Post-Transplant Lymphoproliferative Disorder: A Rare Case of CNS Involvement following Renal Transplant
Abstract
1. Introduction
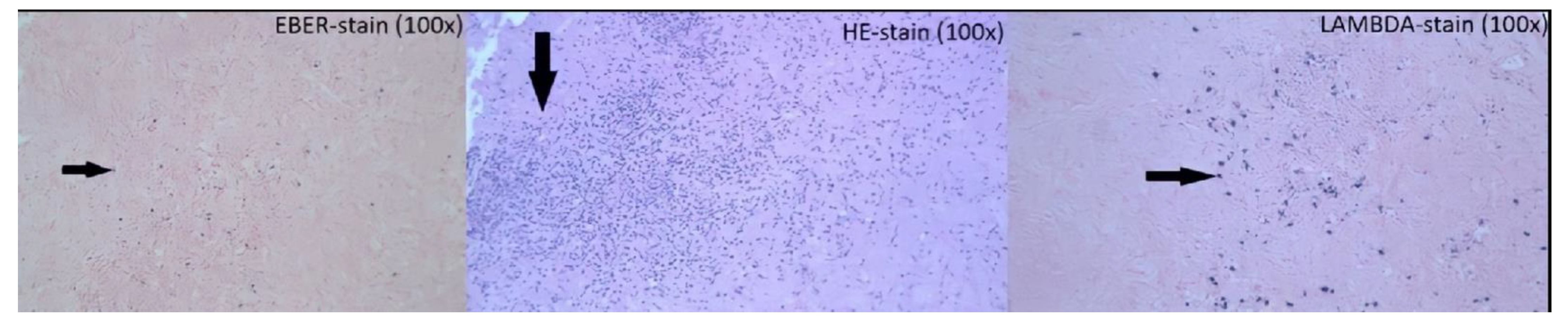
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ng, I.O.; Shek, T.W.; Thung, S.N.; Ye, M.M.; Lo, C.M.; Fan, S.T.; Lee, J.M.; Chan, K.W.; Cheung, A.N. Microsatellite analysis in post-transplantation lymphoproliferative disorder to determine donor/recipient origin. Mod. Pathol. 2000, 13, 1180–1185. [Google Scholar] [CrossRef][Green Version]
- Stubbins, R.J.; Mabilangan, C.; Rojas-Vasquez, M.; Lai, R.L.; Zhu, J.; Preiksaitis, J.P.; Peters, A.C. Classic Hodgkin lymphoma post-transplant lymphoproliferative disorders (PTLD) are often preceded by discordant PTLD subtypes. Leuk Lymphoma 2020, 61, 3319–3330. [Google Scholar] [CrossRef]
- Cheng, J.; Moore, C.A.; Iasella, C.J.; Glanville, A.R.; Morrell, M.R.; Smith, R.B.; McDyer, J.F.; Ensor, C.R. Systematic review and meta-analysis of post-transplant lymphoproliferative disorder in lung transplant recipients. Clin. Transpl. 2018, 32, e13235. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; David, K.A.; Helenowski, I.; Nelson, B.; Kaufman, D.; Kircher, S.M.; Gimelfarb, A.; Hattersley, E.; Mauro, L.A.; Jovanovic, B. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: Outcomes and prognostic factors in the modern era. J. Clin. Oncol. 2010, 28, 1038–1046. [Google Scholar] [CrossRef]
- Luskin, M.R.; Heil, D.S.; Tan, K.S.; Choi, S.; Stadtmauer, E.A.; Schuster, S.J.; Porter, D.L.; Vonderheide, R.H.; Bagg, A.; Heitjan, D.F. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am. J. Transpl. 2015, 15, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.J.; Puglia, J. The neuropathology of liver, heart, and heart-lung transplantation. Transpl. Proc. 1988, 20 (Suppl. S1), 806–809. [Google Scholar]
- Stubbins, R.J.; Lam, R.; Zhu, J.; Ghosh, S.; Mabilangan, C.; Kuruvilla, J.; Goswami, R.S.; Lai, R.; Preiksaitis, J.K.; Jain, M.D.; et al. Tumor Infiltrating Lymphocytes Predict Survival in Solid Organ Transplant Recipients with Monomorphic Post-transplant Lymphoproliferative Disorders. Clin. Lymphoma Myeloma Leuk. 2022, 22, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Lin, C.Y.; Kuo, T.T.; Shih, L.Y.; Chang, C.H.; Chen, W.T.; Yang, C.Y. Cutaneous involvement of polymorphic post-transplant lymphoproliferative disorder in a child after liver transplantation. Pediatr. Dermatol. 2019, 36, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, S.C.; Pfeiffer, R.M.; Morton, L.M.; Engels, E.A. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am. J. Hematol. 2011, 86, 206–209. [Google Scholar] [CrossRef]
- White, M.L.; Moore, D.W.; Zhang, Y.; Mark, K.D.; Greiner, T.C.; Bierman, P.J. Primary central nervous system post-transplant lymphoproliferative disorders: The spectrum of imaging appearances and differential. Insights Imaging 2019, 10, 46. [Google Scholar] [CrossRef]
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef]
- Buell, J.F.; Gross, T.; Alloway, R.R.; Trofe, J.; Woodle, E.S. Central nervous system tumors in donors: Misdiagnosis carries a high morbidity and mortality. Transpl. Proc. 2005, 37, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Gross, T.G.; Hanaway, M.J.; Trofe, J.; Roy-Chaudhury, P.; First, M.R.; Woodle, E.S. Posttransplant lymphoproliferative disorder: Significance of central nervous system involvement. Transpl. Proc. 2005, 37, 954–955. [Google Scholar] [CrossRef]
- Michonneau, D.; Suarez, F.; Lambert, J.; Adam, J.; Brousse, N.; Canioni, D.; Anglicheau, D.; Martinez, F.; Snanoudj, R.; Legendre, C. Late-onset post-transplantation lymphoproliferative disorders after kidney transplantation: A monocentric study over three decades. Nephrol. Dial. Transpl. 2013, 28, 471–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naik, S.; Riches, M.; Hari, P.; Kim, S.; Chen, M.; Bachier, C.; Shaughnessy, P.; Hill, J.; Ljungman, P.; Battiwalla, M.; et al. Survival outcomes of allogeneic hematopoietic cell transplants with EBV-positive or EBV-negative post-transplant lymphoproliferative disorder, A CIBMTR study. Transpl. Infect. Dis. 2019, 21, e13145. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Dohler, B. Lymphomas after solid organ transplantation: A collaborative transplant study report. Am. J. Transpl. 2004, 4, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin. Transpl. 1998, 1, 147–158. [Google Scholar]
- Cavaliere, R.; Petroni, G.; Lopes, M.B.; Schiff, D. International Primary Central Nervous System Lymphoma Collaborative Group. Primary central nervous system post-transplantation lymphoproliferative disorder: An International Primary Central Nervous System Lymphoma Collaborative Group Report. Cancer 2010, 116, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, R.; Bajwa, R.; Franke, A.J.; Skelton, W.P.; Wang, Y.; Patel, N.M.; Slayton, W.B.; Zou, F.; Dang, N.H. Post-transplant lymphoproliferative disorder (PTLD): Single institutional experience of 141 patients. Exp. Hematol. Oncol. 2017, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
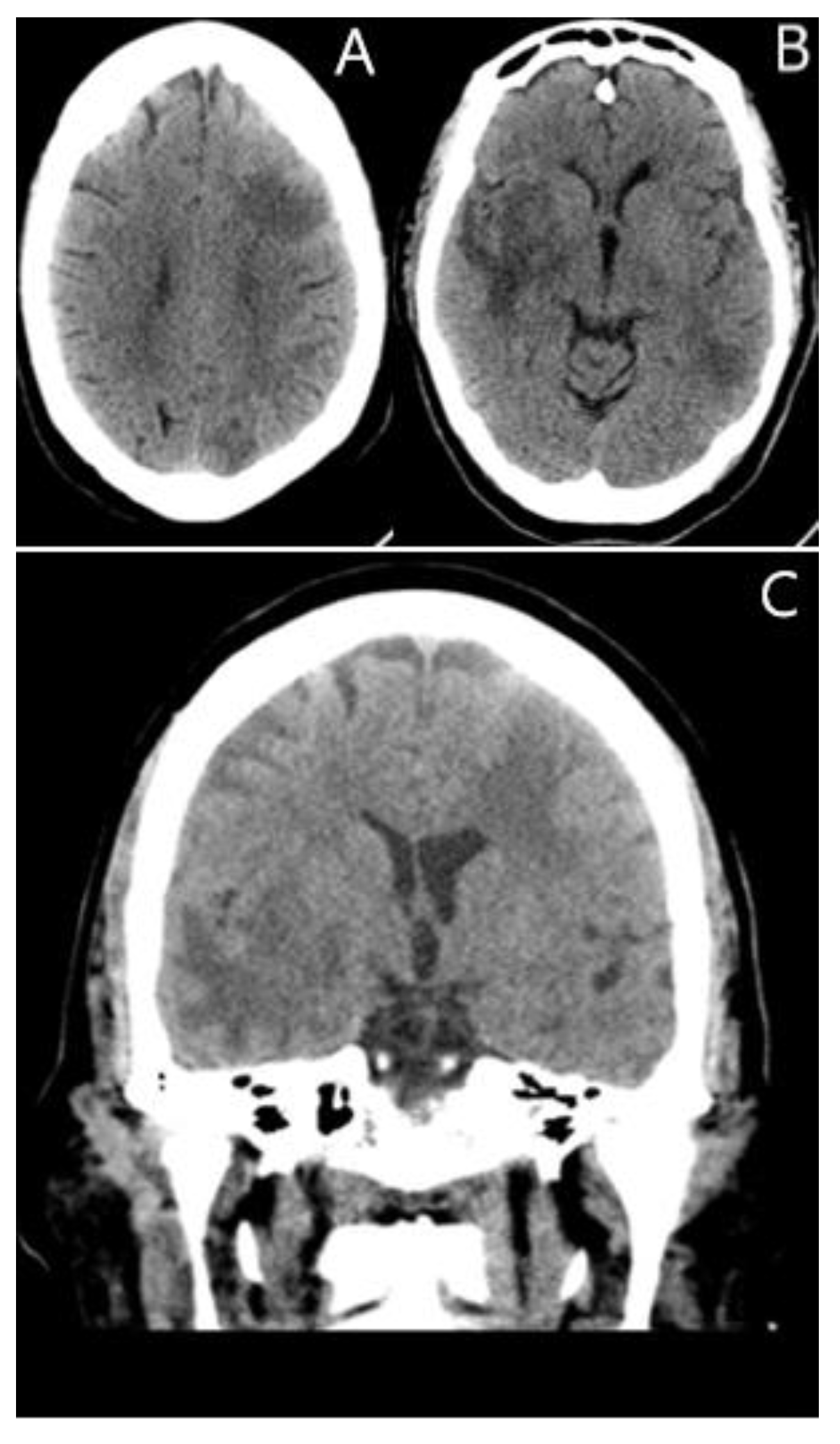
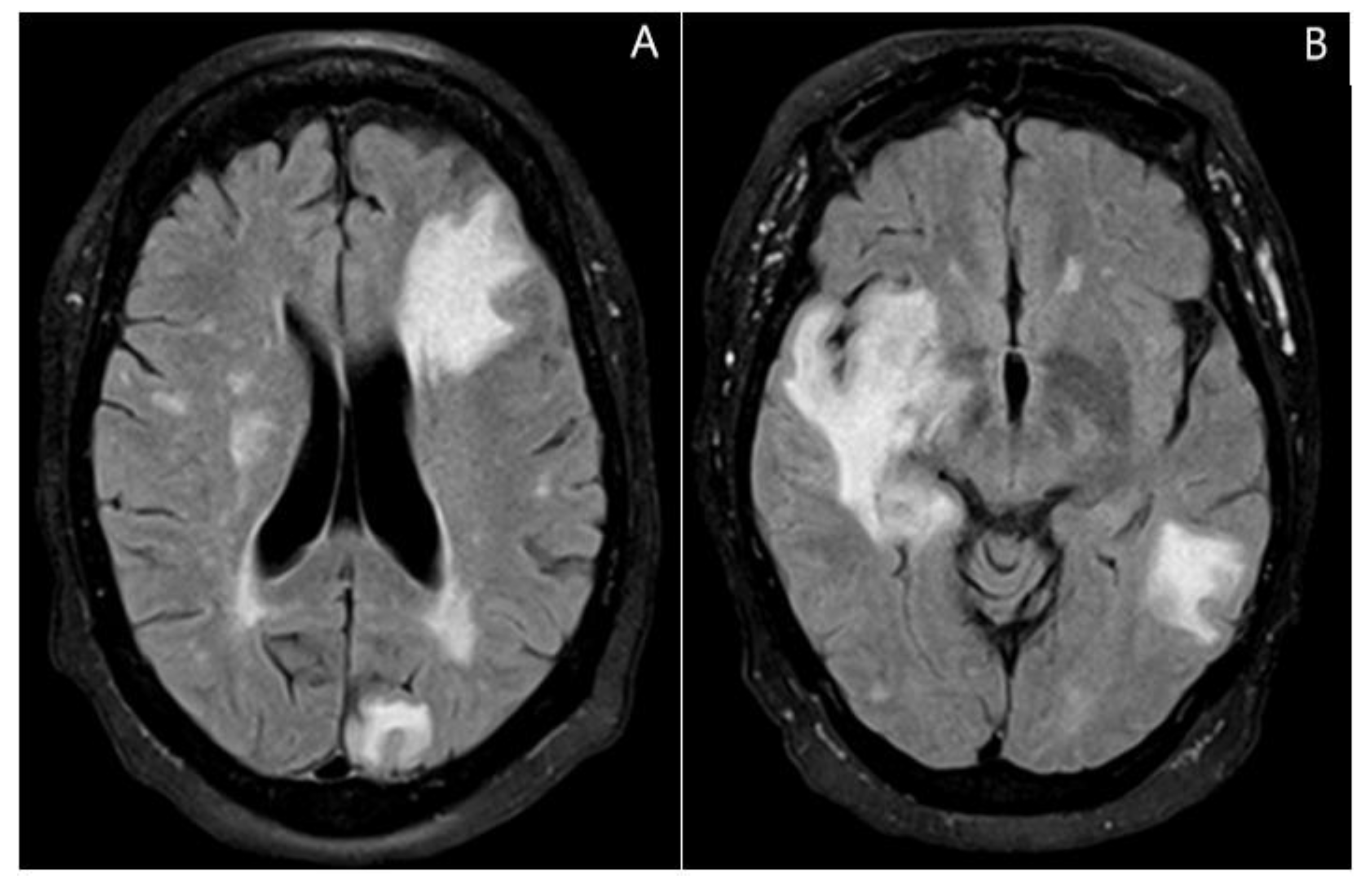
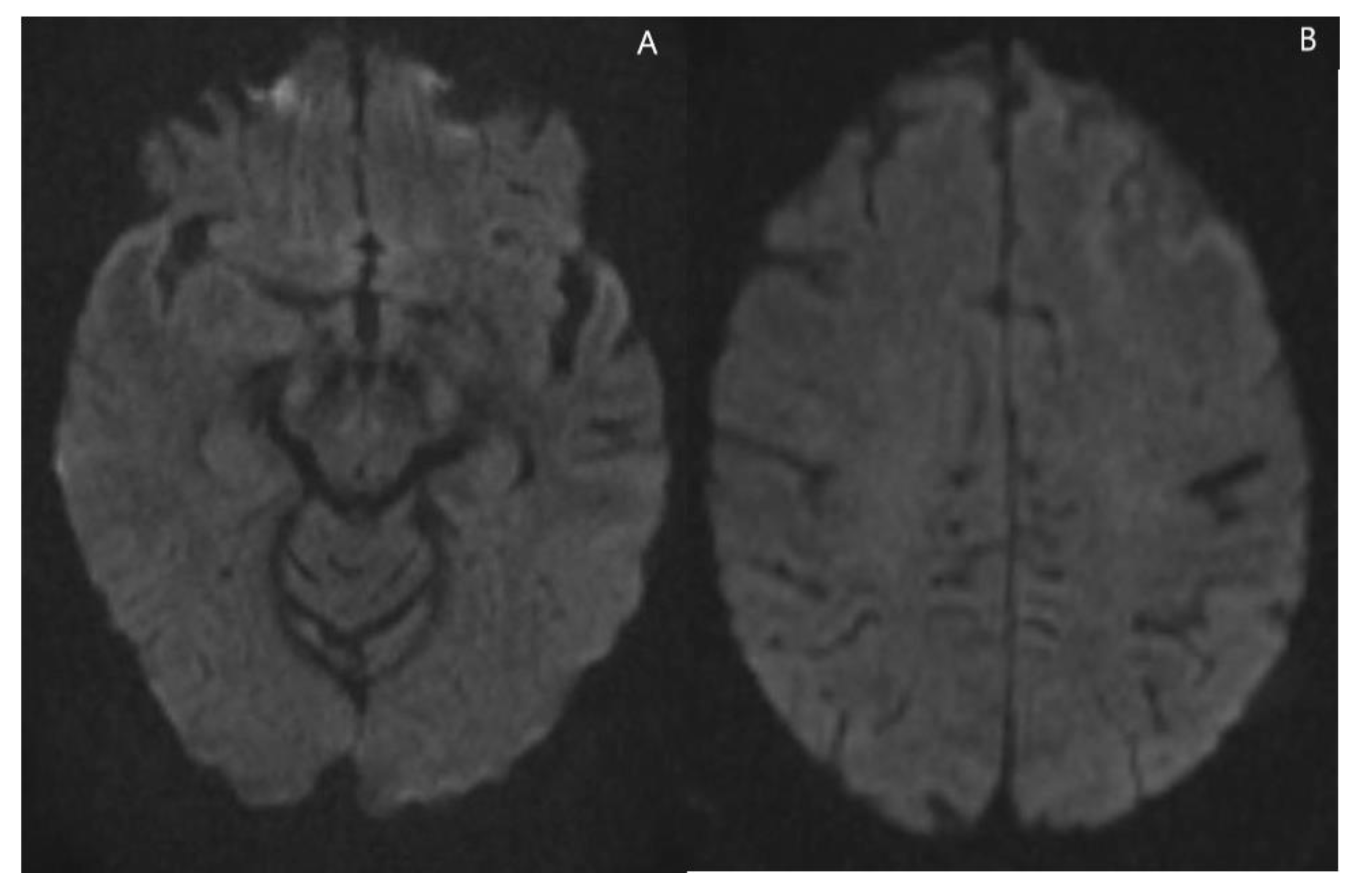
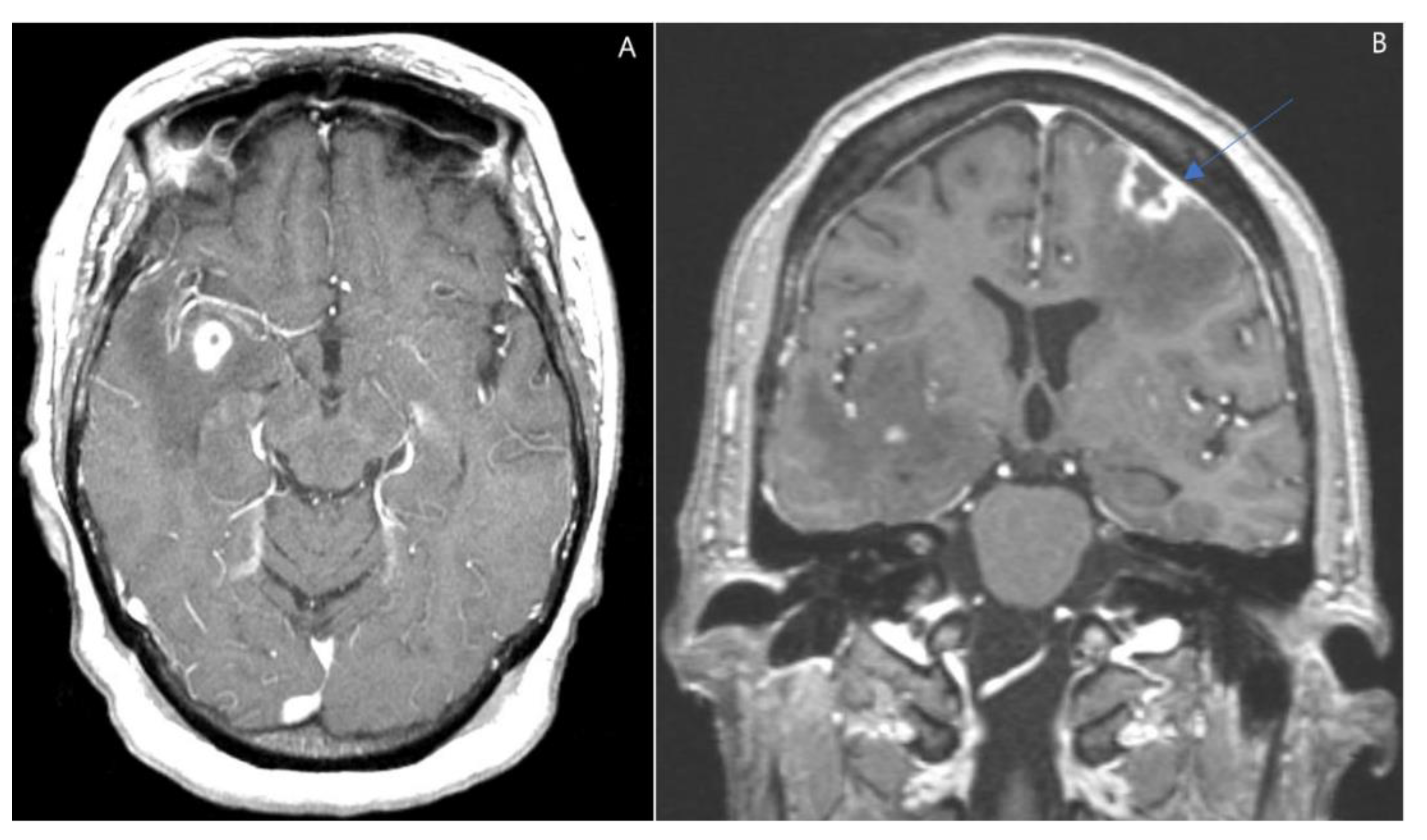
| Clinical Findings | Imaging Findings | Spectroscopy Findings |
|---|---|---|
| Post-transplanted patients of solid organs, stem cells or bone marrow | Partial or diffuse ring enhancement pattern | Increased levels of choline, lipid, and lactate dehydrogenase |
| It can be present as soon as 6 weeks or >10 years post transplantation | Irregular/ill-defined heterogenous margin of enhancement | Preserve or decrease levels of N-acetylaspartate (NAA) Elevated choline (Cho)/creatinine (Cr) ratio Decreased NAA/Cho ratio |
| Usually, multifocal lesions | Hypercellular tumors with a mixed pattern of cystic- central necrosis and hemorrhagic areas | |
| Lobes, basal ganglia, periventricular, brainstem, and cerebellum involvement | Lower perfusion than lymphomas | |
| Some lesions surrounded by edema | ||
| Apparent Diffusion Coefficient (ADC) elevated with areas of restricted diffusion. Susceptibility weighted imaging shows a peripheral pattern of punctate hypo intensities |
| AIDS Lymphomas | PCNS Lymphomas | Glioblastoma | Brain Abscess | Metastasis |
|---|---|---|---|---|
| Multifocal lesions located in the basal ganglia and corpus callosum that show an heterogenous ring enhancement pattern with irregular margins. These lesions show an ADC that is slightly elevated, with increased Cho and decreased NAA and Cr in spectroscopy. | Unifocal periventricular lesions that usually invade the corpus callosum. These lesions show a diffuse and homogeneous solid enhancement pattern and defined margins. PCNS lesions have a lower and homogenous ADC, show elevated perfusion rates, with elevated Cho, lipid and LDH, and decreased NAA and Cr in spectroscopy. | An enhancing lesion with high perfusion that usually invades the corpus callosum; it also tends to show central necrosis and hemorrhagic areas. | Usually, a lesion with a thin rim of enhancement, with high diffusion and restricted ADC in the central area. In the periphery, an abscess shows a low diffusion signal with elevated ADC. The spectroscopy shows amino acids like valine, leucine, isoleucine, acetate, alanine, and succinate present in the abscess cavity | Are enhancing lesions with high perfusion rates. Location of the masses depends on the primary tumor, but in the CNS, they usually do not invade basal ganglia, periventricular areas, or the thalamic regions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feindt, A.; Lara-Velazquez, M.; Alkhasawneh, A.; Rao, D.; Makary, R.; Dombrowski, K.; Tavanaiepour, D.; Rahmathulla, G. Post-Transplant Lymphoproliferative Disorder: A Rare Case of CNS Involvement following Renal Transplant. J. Clin. Med. 2022, 11, 7058. https://doi.org/10.3390/jcm11237058
Feindt A, Lara-Velazquez M, Alkhasawneh A, Rao D, Makary R, Dombrowski K, Tavanaiepour D, Rahmathulla G. Post-Transplant Lymphoproliferative Disorder: A Rare Case of CNS Involvement following Renal Transplant. Journal of Clinical Medicine. 2022; 11(23):7058. https://doi.org/10.3390/jcm11237058
Chicago/Turabian StyleFeindt, Austin, Montserrat Lara-Velazquez, Ahmad Alkhasawneh, Dinesh Rao, Raafat Makary, Keith Dombrowski, Daryoush Tavanaiepour, and Gazanfar Rahmathulla. 2022. "Post-Transplant Lymphoproliferative Disorder: A Rare Case of CNS Involvement following Renal Transplant" Journal of Clinical Medicine 11, no. 23: 7058. https://doi.org/10.3390/jcm11237058
APA StyleFeindt, A., Lara-Velazquez, M., Alkhasawneh, A., Rao, D., Makary, R., Dombrowski, K., Tavanaiepour, D., & Rahmathulla, G. (2022). Post-Transplant Lymphoproliferative Disorder: A Rare Case of CNS Involvement following Renal Transplant. Journal of Clinical Medicine, 11(23), 7058. https://doi.org/10.3390/jcm11237058






