Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Statistics
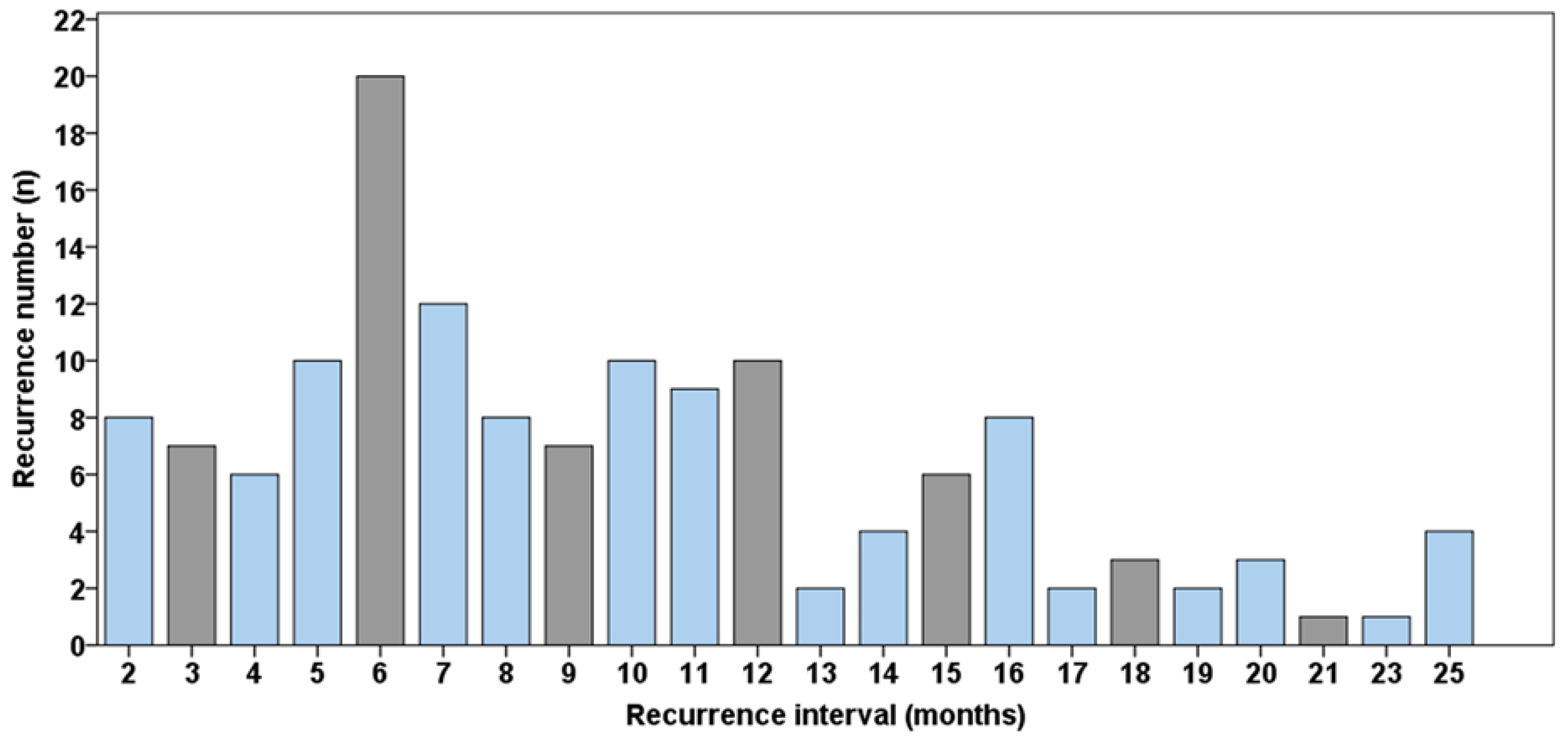
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Boysen, M.; Lövdal, O.; Winther, F.; Tausjö, J. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur. J. Cancer 1992, 28, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Aoki, T.; Karakida, K.; Otsuru, M.; Takahashi, M.; Akamatsu, T.; Sakamoto, H.; Ota, Y. Postoperative follow-up strategy in patients with oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 2011, 69, e105–e111. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Lefebvre, J.-L.; Licitra, L.; Felip, E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v184–v186. [Google Scholar] [CrossRef]
- Pfister, D.G.; Ang, K.; Brockstein, B.; Colevas, A.D.; Ellenhorn, J.; Goepfert, H.; Hicks, W.L.; Hong, W.K.; Kies, M.S.; Lydiatt, W.; et al. NCCN Practice Guidelines for Head and Neck Cancers. Oncology 2000, 14, 163–194. [Google Scholar]
- Zatterstrom, U.; Boysen, M.; Evensen, J.F. Significance of self-reported symptoms as part of follow-up routines in patients treated for oral squamous cell carcinoma. Anticancer Res. 2014, 34, 6593–6599. [Google Scholar]
- Brands, M.T.; Brennan, P.A.; Verbeek, A.L.M.; Merkx, M.A.W.; Geurts, S.M.E. Follow-up after curative treatment for oral squamous cell carcinoma. A critical appraisal of the guidelines and a review of the literature. Eur. J. Surg. Oncol. 2018, 44, 559–565. [Google Scholar] [CrossRef]
- Wolff, K.D.; Follmann, M.; Nast, A. The diagnosis and treatment of oral cavity cancer. Dtsch. Arztebl. Int. 2012, 109, 829–835. [Google Scholar] [CrossRef]
- Blatt, S.; Krüger, M.; Ziebart, T.; Sagheb, K.; Schiegnitz, E.; Goetze, E.; Al-Nawas, B.; Pabst, A.M. Biomarkers in diagnosis and therapy of oral squamous cell carcinoma: A review of the literature. J. Craniomaxillofac. Surg. 2017, 45, 722–730. [Google Scholar] [CrossRef]
- Backes, C.; Bier, H.; Knopf, A. Therapeutic implications of tumor free margins in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 84320–84328. [Google Scholar] [CrossRef]
- Dik, E.A.; Ipenburg, N.A.; Kessler, P.A.; van Es, R.J.J.; Willems, S.M. The value of histological grading of biopsy and resection specimens in early stage oral squamous cell carcinomas. J. Craniomaxillofac. Surg. 2018, 46, 1001–1006. [Google Scholar] [CrossRef]
- Anneroth, G.; Batsakis, J.; Luna, M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand. J. Dent. Res. 1987, 95, 229–249. [Google Scholar] [CrossRef]
- Gleber-Netto, F.O.; Braakhuis, B.J.; Triantafyllou, A.; Takes, R.P.; Kelner, N.; Rodrigo, J.P.; Strojan, P.; Poorten, V.V.; Rapidis, A.D.; Rinaldo, A.; et al. Molecular events in relapsed oral squamous cell carcinoma: Recurrence vs. secondary primary tumor. Oral. Oncol. 2015, 51, 738–744. [Google Scholar] [CrossRef]
- Loeffelbein, D.; Eiber, M.; Mayr, P.; Souvatzoglou, M.; Mücke, T.; von Bomhard, A.; Kesting, M.; Wolff, K.-D. Loco-regional recurrence after surgical treatment of oral squamous cell carcinoma: Proposals for follow-up imaging based on literature, national guidelines and institutional experience. J. Craniomaxillofac. Surg. 2015, 43, 1546–1552. [Google Scholar] [CrossRef]
- Kerawala, C.J.; Newlands, C.; Coombes, D. Follow-up after treatment of squamous cell carcinoma of the oral cavity: Current maxillofacial practice in the United Kingdom. Br. J. Oral Maxillofac. Surg. 2007, 45, 361–363. [Google Scholar] [CrossRef]
- Kissun, D.; Magennis, P.; Lowe, D.; Brown, J.; Vaughan, E.; Rogers, S.N. Timing and presentation of recurrent oral and oropharyngeal squamous cell carcinoma and awareness in the outpatient clinic. Br. J. Oral Maxillofac. Surg. 2006, 44, 371–376. [Google Scholar] [CrossRef]
- Wensing, B.M.; Merkx, M.A.W.; Krabbe, P.F.M.; Marres, H.A.M.; Hoogen, F.J.A.V.D. Oral squamous cell carcinoma and a clinically negative neck: The value of follow-up. Head Neck. 2011, 33, 1400–1405. [Google Scholar] [CrossRef]
- Collan, J.; Lundberg, M.; Vaalavirta, L.; Bäck, L.; Kajanti, M.; Mäkitie, A.; Tenhunen, M.; Saarilahti, K. Patterns of relapse following surgery and postoperative intensity modulated radiotherapy for oral and oropharyngeal cancer. Acta. Oncol. 2011, 50, 1119–1125. [Google Scholar] [CrossRef]
- Liu, G.; Dierks, E.J.; Bell, R.B.; Bui, T.G.; Potter, B.E. Post-therapeutic surveillance schedule for oral cancer: Is there agreement? Oral. Maxillofac. Surg. 2012, 16, 327–340. [Google Scholar] [CrossRef]
- Kanatas, A.; Bala, N.; Lowe, D.; Rogers, S.N. Outpatient follow-up appointments for patients having curative treatment for cancer of the head and neck: Are the current arrangements in need of change? Br. J. Oral. Maxillofac. Surg. 2014, 52, 681–687. [Google Scholar] [CrossRef]
- Guo, Z.; Yamaguchi, K.; Sanchez-Cespedes, M.; Westra, W.H.; Koch, W.M.; Sidransky, D. Allelic losses in OraTest-directed biopsies of patients with prior upper aerodigestive tract malignancy. Clin. Cancer Res. 2001, 7, 1963–1968. [Google Scholar]
- Epstein, J.B.; Sciubba, J.; Silverman, S.; Sroussi, H.Y. Utility of toluidine blue in oral premalignant lesions and squamous cell carcinoma: Continuing research and implications for clinical practice. Head Neck 2007, 29, 948–958. [Google Scholar] [CrossRef]
- Morikawa, T.; Shibahara, T.; Nomura, T.; Katakura, A.; Takano, M. Non-Invasive Early Detection of Oral Cancers Using Fluorescence Visualization with Optical Instruments. Cancers 2020, 12, 2771. [Google Scholar] [CrossRef]
- Blatt, S.; Ziebart, T.; Krüger, M.; Pabst, A.M. Diagnosing oral squamous cell carcinoma: How much imaging do we really need? A review of the current literature. J. Craniomaxillofac. Surg. 2016, 44, 538–549. [Google Scholar] [CrossRef]
- Lonneux, M.; Lawson, G.; Ide, C.; Bausart, R.; Remacle, M.; Pauwels, S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope 2000, 110, 1493–1497. [Google Scholar] [CrossRef]
- Kim, S.; Roh, J.; Kim, J.S.; Lee, J.H.; Lee, S.H.; Choi, S.; Nam, S.Y.; Kim, S.Y. 18F-FDG PET/CT surveillance for the detection of recurrence in patients with head and neck cancer. Eur. J. Cancer 2017, 72, 62–70. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Kaur, J.; Kumar, M.; Matta, A.; Srivastava, G.; Alyass, A.; Assi, J.; Leong, I.; MacMillan, C.; Witterick, I.; et al. Prediction of recurrence-free survival using a protein expression-based risk classifier for head and neck cancer. Oncogenesis 2015, 4, e147. [Google Scholar] [CrossRef]
- Sato, J.; Ohuchi, M.; Abe, K.; Satoh, T.; Abe, T.; Yamazaki, Y.; Satoh, A.; Notani, K.-I.; Kitagawa, Y. Correlation between salivary interleukin-6 levels and early locoregional recurrence in patients with oral squamous cell carcinoma: Preliminary study. Head Neck 2013, 35, 889–894. [Google Scholar] [CrossRef]
- Rakesh, N.; Devi, Y.; Majumdar, K.; Reddy, S.S.; Agarwal, K. Tumour associated tissue eosinophilia as a predictor of locoregional recurrence in oral squamous cell carcinoma. J. Clin. Exp. Dent. 2015, 7, e1–e6. [Google Scholar] [CrossRef]
- Safi, A.-F.; Kauke, M.; Grandoch, A.; Nickenig, H.-J.; Zöller, J.E.; Kreppel, M. Analysis of clinicopathological risk factors for locoregional recurrence of oral squamous cell carcinoma-Retrospective analysis of 517 patients. J. Craniomaxillofac. Surg. 2017, 45, 1749–1753. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.; Li, B.; Li, J.; Mao, M.; Qin, L.; Li, H.; Huang, X.; Han, Z.; Feng, Z. Prognostic value of pathologic grade for patients with oral squamous cell carcinoma. Oral Dis. 2018, 24, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, S.; Yue, K.; Wang, X.-D. The recurrence and survival of oral squamous cell carcinoma: A report of 275 cases. Chin. J. Cancer 2013, 32, 614–618. [Google Scholar] [CrossRef]
- Sklenicka, S.; Gardiner, S.; Dierks, E.J.; Potter, B.E.; Bell, R.B. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: Does surgical salvage affect outcome? J. Oral Maxillofac. Surg. 2010, 68, 1270–1275. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Knösel, T.; Woodlock, T.; Troeltzsch, M.; Pianka, A.; Probst, F.A.; Mast, G.; Ehrenfeld, M.; Otto, S. Are There Clinical or Pathological Parameters of Maxillary Oral Squamous Cell Carcinoma with an Influence on the Occurrence of Neck Node Metastasis? An Appraisal of 92 Patients. J. Oral Maxillofac. Surg. 2016, 74, 79–86. [Google Scholar] [CrossRef]
- Day, G.L.; Blot, W.J. Second primary tumors in patients with oral cancer. Cancer 1992, 70, 14–19. [Google Scholar] [CrossRef]
- Rennemo, E.; Zätterström, U.; Evensen, J.; Boysen, M. Reduced risk of head and neck second primary tumors after radiotherapy. Radiother. Oncol. 2009, 93, 559–562. [Google Scholar] [CrossRef]
- Boakye, E.A.; Buchanan, P.; Hinyard, L.; Osazuwa-Peters, N.; Schootman, M.; Piccirillo, J.F. Incidence and Risk of Second Primary Malignant Neoplasm After a First Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 727–737. [Google Scholar] [CrossRef]
- Morris, L.G.; Sikora, A.G.; Patel, S.G.; Hayes, R.B.; Ganly, I. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol. 2011, 29, 739–746. [Google Scholar] [CrossRef]
- Kruger, M.; Krüger, M.; Pabst, A.M.; Walter, C.; Sagheb, K.; Günther, C.; Blatt, S.; Weise, K.; Al-Nawas, B.; Ziebart, T. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: A retrospective analysis of 88 patients and literature overview. J. Craniomaxillofac. Surg. 2014, 42, 1506–1514. [Google Scholar] [CrossRef]

| n = 760 | ||
|---|---|---|
| Patients | Mean age | 62 ± 13 years |
| Female | n = 258 (34%) | |
| Male | n = 502 (66%) | |
| Risk factors | No risk factors Smoking and alcohol abuse Smoking Alcohol abuse | n = 191 (25%) n = 374 (49%) n = 96 (13%) n = 99 (13%) |
| Primary tumor sites | Floor of the mouth Tongue Mandible Maxilla Cheek | n = 226 (30%) n = 209 (27%) n = 167 (22%) n = 111 (15%) n = 47 (6%) |
| Primary Tumor stadium (UICC, = Union for International Cancer Control) | Tumor stadium 1 Tumor stadium 2 Tumor stadium 3 Tumor stadium 4 | n = 263 (33%) n = 144 (17%) n = 91 (12%) n = 319 (38%) |
| Primary Tumor size | T1 T2 T3 T4 | n = 311 (41%) n = 231 (30%) n = 43 (6%) n = 175 (23%) |
| Primary lymph node status | N0 N1 N2a N2b N2c | n = 502 (66%) n = 98 (13%) n = 6 (1%) n = 111 (15%) n = 43 (5%) |
| Histopathological grading | G1 G2 G3 | n = 125 (17%) n = 541 (71%) n = 94 (12%) |
| Resection margin | R0 R1 R2 | n = 643 (85%) n = 110 (14%) n = 7 (1%) |
| Therapy of primary tumor | Surgery only Adjuvant radiation Adjuvant radiochemotherapy | n = 423 (56%) n = 146 (19%) n = 191 (25%) |
| Tumor recurrence | Mean recurrence interval No recurrence Local recurrence Lymph node metastases Distant metastases Second oral cavity cancer combination | n = 216 (28%) 24 ± 26 months n = 544 (72%) n = 75 (10%) n = 50 (7%) n = 26 (3%) n = 58 (7%) n = 7 (1%) |
| Recurrence number in each patient | n = 0 n = 1 n = 2 n = 3 n = 4 | n = 544 (72%) n = 181 (24%) n = 32 (4%) n = 2 (<1%) n = 1 (<1%) |
| Tumor recurrence therapy | Surgery only Either radio-, chemo- or both therapies Best supportive care | n = 127 (59%) n = 58 (27%) n = 31 (14%) |
| Detection of tumor recurrence | Clinical examination Radiological diagnosis Ultrasonography Self-reported symptoms | n = 123 (57%) n = 57 (27%) n = 18 (8%) n = 18 (8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blatt, S.; Krüger, M.; Sagheb, K.; Barth, M.; Kämmerer, P.W.; Al-Nawas, B.; Sagheb, K. Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma. J. Clin. Med. 2022, 11, 7061. https://doi.org/10.3390/jcm11237061
Blatt S, Krüger M, Sagheb K, Barth M, Kämmerer PW, Al-Nawas B, Sagheb K. Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma. Journal of Clinical Medicine. 2022; 11(23):7061. https://doi.org/10.3390/jcm11237061
Chicago/Turabian StyleBlatt, Sebastian, Maximilian Krüger, Kawe Sagheb, Marie Barth, Peer W. Kämmerer, Bilal Al-Nawas, and Keyvan Sagheb. 2022. "Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma" Journal of Clinical Medicine 11, no. 23: 7061. https://doi.org/10.3390/jcm11237061
APA StyleBlatt, S., Krüger, M., Sagheb, K., Barth, M., Kämmerer, P. W., Al-Nawas, B., & Sagheb, K. (2022). Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma. Journal of Clinical Medicine, 11(23), 7061. https://doi.org/10.3390/jcm11237061






