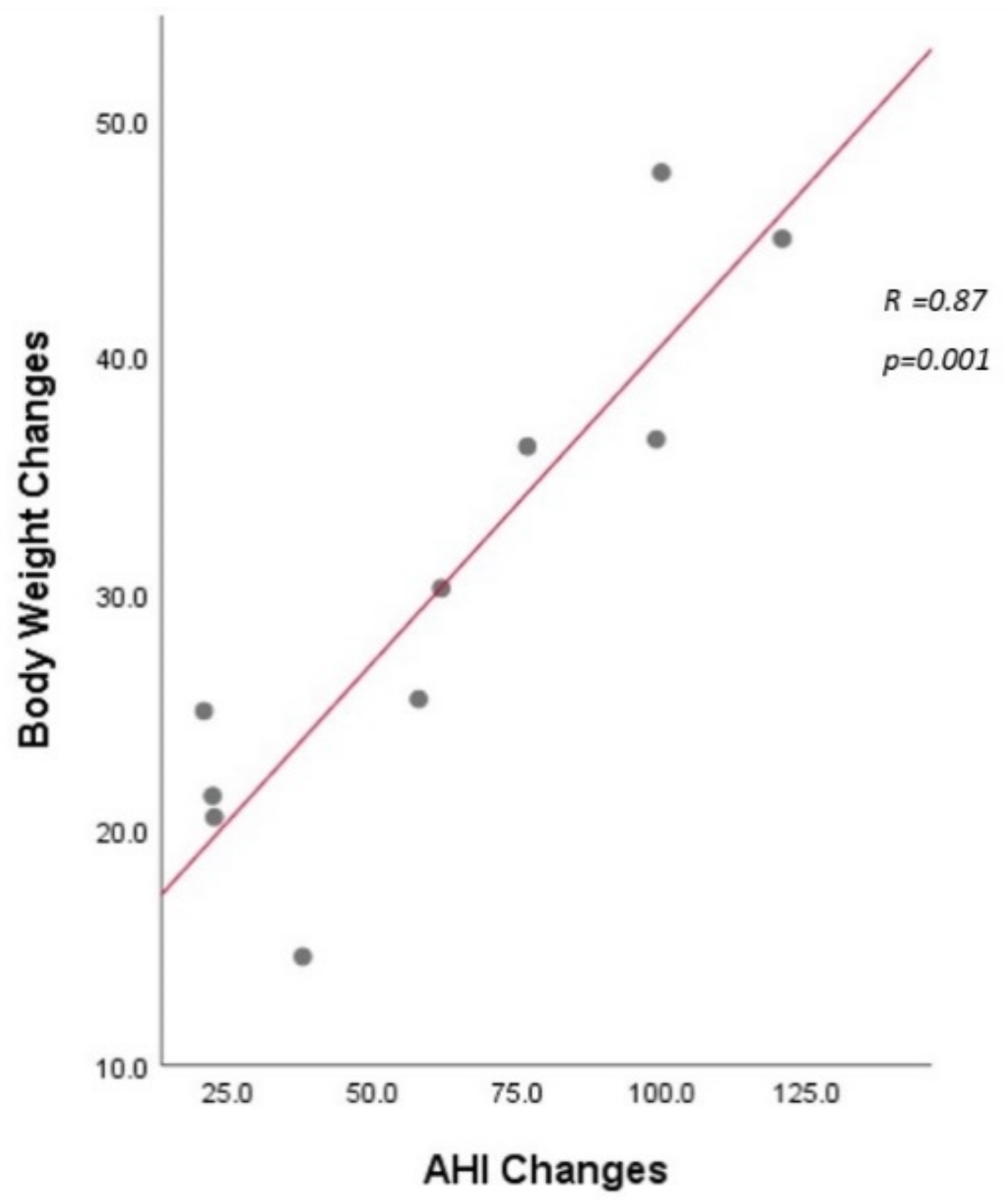
| AHI (event/hour) | 75.1 (30.9–105.1) | 4.5 (1.8–13.5) | 0.0004 |
| Supine AHI | 58.5 (24.1–104.9) | 6.3 (2.2–33.3) | 0.003 |
| Non-supine AHI | 17.9 (0–97.7) | 2.6 (1.4–6.3) | 0.02 |
| AI (event/hour) | 52.8 (3.8–84.1) | 1.1 (0.2–3.4) | 0.009 |
| Mean SAT (%) | 87.0 (78.3–94.0) | 94.0 (93.8–95.3) | 0.008 |
| Mini SAT (%) | 58.5 (40.8–77.3) | 87 (75.5–90.0) | 0.001 |
| ODI (event/hour) | 66.4 (31.2–99.2) | 5 (2.3–12.9) | 0.0003 |
| <90% SAT (%) | 30 (4.5–75.5) | 1 (0–9.3) | 0.006 |
| SI (event/hour) | 479.1 (355.5–592) | 20 (6.9–168.7) | 0.0001 |
| HR (beat/minute) | 77 (72–86) | 62 (55–66) | <0.0001 |
| Max HR (beat/min) | 114.0 (110–181) | 94.3 (88.3–100) | 0.0005 |
| Body weight (kg) | 114.6 (95.5–127.5) | 80.2 (72.9–90.8) | <0.0001 |
| BMI (kg/m2) | 40.4 (34.1–42.4) | 28.4 (25.6–30.1) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-C.; Liu, K.-H.; Lee, L.-A.; Chuang, L.-P.; Lin, Y.-S.; Hsin, L.-J.; Lin, W.-N.; Chiang, Y.-T.; Cheng, W.-N.; Li, H.-Y. Combined Airway and Bariatric Surgery (CABS) for Obstructive Sleep Apnea Patients with Morbid Obesity: A Comprehensive Alternative Preliminary Study. J. Clin. Med. 2022, 11, 7078. https://doi.org/10.3390/jcm11237078
Lin C-C, Liu K-H, Lee L-A, Chuang L-P, Lin Y-S, Hsin L-J, Lin W-N, Chiang Y-T, Cheng W-N, Li H-Y. Combined Airway and Bariatric Surgery (CABS) for Obstructive Sleep Apnea Patients with Morbid Obesity: A Comprehensive Alternative Preliminary Study. Journal of Clinical Medicine. 2022; 11(23):7078. https://doi.org/10.3390/jcm11237078
Chicago/Turabian StyleLin, Chia-Chen, Keng-Hao Liu, Li-Ang Lee, Li-Pang Chuang, Yu-Sheng Lin, Li-Jen Hsin, Wan-Ni Lin, Yen-Ting Chiang, Wen-Nuan Cheng, and Hsueh-Yu Li. 2022. "Combined Airway and Bariatric Surgery (CABS) for Obstructive Sleep Apnea Patients with Morbid Obesity: A Comprehensive Alternative Preliminary Study" Journal of Clinical Medicine 11, no. 23: 7078. https://doi.org/10.3390/jcm11237078
APA StyleLin, C.-C., Liu, K.-H., Lee, L.-A., Chuang, L.-P., Lin, Y.-S., Hsin, L.-J., Lin, W.-N., Chiang, Y.-T., Cheng, W.-N., & Li, H.-Y. (2022). Combined Airway and Bariatric Surgery (CABS) for Obstructive Sleep Apnea Patients with Morbid Obesity: A Comprehensive Alternative Preliminary Study. Journal of Clinical Medicine, 11(23), 7078. https://doi.org/10.3390/jcm11237078







