Outcomes of Patients Undergoing Closed Traction Coronary Endarterectomy: A Long-Term Single Center Study †
Abstract
1. Introduction
2. Methods
2.1. Study Design
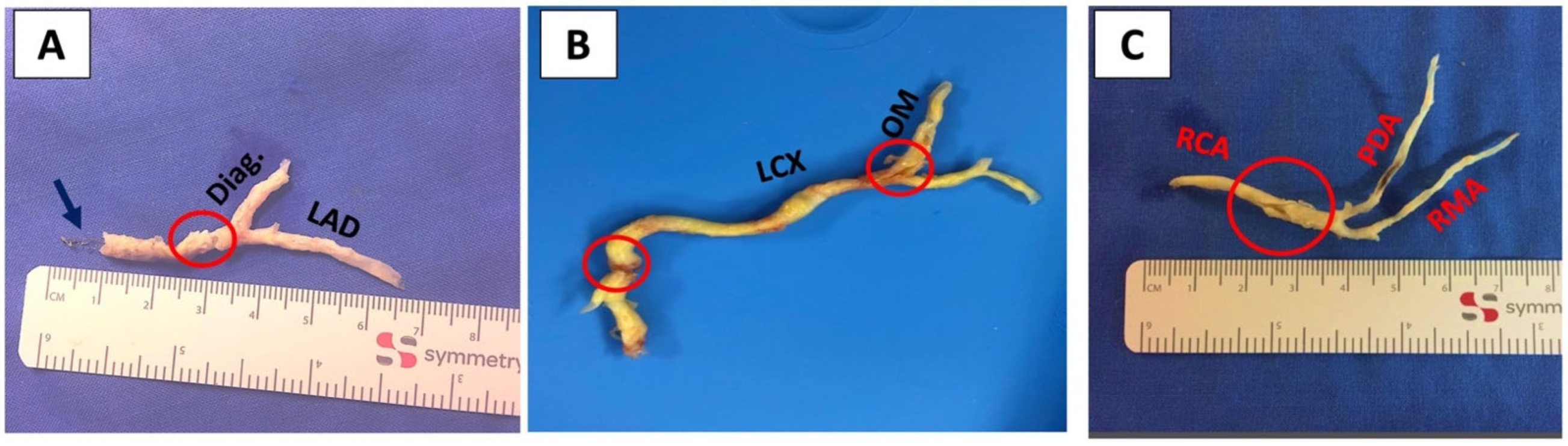
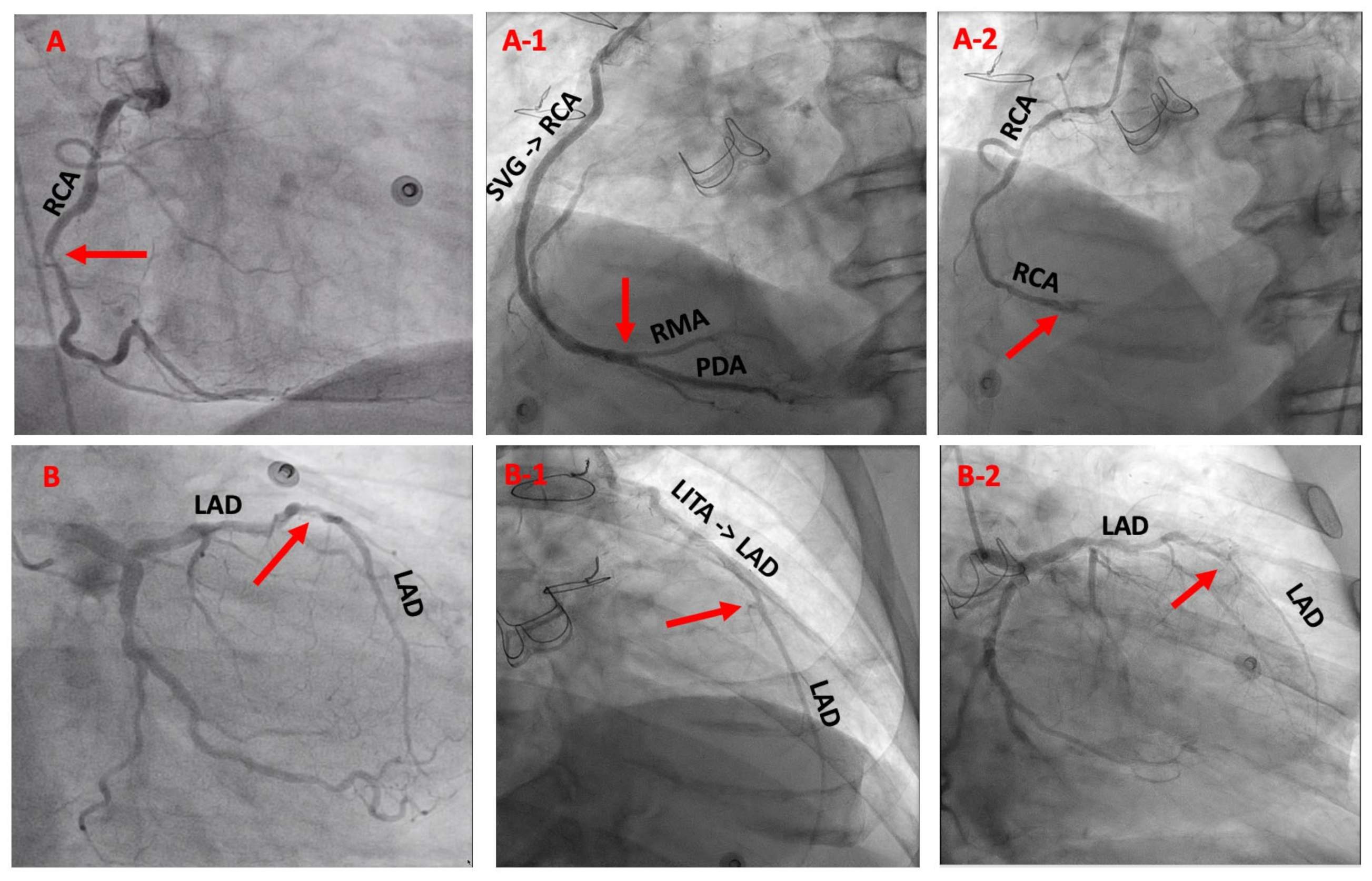
2.2. Indication and Surgical Technique
2.3. Postoperative Treatment
2.4. Study Endpoints and Definitions
2.5. Statistics
3. Results
3.1. Preoperative Data
3.2. Postoperative Outcomes
3.3. Long-Term Outcomes
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, C.P.; May, A.; Lemmon, W.M. Survival after coronary endarterectomy in man. J. Am. Med. Assoc. 1957, 164, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, C.; Yu, W.; Gao, M.; Yu, Y. Short- and Long-Term Patient Outcomes From Combined Coronary Endarterectomy and Coronary Artery Bypass Grafting: A Meta-Analysis of 63,730 Patients (PRISMA). Medicine 2015, 94, e1781. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.; Harling, L.; Ashrafian, H.; Casula, R.; Kokotsakis, J.; Athanasiou, T. Adjunct coronary endarterectomy increases myocardial infarction and early mortality after coronary artery bypass grafting: A meta-analysis. Interact. Cardio Vascular Thorac. Surg. 2014, 19, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Lemma, M.; Beretta, L.; Vanelli, P.; Santoli, C. Open coronary endarterectomy, saphenous vein patch reconstruction, and internal mammary artery grafting. Ann. Thorac. Surg. 1992, 53, 1151–1152. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N.; Lumia, F.J.; Gottdiener, J.S.; Germon, P.; Lemole, G.M. Adjunct endarterectomy of the left anterior descending coronary artery. Ann. Thorac. Surg. 1988, 46, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Erdil, N.; Cetin, L.; Kucuker, S.; Demirkilic, U.; Sener, E.; Tatar, H. Closed endarterectomy for diffuse right coronary artery disease: Early results with angiographic controls. J. Card. Surg. 2002, 17, 261–266. [Google Scholar] [CrossRef]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Shehada, S.E.; Mourad, F.; Balaj, I.; El Gabry, M.; Wendt, D.; Thielmann, M.; Schlosser, T.; Jakob, H. Long-Term Outcomes of Coronary Endarterectomy in Patients with Complete Imaging Follow-up. In Seminars in Thoracic and Cardiovascular Surgery; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Jakob, H.; Mourad, F.; Lubarski, J.; Schlosser, T.; Shehada, S.-E. A Case of Diffuse Three-Vessel Coronary Artery Disease With Triple Coronary Endarterectomy in a Patient Undergoing Coronary Artery Bypass Grafting; CTSNet, Inc.: Valhalla, NY, USA, 2019. [Google Scholar] [CrossRef]
- Wendt, D.; Shehada, S.E.; Mourad, F.; Machulla, R.; Demircioglu, E.; Marx, P.; Demircioglu, A.; Tsagakis, K.; Thielmann, M.; Jakob, H.G.; et al. Transit time flow measurement and high frequency ultrasound epi-cardiac imaging to guide coronary artery bypass surgery. J. Cardiovasc. Surg. (Torino) 2018, 60, 245–250. [Google Scholar] [CrossRef]
- Petricevic, M.; Biocina, B.; Milicic, D.; Konosic, S.; Ivancan, V.; Milosevic, M.; Burcar, I.; Gasparovic, H. Bleeding risk assessment using multiple electrode aggregometry in patients following coronary artery bypass surgery. J. Thromb. Thrombolysis 2013, 35, 31–40. [Google Scholar] [CrossRef]
- Petricevic, M.; Konosic, S.; Biocina, B.; Dirkmann, D.; White, A.; Mihaljevic, M.Z.; Ivancan, V.; Konosic, L.; Svetina, L.; Gorlinger, K. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM((R)) platelet and Multiplate((R)) impedance aggregometry. Anaesthesia 2016, 71, 636–647. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; Thygesen, K.; Alpert, J.S.; Simoons, M.L.; et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Effler, D.B.; Groves, L.K.; Sones, F.M., Jr.; Shirey, E.K. Endarterectomy in the Treatment of Coronary Artery Disease. J. Thorac. Cardiovasc. Surg. 1964, 47, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Minale, C.; Nikol, S.; Zander, M.; Uebis, R.; Effert, S.; Messmer, B.J. Controversial aspects of coronary endarterectomy. Ann. Thorac. Surg. 1989, 48, 235–241. [Google Scholar] [CrossRef]
- Myers, P.O.; Tabata, M.; Shekar, P.S.; Couper, G.S.; Khalpey, Z.I.; Aranki, S.F. Extensive endarterectomy and reconstruction of the left anterior descending artery: Early and late outcomes. J. Thorac. Cardiovasc. Surg. 2012, 143, 1336–1340. [Google Scholar] [CrossRef]
- Nishigawa, K.; Fukui, T.; Yamazaki, M.; Takanashi, S. Ten-Year Experience of Coronary Endarterectomy for the Diffusely Diseased Left Anterior Descending Artery. Ann. Thorac. Surg. 2017, 103, 710–716. [Google Scholar] [CrossRef]
- Beretta, L.; Lemma, M.; Vanelli, P.; DiMattia, D.; Bozzi, G.; Broso, P.; Salvaggio, A.; Santoli, C. Coronary “open” endarterectomy and reconstruction: Short- and long-term results of the revascularization with saphenous vein versus IMA-graft. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 1992, 6, 382–386, discussion 387. [Google Scholar] [CrossRef]
- Sommerhaug, R.G.; Wolfe, S.F.; Reid, D.A.; Lindsey, D.E. Early clinical results of long coronary arteriotomy, endarterectomy and reconstruction combined with multiple bypass grafting for severe coronary artery disease. Am. J. Cardiol. 1990, 66, 651–659. [Google Scholar] [CrossRef]
- Loffler, A.I.; Kramer, C.M. Myocardial Viability Testing to Guide Coronary Revascularization. Interv. Cardiol. Clin. 2018, 7, 355–365. [Google Scholar] [CrossRef]
- Previtali, M.; Lanzarini, L.; Poli, A.; Fetiveau, R.; Barberis, P. Dobutamine stress echocardiography early after myocardial infarction treated with thrombolysis. Identification of myocardial viability and ischemia and relation to spontaneous functional recovery. Int. J. Card. Imaging 1996, 12, 97–104. [Google Scholar] [CrossRef]
- Castini, D.; Garbin, M.; Bigi, R.; Occhi, G.; Concardi, G.; Belletti, S.; Gioventu, M.; Sponzilli, C.; Fiorentini, C. Early assessment of viable myocardium after acute myocardial infarction by low-dose echo-dobutamine. G. Ital. Cardiol. 1998, 28, 1215–1224. [Google Scholar] [PubMed]
- Adelborg, K.; Horvath-Puho, E.; Schmidt, M.; Munch, T.; Pedersen, L.; Nielsen, P.H.; Botker, H.E.; Toft Sorensen, H. Thirty-Year Mortality After Coronary Artery Bypass Graft Surgery: A Danish Nationwide Population-Based Cohort Study. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e002708. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Caputo, M.; Gaudino, M.; Mariscalco, G.; Bryan, A.; Angelini, G.D. Is the right internal thoracic artery superior to saphenous vein for grafting the right coronary artery? A propensity score-based analysis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1269–1275.e1265. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef]
| All Patients (n = 326) | |
|---|---|
| Age, years | 66.7 ± 9.3 |
| Gender, males | 287 (88) |
| Body mass index, kg/m2 | 27.4 ± 4.1 |
| Diabetes mellitus | 125 (38.3) |
| Nicotine abuse | 115 (35.3) |
| Hypercholesterinemia | 204 (62.6) |
| Chronic obstructive pulmonary diseases | 38 (11.6) |
| Peripheral vascular disease | 53 (16.3) |
| Preoperative dialysis | 6 (1.8) |
| Previous cerebrovascular event | 25 (7.7) |
| Previous myocardial infarction | 123 (37.7) |
| Previous CABG | 18 (5.5) |
| NYHA class III–IV | 108 (33.1) |
| Nonelective surgery | 99 (30.4) |
| Extent of coronary artery disease | |
| Three-vessel disease | 302 (93) |
| Two-vessel disease | 21 (6) |
| One-vessel disease | 3 (1) |
| SYNTAX score | 33.1 ± 12 |
| Left ventricular function | |
| EF > 50% | 143 (43.9) |
| EF = 35–50% | 91 (27.9) |
| EF < 30% | 92 (28.2) |
| All Patients (n = 326) | |
|---|---|
| Aortic cross-clamp time | 84.3 ± 19.2 |
| Number of grafts for each patient | 4.3 ± 1.1 |
| Total number of CEA grafts | 394 |
| One CEA | 269 (82.5) |
| Two CEAs | 47 (14.4) |
| Three CEAs | 9 (2.8) |
| Four CEAs | 1 (0.3) |
| Indication for CEA | |
| Totally occluded vessel | 111 (28.2) |
| Sub-totally occluded vessel | 283 (71.8) |
| CEA vessel | |
| LAD territory | 174 (44.2) |
| RCA territory | 153 (38.8) |
| LCX territory | 67 (17) |
| Graft used after CEA | |
| Arterial graft | 145 (36.8) |
| Venous graft | 249 (63.2) |
| TTFM after CEA, mL/min | 67.5 ± 39.7 |
| All Patients (n = 326) | |
|---|---|
| Low cardiac output syndrome | |
| Need for IABP | 20 (6.1) |
| Need for ECMO | 5 (1.5) |
| Myocardial infarction | 8 (2.4) |
| In-hospital mortality | 13 (4) |
| Cardiac-related mortality | 11 (3.3) |
| Cerebrovascular events | |
| Transient ischemic attack | 3 (0.9) |
| Stroke | 8 (2.4) |
| Re-exploration for bleeding | 18 (5.5) |
| ICU stay, days | 2.4 ± 2.1 |
| Postoperative dialysis | 22 (6.7) |
| Respiratory complications | |
| Need for reintubation | 23 (7) |
| Need for tracheostomy | 22 (6.7) |
| Antiplatelet therapy (APT) | |
| Single APT | 152 (46.6) |
| Dual APT | 174 (53.4) |
| Follow-Up Results | All Patients 326 (100) | Single-APT (n = 152) | Dual-APT (n = 174) | p-Value |
|---|---|---|---|---|
| Lost during follow-up | 5 (1.5) | 3 (2) | 2 (1.1) | |
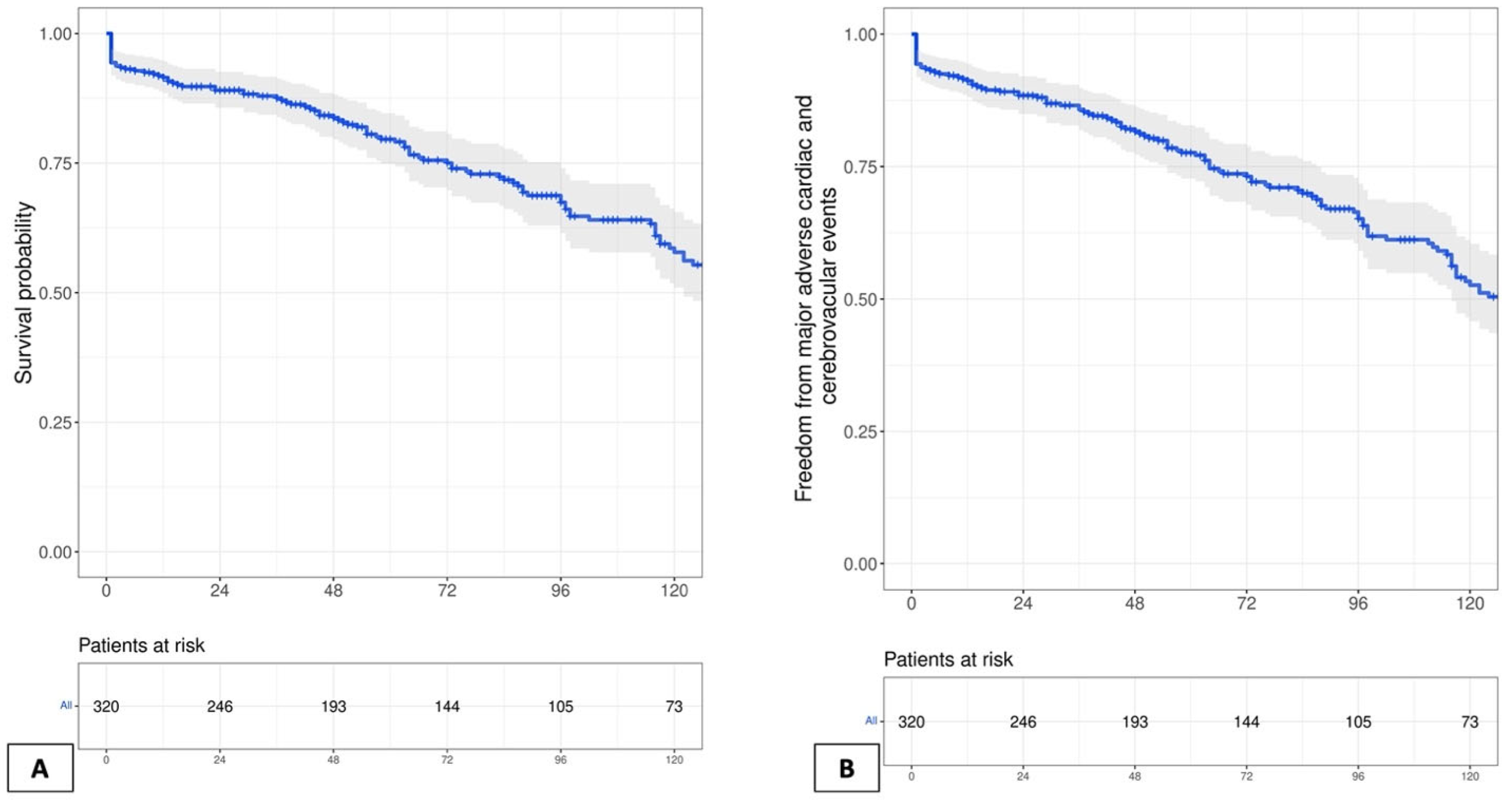
| One-year mortality | 27 (8.3) | 15 (9.8) | 12 (6.9) | 0.32 |
| Ten-year mortality | 90 (27.6) | 56 (36.8) | 34 (19.5) | <0.001 |
| Overall mortality at last follow-up | 112 (34.4) | 76 (50) | 36 (20.7) | <0.001 |
| Overall MACCE at last follow-up | 135 (41.4) | 88 (57.9) | 47 (27) | <0.001 |
| Follow-up, median (IQR) years | 8.9 (3–12) | |||
| Survivors’ clinical follow-up | 209 (100) | (n = 73) | (n = 136) | |
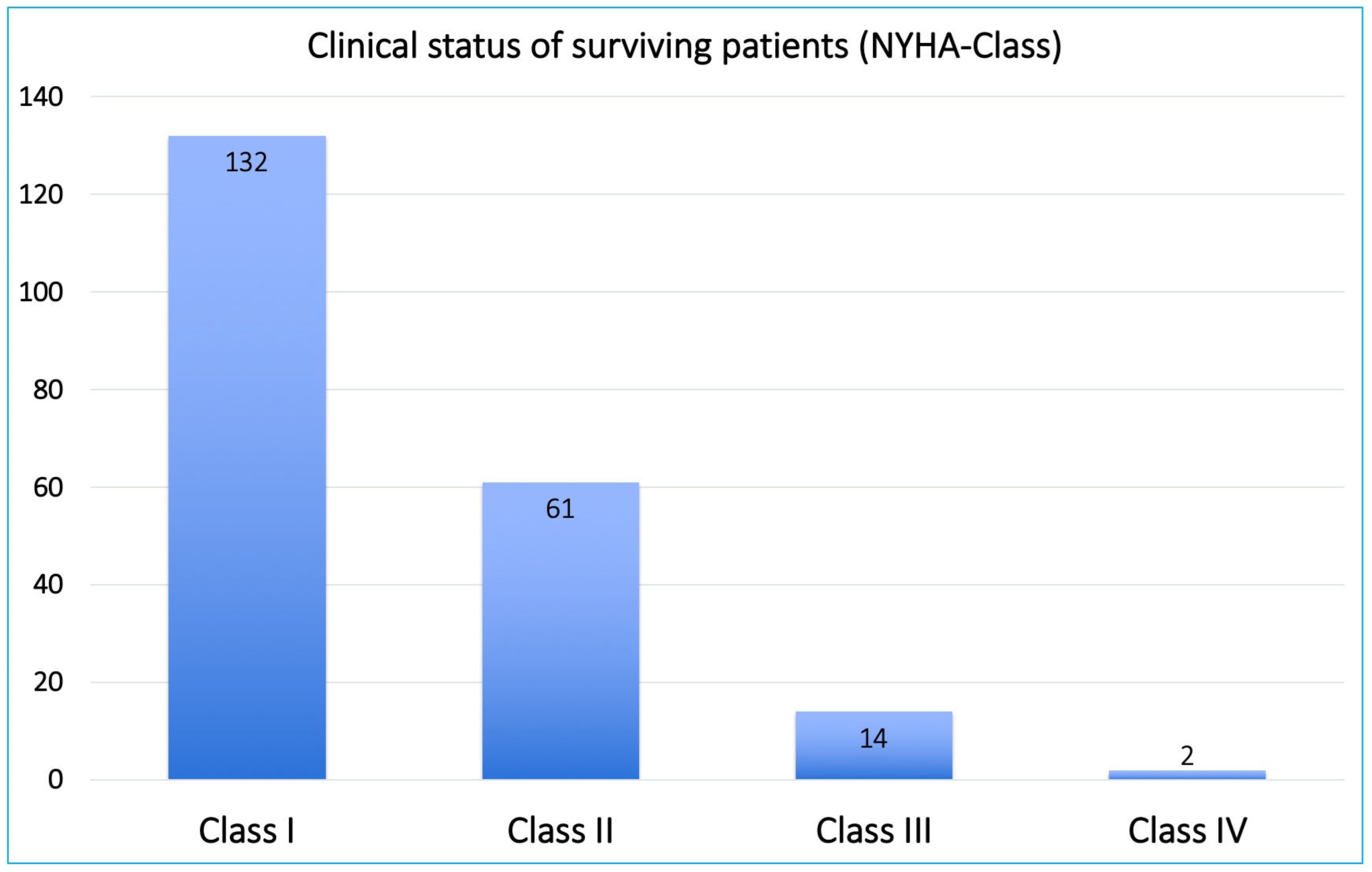
| NYHA class I–II | 193 (92.3) | 59 (80.8) | 129 (94.9) | 0.018 |
| NYHA class III–IV | 16 (7.6) | 9 (12.3) | 7 (5.1) | 0.018 |
| Stroke | 11 (5.2) | 5 (6.8) | 6 (4.4) | 0.25 |
| Myocardial infarction | 10 (4.8) | 7 (9.6) | 3 (2.2) | 0.006 |
| PCI/stenting | 15 (7.1) | 9 (12.3) | 6 (4.4) | 0.018 |
| Re-CABG | 0 | |||
| Other cardiac surgery | 5 (2.4) | |||
| Pacemaker implantation | 10 (4.8) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehada, S.-E.; Mourad, F.; Haddad, A.; Darwish, B.; Ryadi, N.; Balaj, I.; Jakob, H.; Ruhparwar, A. Outcomes of Patients Undergoing Closed Traction Coronary Endarterectomy: A Long-Term Single Center Study. J. Clin. Med. 2022, 11, 7026. https://doi.org/10.3390/jcm11237026
Shehada S-E, Mourad F, Haddad A, Darwish B, Ryadi N, Balaj I, Jakob H, Ruhparwar A. Outcomes of Patients Undergoing Closed Traction Coronary Endarterectomy: A Long-Term Single Center Study. Journal of Clinical Medicine. 2022; 11(23):7026. https://doi.org/10.3390/jcm11237026
Chicago/Turabian StyleShehada, Sharaf-Eldin, Fanar Mourad, Ali Haddad, Belal Darwish, Noura Ryadi, Ilir Balaj, Heinz Jakob, and Arjang Ruhparwar. 2022. "Outcomes of Patients Undergoing Closed Traction Coronary Endarterectomy: A Long-Term Single Center Study" Journal of Clinical Medicine 11, no. 23: 7026. https://doi.org/10.3390/jcm11237026
APA StyleShehada, S.-E., Mourad, F., Haddad, A., Darwish, B., Ryadi, N., Balaj, I., Jakob, H., & Ruhparwar, A. (2022). Outcomes of Patients Undergoing Closed Traction Coronary Endarterectomy: A Long-Term Single Center Study. Journal of Clinical Medicine, 11(23), 7026. https://doi.org/10.3390/jcm11237026









