Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects
Abstract
1. Introduction
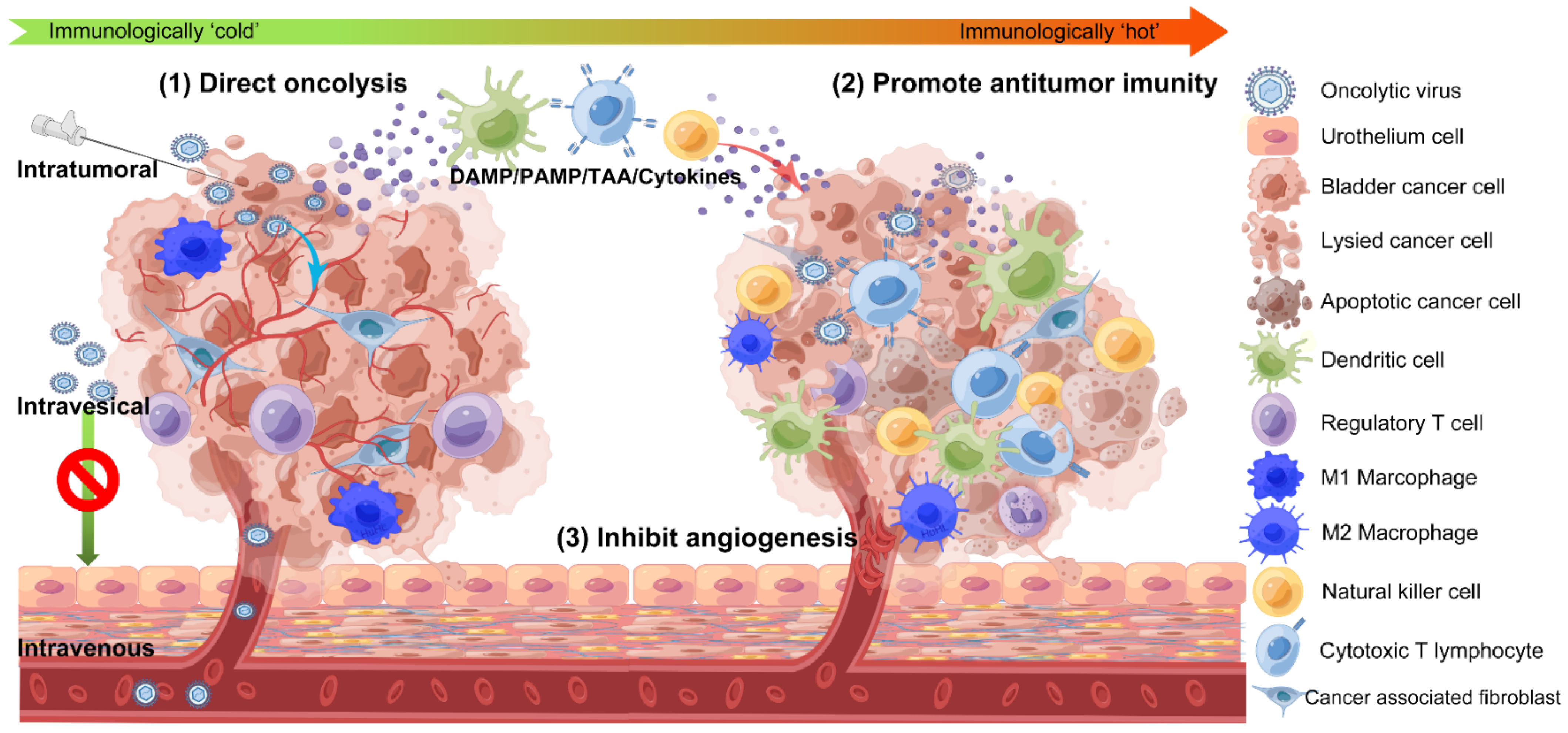
2. OVs and Their Antitumor Mechanisms in BC
2.1. Direct Oncolysis
2.2. Promoting Antitumor Immunity
2.3. Inhibition of Intratumor Angiogenesis
3. OVs for BC
3.1. Adenovirus
3.2. Herpesvirus
3.3. Coxsackievirus
3.4. Vesicular Stomatitis Virus
3.5. Alphavirus
3.6. Newcastle Disease Virus
3.7. Reovirus
3.8. Vaccinia Virus
4. Clinical Trials of OVs for BC
5. Combination Therapy
5.1. OVT in Combination with Chemotherapy
5.2. OVT in Combination with Radiotherapy
5.3. OVT in Combination with ICIs
5.4. OVT in Combination with Adoptive Cell Therapy
6. Challenges and Prospects
6.1. Improving the Delivery Efficiency
6.2. Combination Therapy
6.3. Tumor Imaging
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Naghavi, M.; Global Burden of Disease Bladder Cancer, C. Global, regional and national burden of bladder cancer and its attributable risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. BMJ Glob. Health 2021, 6, e004128. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European association of urology guidelines on non-muscle-invasive bladder cancer (ta, t1, and carcinoma in situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, R.; Lorch, A.; Bruins, H.M.; Comperat, E.M.; Cowan, N.C.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Hernandez, V.; Espinos, E.L.; et al. The 2021 updated european association of urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 2022, 81, 95–103. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Cao, G.D.; He, X.B.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.P.; Chen, B.; Xiong, M.M. The oncolytic virus in cancer diagnosis and treatment. Front. Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Ylosmaki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Fong, Y.; Warner, S.G. Oncolytic virotherapy for cancer: Clinical experience. Biomedicines 2021, 9, 419. [Google Scholar] [CrossRef]
- Jhawar, S.R.; Thandoni, A.; Bommareddy, P.K.; Hassan, S.; Kohlhapp, F.J.; Goyal, S.; Schenkel, J.M.; Silk, A.W.; Zloza, A. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front. Oncol. 2017, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Maroun, J.; Munoz-Alia, M.; Ammayappan, A.; Schulze, A.; Peng, K.W.; Russell, S. Designing and building oncolytic viruses. Future Virol. 2017, 12, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Potts, K.G.; Hitt, M.M.; Moore, R.B. Oncolytic viruses in the treatment of bladder cancer. Adv. Urol. 2012, 2012, 404581. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Shen, T.; Wientjes, M.G.; O’Donnell, M.A.; Au, J.L. Intravesical treatments of bladder cancer: Review. Pharm. Res. 2008, 25, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Garmaroudi, G.A.; Karimi, F.; Naeini, L.G.; Kokabian, P.; Givtaj, N. Therapeutic efficacy of oncolytic viruses in fighting cancer: Recent advances and perspective. Oxid. Med. Cell. Longev. 2022, 2022, 3142306. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.C.; Strong, J.E.; Forsyth, P.A.; Lee, P.W. Reovirus therapy of tumors with activated ras pathway. Science 1998, 282, 1332–1334. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef]
- Guse, K.; Dias, J.D.; Bauerschmitz, G.J.; Hakkarainen, T.; Aavik, E.; Ranki, T.; Pisto, T.; Sarkioja, M.; Desmond, R.A.; Kanerva, A.; et al. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Ther. 2007, 14, 902–911. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; et al. Identification and characterization of alphavirus m1 as a selective oncolytic virus targeting zap-defective human cancers. Proc. Natl. Acad. Sci. USA 2014, 111, E4504–E4512. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Pathak, D.C.; D’Silva, A.L.; Batheja, R.; Mariappan, A.K.; Vakharia, V.N.; Chellappa, M.M.; Dey, S. Evaluation of the oncolytic property of recombinant newcastle disease virus strain r2b in 4t1 and b16-f10 cells in-vitro. Res. Vet. Sci. 2021, 139, 159–165. [Google Scholar] [CrossRef]
- Li, Q.; Oduro, P.K.; Guo, R.; Li, R.; Leng, L.; Kong, X.; Wang, Q.; Yang, L. Oncolytic viruses: Immunotherapy drugs for gastrointestinal malignant tumors. Front. Cell. Infect. Microbiol. 2022, 12, 921534. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, X.; Yu, J.; Ge, S.; Fan, X. Oncolytic virotherapy: From bench to bedside. Front. Cell Dev. Biol. 2021, 9, 790150. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chard Dunmall, L.S.; Cheng, Z.; Wang, Y. Remodeling the tumor microenvironment by oncolytic viruses: Beyond oncolysis of tumor cells for cancer treatment. J. Immunother. Cancer 2022, 10, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Davola, M.E.; Mossman, K.L. Oncolytic viruses: How “lytic” must they be for therapeutic efficacy? Oncoimmunology 2019, 8, e1581528. [Google Scholar] [CrossRef] [PubMed]
- Coffin, R.S. Oncolytic immunotherapy: An emerging new modality for the treatment of cancer. Ann. Oncol. 2016, 27, 1805–1808. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.H.; et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013, 73, 1265–1275. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Paterson, J.M.; Lemay, C.G.; Falls, T.J.; McGuire, A.; Parato, K.A.; Stojdl, D.F.; Daneshmand, M.; Speth, K.; Kirn, D.; et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 2007, 15, 1686–1693. [Google Scholar] [CrossRef]
- Angarita, F.A.; Acuna, S.A.; Ottolino-Perry, K.; Zerhouni, S.; McCart, J.A. Mounting a strategic offense: Fighting tumor vasculature with oncolytic viruses. Trends Mol. Med. 2013, 19, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, S.; Zakay-Rones, Z.; Panet, A. Therapeutic potential of oncolytic newcastle disease virus: A critical review. Oncolytic Virotherapy 2015, 4, 49–62. [Google Scholar] [PubMed]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Annels, N.E.; Arif, M.; Simpson, G.R.; Denyer, M.; Moller-Levet, C.; Mansfield, D.; Butler, R.; Shafren, D.; Au, G.; Knowles, M.; et al. Oncolytic immunotherapy for bladder cancer using coxsackie A21 virus. Mol. Ther. Oncolytics 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Cao, W.; Tian, J.; Li, C.; Gao, Y.; Liu, X.; Lu, J.; Wang, Y.; Wang, Z.; Svatek, R.S.; Rodriguez, R. A novel bladder cancer-specific oncolytic adenovirus by cd46 and its effect combined with cisplatin against cancer cells of car negative expression. Virol. J. 2017, 14, 149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gotoh, A.; Nagaya, H.; Kanno, T.; Tagawa, M.; Nishizaki, T. Fiber-substituted conditionally replicating adenovirus ad5f35 induces oncolysis of human bladder cancer cells in in vitro analysis. Urology 2013, 81, 920.e7–920.e11. [Google Scholar] [CrossRef]
- Hadaschik, B.A.; Zhang, K.; So, A.I.; Fazli, L.; Jia, W.; Bell, J.C.; Gleave, M.E.; Rennie, P.S. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008, 68, 4506–4510. [Google Scholar] [CrossRef]
- Hanel, E.G.; Xiao, Z.; Wong, K.K.; Lee, P.W.; Britten, R.A.; Moore, R.B. A novel intravesical therapy for superficial bladder cancer in an orthotopic model: Oncolytic reovirus therapy. J. Urol. 2004, 172, 2018–2022. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Lin, Y.; Liang, J.K.; Zhong, W.W.; Li, K.; Huang, W.T.; Wang, D.J.; Yan, G.M.; Zhu, W.B.; et al. Intravenous injections of the oncolytic virus m1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018, 9, 274. [Google Scholar] [CrossRef]
- Joo, K.J.; Li, H.; Zhang, X.; Lerner, S.P. Therapeutic effect on bladder cancer with a conditionally replicating oncolytic virus derived from type ii herpes simplex virus. Bladder Cancer 2015, 1, 81–90. [Google Scholar] [CrossRef]
- Kilani, R.T.; Tamimi, Y.; Hanel, E.G.; Wong, K.K.; Karmali, S.; Lee, P.W.; Moore, R.B. Selective reovirus killing of bladder cancer in a co-culture spheroid model. Virus Res. 2003, 93, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Luo, C.; Goshima, F.; Nishiyama, Y.; Sata, T.; Ono, Y. Herpes simplex virus type 1 mutant hf10 oncolytic viral therapy for bladder cancer. Urology 2005, 66, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, F.; Zhai, Z.; Fu, S.; Lu, J.; Zhang, H.; Guo, H.; Hu, X.; Li, R.; Wang, Z.; et al. Synergistic effect of bladder cancer-specific oncolytic adenovirus in combination with chemotherapy. Oncol. Lett. 2017, 14, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, E.; Koll, F.; Haas, H.; Mantwill, K.; Janssen, K.P.; Laschinger, M.; Gschwend, J.; Steiger, K.; Black, P.C.; Moskalev, I.; et al. The oncolytic adenovirus xvir-n-31 as a novel therapy in muscle-invasive bladder cancer. Hum. Gene Ther. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Zhu, W.B.; Zhang, H.; Huang, W.T.; Liu, X.C.; Lin, Y.; Cai, J.; Yan, G.M.; Qiu, J.G.; et al. Suppression of ccdc6 sensitizes tumor to oncolytic virus m1. Neoplasia 2021, 23, 158–168. [Google Scholar] [CrossRef]
- Lu, C.S.; Hsieh, J.L.; Lin, C.Y.; Tsai, H.W.; Su, B.H.; Shieh, G.S.; Su, Y.C.; Lee, C.H.; Chang, M.Y.; Wu, C.L.; et al. Potent antitumor activity of oct4 and hypoxia dual-regulated oncolytic adenovirus against bladder cancer. Gene Ther. 2015, 22, 305–315. [Google Scholar] [CrossRef]
- Lu, K.; Wang, F.; Ma, B.; Cao, W.; Guo, Q.; Wang, H.; Rodriguez, R.; Wang, Z. Teratogenic toxicity evaluation of bladder cancer-specific oncolytic adenovirus on mice. Curr. Gene Ther. 2021, 21, 160–166. [Google Scholar] [CrossRef]
- Mullerad, M.; Bochner, B.H.; Adusumilli, P.S.; Bhargava, A.; Kikuchi, E.; Hui-Ni, C.; Kattan, M.W.; Chou, T.C.; Fong, Y. Herpes simplex virus based gene therapy enhances the efficacy of mitomycin c for the treatment of human bladder transitional cell carcinoma. J. Urol. 2005, 174, 741–746. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Ricca, J.M.; Gigoux, M.; Ko, B.; Redelman-Sidi, G.; Walther, T.; Liu, C.; Iyer, G.; Merghoub, T.; Wolchok, J.D.; et al. Lysis-independent potentiation of immune checkpoint blockade by oncolytic virus. Oncotarget 2018, 9, 28702–28716. [Google Scholar] [CrossRef][Green Version]
- Potts, K.G.; Irwin, C.R.; Favis, N.A.; Pink, D.B.; Vincent, K.M.; Lewis, J.D.; Moore, R.B.; Hitt, M.M.; Evans, D.H. Deletion of f4l (ribonucleotide reductase) in vaccinia virus produces a selective oncolytic virus and promotes anti-tumor immunity with superior safety in bladder cancer models. EMBO Mol. Med. 2017, 9, 638–654. [Google Scholar] [CrossRef]
- Ramesh, N.; Ge, Y.; Ennist, D.L.; Zhu, M.; Mina, M.; Ganesh, S.; Reddy, P.S.; Yu, D.C. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Rangsitratkul, C.; Lawson, C.; Bernier-Godon, F.; Niavarani, S.R.; Boudaud, M.; Rouleau, S.; Gladu-Corbin, A.O.; Surendran, A.; Ekindi-Ndongo, N.; Koti, M.; et al. Intravesical immunotherapy with a gm-csf armed oncolytic vesicular stomatitis virus improves outcome in bladder cancer. Mol. Ther. Oncolytics 2022, 24, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Reichard, K.W.; Lorence, R.M.; Cascino, C.J.; Peeples, M.E.; Walter, R.J.; Fernando, M.B.; Reyes, H.M.; Greager, J.A. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 1992, 52, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.L.; Lin, Y.P.; Shieh, G.S.; Su, C.H.; Wu, W.L.; Tsai, Y.S.; Cheng, C.W.; Lai, M.D.; Wu, C.L. Development of a conditionally replicating pseudorabies virus for her-2/neu-overexpressing bladder cancer therapy. Mol. Ther. 2007, 15, 131–138. [Google Scholar] [CrossRef]
- Terao, S.; Shirakawa, T.; Kubo, S.; Bishunu, A.; Lee, S.J.; Goda, K.; Tsukuda, M.; Hamada, K.; Tagawa, M.; Takenaka, A.; et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology 2007, 70, 1009–1013. [Google Scholar] [CrossRef]
- Van der Poel, H.G.; Molenaar, B.; van Beusechem, V.W.; Haisma, H.J.; Rodriguez, R.; Curiel, D.T.; Gerritsen, W.R. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J. Urol. 2002, 168, 266–272. [Google Scholar] [CrossRef]
- Wang, H.; Cai, Z.; Yang, F.; Luo, J.; Satoh, M.; Arai, Y.; Li, D. Enhanced antitumor efficacy of integrin-targeted oncolytic adenovirus axdadb3-f/rgd on bladder cancer. Urology 2014, 83, 508.e13–508.e19. [Google Scholar] [CrossRef]
- Wang, H.; Satoh, M.; Abe, H.; Sunamura, M.; Moriya, T.; Ishidoya, S.; Saito, S.; Hamada, H.; Arai, Y. Oncolytic viral therapy by bladder instillation using an e1a, e1b double-restricted adenovirus in an orthotopic bladder cancer model. Urology 2006, 68, 674–681. [Google Scholar] [CrossRef]
- Wu, C.L.; Shieh, G.S.; Chang, C.C.; Yo, Y.T.; Su, C.H.; Chang, M.Y.; Huang, Y.H.; Wu, P.; Shiau, A.L. Tumor-selective replication of an oncolytic adenovirus carrying oct-3/4 response elements in murine metastatic bladder cancer models. Clin. Cancer Res. 2008, 14, 1228–1238. [Google Scholar] [CrossRef]
- Zhang, J.; Ramesh, N.; Chen, Y.; Li, Y.; Dilley, J.; Working, P.; Yu, D.C. Identification of human uroplakin ii promoter and its use in the construction of CG8840, a urothelium-specific adenovirus variant that eliminates established bladder tumors in combination with docetaxel. Cancer Res. 2002, 62, 3743–3750. [Google Scholar]
- Zhao, G.Z.; Tan, W.L.; Zheng, S.B.; Wu, Y.D.; Xie, Y.; Zhu, W.H. cytotoxic effect of oncolytic virus combined with mitomycin against human bladder cancer cells in vitro and in vivo. Nan Fang Yi Ke Da Xue Xue Bao 2006, 26, 1623–1625, 1628. [Google Scholar] [PubMed]
- Wang, L.; Zhang, Y.; Zhao, J.; Xiao, E.; Lu, J.; Fu, S.; Wang, Z. Combination of bladder cancer-specific oncolytic adenovirus gene therapy with cisplatin on bladder cancer in vitro. Tumour Biol. 2014, 35, 10879–10890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, F.; Mao, C.; Zhang, Z.; Fu, S.; Lu, J.; Zhai, Z.; Li, R.; Li, S.; Rodriguez, R.; et al. Effect of combined treatment of radiation and tissue-specific recombinant oncolytic adenovirus on bladder cancer cells. Int. J. Radiat. Biol. 2017, 93, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, H.; Shen, J.; Yang, Y.; Wu, S.; Xiao, J.; Xu, Y.; Liu, X.Y.; Chu, L. Rgd-modifided oncolytic adenovirus exhibited potent cytotoxic effect on car-negative bladder cancer-initiating cells. Cell Death Dis. 2015, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Matsui, Y.; Lee, C.; Osamu, O.; Skinner, L.; Wang, J.; So, A.; Rennie, P.S.; Jia, W.W. Intravesical treatment of advanced urothelial bladder cancers with oncolytic hsv-1 co-regulated by differentially expressed micrornas. Gene Ther. 2016, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Matsui, Y.; Hadaschik, B.A.; Lee, C.; Jia, W.; Bell, J.C.; Fazli, L.; So, A.I.; Rennie, P.S. Down-regulation of type i interferon receptor sensitizes bladder cancer cells to vesicular stomatitis virus-induced cell death. Int. J. Cancer 2010, 127, 830–838. [Google Scholar] [CrossRef]
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A first in human phase 1 study of CG0070, a gm-csf expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L., 3rd; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase ii multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Shi, J.; Fu, S.; Wang, L.; Tao, Y.; Rodriguez, R.; Wang, Z. Lentivirus-mediated p21/waf-1 short hairpin rna enhances the cytotoxic effects and replicative potential of a bladder cancer-specific oncolytic adenovirus in vitro. Anticancer Drugs 2017, 28, 88–96. [Google Scholar] [CrossRef]
- Hong, B.; Sahu, U.; Mullarkey, M.P.; Kaur, B. Replication and spread of oncolytic herpes simplex virus in solid tumors. Viruses 2022, 14, 118. [Google Scholar] [CrossRef]
- Watanabe, D.; Goshima, F. Oncolytic virotherapy by hsv. Adv. Exp. Med. Biol. 2018, 1045, 63–84. [Google Scholar] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; He, H.; Wang, H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.J.; Malhotra, S.; McAuliffe, P.; Kooby, D.A.; Federoff, H.J.; Huryk, B.; Johnson, P.; Scardino, P.T.; Heston, W.D.; Fong, Y. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses g207 and nv1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J. 2001, 15, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Mineta, T.; Rabkin, S.D.; Yazaki, T.; Hunter, W.D.; Martuza, R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995, 1, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.C.; Price, K.H.; Parker, J.N.; Samuel, S.L.; Meleth, S.; Cassady, K.A.; Gillespie, G.Y.; Whitley, R.J.; Markert, J.M. Serial passage through human glioma xenografts selects for a deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors. J. Virol. 2006, 80, 7308–7315. [Google Scholar] [CrossRef]
- Enquist, L.W.; Husak, P.J.; Banfield, B.W.; Smith, G.A. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 1998, 51, 237–347. [Google Scholar]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current status and challenge of pseudorabies virus infection in china. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef]
- Jimenez, R.E.; Hussain, M.; Bianco, F.J., Jr.; Vaishampayan, U.; Tabazcka, P.; Sakr, W.A.; Pontes, J.E.; Wood, D.P., Jr.; Grignon, D.J. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: Prognostic significance and comparative analysis in primary and metastatic tumors. Clin. Cancer Res. 2001, 7, 2440–2447. [Google Scholar]
- Su, W.P.; Tu, I.H.; Hu, S.W.; Yeh, H.H.; Shieh, D.B.; Chen, T.Y.; Su, W.C. Her-2/neu raises shp-2, stops ifn-gamma anti-proliferation in bladder cancer. Biochem. Biophys. Res. Commun. 2007, 356, 181–186. [Google Scholar] [CrossRef]
- Bradley, S.; Jakes, A.D.; Harrington, K.; Pandha, H.; Melcher, A.; Errington-Mais, F. Applications of coxsackievirus A21 in oncology. Oncolytic Virotherapy 2014, 3, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Relph, K.; Annels, N.; Smith, C.; Kostalas, M.; Pandha, H. Oncolytic immunotherapy for bladder cancer using coxsackie A21 virus: Using a bladder tumor precision-cut slice model system to assess viral efficacy. Methods Mol. Biol. 2020, 2058, 249–259. [Google Scholar] [PubMed]
- Annels, N.E.; Mansfield, D.; Arif, M.; Ballesteros-Merino, C.; Simpson, G.R.; Denyer, M.; Sandhu, S.S.; Melcher, A.A.; Harrington, K.J.; Davies, B.; et al. Phase i trial of an icam-1-targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin. Cancer Res. 2019, 25, 5818–5831. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cao, W.; Salawudeen, A.; Zhu, W.; Emeterio, K.; Safronetz, D.; Banadyga, L. Vesicular stomatitis virus: From agricultural pathogen to vaccine vector. Pathogens 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type i interferon in chronic virus infection and cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, K.; Bourgeois-Daigneault, M.C. The pros and cons of interferons for oncolytic virotherapy. Cytokine Growth Factor Rev. 2020, 56, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type i interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.D.; tenOever, B.R.; Paterson, J.M.; Power, A.T.; Knowles, S.; Marius, R.; Reynard, J.; Poliquin, L.; Atkins, H.; et al. Vsv strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003, 4, 263–275. [Google Scholar] [CrossRef]
- Lundstrom, K. Oncolytic alphaviruses in cancer immunotherapy. Vaccines 2017, 5, 9. [Google Scholar] [CrossRef]
- Wen, J.S.; Zhao, W.Z.; Liu, J.W.; Zhou, H.; Tao, J.P.; Yan, H.J.; Liang, Y.; Zhou, J.J.; Jiang, L.F. Genomic analysis of a chinese isolate of getah-like virus and its phylogenetic relationship with other alphaviruses. Virus Genes 2007, 35, 597–603. [Google Scholar] [CrossRef]
- Cai, J.; Yan, G. The identification and development of a novel oncolytic virus: Alphavirus m1. Hum. Gene Ther. 2021, 32, 138–149. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Qiu, J.; Lin, Y.; Liang, J.; Xiao, X.; Fu, L.; Wang, F.; Cai, J.; Tan, Y.; et al. Activation of cyclic adenosine monophosphate pathway increases the sensitivity of cancer cells to the oncolytic virus m1. Mol. Ther. 2016, 24, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Muller, L.; Berkeley, R.; Barr, T.; Ilett, E.; Errington-Mais, F. Past, present and future of oncolytic reovirus. Cancers 2020, 12, 3219. [Google Scholar] [CrossRef]
- Gong, J.; Sachdev, E.; Mita, A.C.; Mita, M.M. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J. Methodol. 2016, 6, 25–42. [Google Scholar] [CrossRef]
- Colamonici, O.R.; Domanski, P.; Sweitzer, S.M.; Larner, A.; Buller, R.M. Vaccinia virus b18r gene encodes a type i interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 1995, 270, 15974–15978. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.S.; Yoo, S.Y. Oncolytic vaccinia virus in lung cancer vaccines. Vaccines 2022, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Al Yaghchi, C.; Zhang, Z.; Alusi, G.; Lemoine, N.R.; Wang, Y. Vaccinia virus, a promising new therapeutic agent for pancreatic cancer. Immunotherapy 2015, 7, 1249–1258. [Google Scholar] [CrossRef]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The rigvir(r) story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef]
- Wei, D.; Xu, J.; Liu, X.Y.; Chen, Z.N.; Bian, H. Fighting cancer with viruses: Oncolytic virus therapy in china. Hum. Gene Ther. 2018, 29, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.S.; Hecht, J.R.; Chan, E. Talimogene laherparepvec: Review of its mechanism of action and clinical efficacy and safety. Immunotherapy 2019, 11, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Yoo, J.Y.; Shimizu, T.; Kurozumi, K.; Date, I.; Kaur, B. Implications of immune cells in oncolytic herpes simplex virotherapy for glioma. Brain Tumor Pathol. 2022, 39, 57–64. [Google Scholar] [CrossRef]
- OH2 Oncolytic Viral Therapy in Non-Muscle-Invasive Bladder Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05232136 (accessed on 15 November 2022).
- Wang, Y.; Jin, J.; Wu, Z.; Hu, S.; Hu, H.; Ning, Z.; Li, Y.; Dong, Y.; Zou, J.; Mao, Z.; et al. Stability and anti-tumor effect of oncolytic herpes simplex virus type 2. Oncotarget 2018, 9, 24672–24683. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Salazar, R.; Duran, I.; Osman-Garcia, I.; Paz-Ares, L.; Bozada, J.M.; Boni, V.; Blanc, C.; Seymour, L.; Beadle, J.; et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 2017, 5, 71. [Google Scholar] [CrossRef]
- Trial of Intravesical Measles Virotherapy in Patients with Bladder Cancer Who Are Undergoing Radical Cystectomy. Available online: https://clinicaltrials.gov/ct2/show/NCT03171493 (accessed on 15 November 2022).
- OH2 Oncolytic Viral Therapy in Advanced Bladder Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05248789 (accessed on 15 November 2022).
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of oncolytic virotherapy: From genetic modification to combination therapy. Front. Med. 2020, 14, 160–184. [Google Scholar] [CrossRef]
- Pandha, H.; Harrington, K.; Ralph, C.; Melcher, A.; Gupta, S.; Akerley, W.; Sandborn, R.E.; Rudin, C.; Rosenberg, J.; Kaufman, D.; et al. Phase 1b keynote 200 (storm study): A study of an intravenously delivered oncolytic virus, coxsackievirus A21 in combination with pembrolizumab in advanced cancer patients. Cancer Res. 2017, 77, CT115. [Google Scholar] [CrossRef]
- Bhindi, B.; Kool, R.; Kulkarni, G.S.; Siemens, D.R.; Aprikian, A.G.; Breau, R.H.; Brimo, F.; Fairey, A.; French, C.; Hanna, N.; et al. Canadian urological association guideline on the management of non-muscle-invasive bladder cancer—Full-text. Can. Urol. Assoc. J. 2021, 15, E424–E460. [Google Scholar]
- Yano, S.; Tazawa, H.; Hashimoto, Y.; Shirakawa, Y.; Kuroda, S.; Nishizaki, M.; Kishimoto, H.; Uno, F.; Nagasaka, T.; Urata, Y.; et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells in s/g2/m phases. Clin. Cancer Res. 2013, 19, 6495–6505. [Google Scholar] [CrossRef]
- Roulstone, V.; Twigger, K.; Zaidi, S.; Pencavel, T.; Kyula, J.N.; White, C.; McLaughlin, M.; Seth, R.; Karapanagiotou, E.M.; Mansfield, D.; et al. Synergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapy. Gene Ther. 2013, 20, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Hossain, E.; Yanagawa-Matsuda, A.; Chowdhury, A.; Tsuda, M.; Zaman, A.U.; Tanaka, S.; Higashino, F. Cisplatin relocalizes rna binding protein hur and enhances the oncolytic activity of e4orf6 deleted adenovirus. Cancers 2020, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current best practice for bladder cancer: A narrative review of diagnostics and treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Vassaux, G.; Harrington, K.J. Oncolytic viruses in radiation oncology. Radiother. Oncol. 2011, 99, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Audisio, A.; Buttigliero, C.; Delcuratolo, M.D.; Parlagreco, E.; Audisio, M.; Ungaro, A.; Di Stefano, R.F.; Di Prima, L.; Turco, F.; Tucci, M. New perspectives in the medical treatment of non-muscle-invasive bladder cancer: Immune checkpoint inhibitors and beyond. Cells 2022, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune checkpoint inhibitors as a neoadjuvant/adjuvant treatment of muscle-invasive bladder cancer: A systematic review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef]
- Marchini, A.; Scott, E.M.; Rommelaere, J. Overcoming barriers in oncolytic virotherapy with hdac inhibitors and immune checkpoint blockade. Viruses 2016, 8, 9. [Google Scholar] [CrossRef]
- Shi, T.; Song, X.; Wang, Y.; Liu, F.; Wei, J. Combining oncolytic viruses with cancer immunotherapy: Establishing a new generation of cancer treatment. Front. Immunol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Hwang, J.K.; Hong, J.; Yun, C.O. Oncolytic viruses and immune checkpoint inhibitors: Preclinical developments to clinical trials. Int. J. Mol. Sci. 2020, 21, 8627. [Google Scholar] [CrossRef]
- Chiu, M.; Armstrong, E.J.L.; Jennings, V.; Foo, S.; Crespo-Rodriguez, E.; Bozhanova, G.; Patin, E.C.; McLaughlin, M.; Mansfield, D.; Baker, G.; et al. Combination therapy with oncolytic viruses and immune checkpoint inhibitors. Expert Opin. Biol. Ther. 2020, 20, 635–652. [Google Scholar] [CrossRef]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic viruses and immune checkpoint inhibition: The best of both worlds. Mol. Ther. Oncolytics 2019, 13, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. Immunother. Cancer 2021, 9, e002723. [Google Scholar] [CrossRef] [PubMed]
- Vishwasrao, P.; Li, G.; Boucher, J.C.; Smith, D.L.; Hui, S.K. Emerging car t cell strategies for the treatment of aml. Cancers 2022, 14, 1241. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.A.; Bazargan, S.; Pilon-Thomas, S.; Poch, M.; Chahoud, J. Current state of cell therapies for genitourinary malignancies. Cancer J. 2022, 28, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, cars and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Alemany, R. Car-t cells and oncolytic viruses: Joining forces to overcome the solid tumor challenge. Front. Immunol. 2018, 9, 2460. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.; Gao, Y.; Liu, K.; Xu, B.; Qin, W. The clinical significance and prognostic value of her2 expression in bladder cancer: A meta-analysis and a bioinformatic analysis. Front. Oncol. 2021, 11, 653491. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, S.; Shu, Q.; Wu, S. Strategies to get drugs across bladder penetrating barriers for improving bladder cancer therapy. Pharmaceutics 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Memarzadeh, B.; Ge, Y.; Frey, D.; VanRoey, M.; Rojas, V.; Yu, D.C. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Mol. Ther. 2004, 10, 697–705. [Google Scholar] [CrossRef]
- Tang, B.; Guo, Z.S.; Bartlett, D.L.; Liu, J.; McFadden, G.; Shisler, J.L.; Roy, E.J. A cautionary note on the selectivity of oncolytic poxviruses. Oncolytic Virother. 2019, 8, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Negahdari, B.; Ghorghanlu, S.; Jahangiri, S.; Arashkia, A. Strategies for enhancing intratumoral spread of oncolytic adenoviruses. Pharmacol. Ther. 2020, 213, 107586. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Calistri, A.; Altomonte, J. Giving oncolytic viruses a free ride: Carrier cells for oncolytic virotherapy. Pharmaceutics 2021, 13, 2192. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fukuhara, H.; Yamamoto, S.; Karashima, T.; Kurabayashi, A.; Furihata, M.; Hanazaki, K.; Lai, H.W.; Ogura, S.I. Current status of photodynamic technology for urothelial cancer. Cancer Sci. 2022, 113, 392–398. [Google Scholar] [CrossRef]
- Kubrak, T.; Karakula, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in management of bladder cancer-the role of photodynamic therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef]
- Takehara, K.; Tazawa, H.; Okada, N.; Hashimoto, Y.; Kikuchi, S.; Kuroda, S.; Kishimoto, H.; Shirakawa, Y.; Narii, N.; Mizuguchi, H.; et al. Targeted photodynamic virotherapy armed with a genetically encoded photosensitizer. Mol. Cancer Ther. 2016, 15, 199–208. [Google Scholar] [CrossRef]
- Takehara, K.; Yano, S.; Tazawa, H.; Kishimoto, H.; Narii, N.; Mizuguchi, H.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Eradication of melanoma in vitro and in vivo via targeting with a killer-red-containing telomerase-dependent adenovirus. Cell Cycle 2017, 16, 1502–1508. [Google Scholar] [CrossRef][Green Version]
- Yano, S.; Tazawa, H.; Kishimoto, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Real-time fluorescence image-guided oncolytic virotherapy for precise cancer treatment. Int. J. Mol. Sci. 2021, 22, 879. [Google Scholar] [CrossRef]
- Quillien, L.; Top, S.; Kappler-Gratias, S.; Redoute, A.; Dusetti, N.; Quentin-Froignant, C.; Lulka, H.; Camus-Bouclainville, C.; Buscail, L.; Gallardo, F.; et al. A novel imaging approach for single-cell real-time analysis of oncolytic virus replication and efficacy in cancer cells. Hum. Gene Ther. 2021, 32, 166–177. [Google Scholar] [CrossRef]
| Parent Virus | Oncolytic Viruses | Virus Description | Reference |
|---|---|---|---|
| Adenovirus | CG8840 | An adenovirus variant engineered with a UII promoter that can drive E1A and E1B expression | [60] |
| Ad5-d55K; Ad5-d24 | Serotype-5 adenoviruses that have been weakened to selectively replicate in p53-deficient (Ad5-d55K) cells or retinoblastoma pRb (Ad5-d24) cells | [56] | |
| ONYX-015 | Adenovirus that has had its E1B 55-kDa gene removed and has been modified to replicate only in and lyse the cancer cells lacking p53 | [61] | |
| AxdAdB-3 | A double-restricted oncolytic virus that carries an E1A mutation and an E1B-55KD deletion | [58] | |
| CG0070 | Adenoviruses armed with GM-CSF | [51] | |
| Ad-MK-E1a | A conditionally replicating and MK promoter-regulated adenovirus | [55] | |
| Ad.9OC | Nine copies of Oct-3/4 response element-derived adenovirus with the E1B-55 kDa deletion | [59] | |
| Ad5F35/MKp-E1 | A conditionally replicating chimeric adenovirus that has the E1 gene under the control of the human midkine promoter and replaces the fiber knob on Ad5 with that on Ad35 | [36] | |
| Ad/PSCAE/UPII/E1A | An adenovirus with the human UPII promoter PSCAE-regulated E1A gene | [62] | |
| [63] | |||
| AxdAdB3-F/RGD | An RGD-fiber modified oncolytic adenovirus with E1A and E1B mutations | [57] | |
| Onco(Ad).RGD-hTERT-TRAIL | An RGD-fiber modified oncolytic adenovirus carrying TNF-related apoptosis-inducing ligand gene and EGFP | [64] | |
| AdLCY | A hypoxia and Oct4 dual-regulated oncolytic adenovirus | [46] | |
| Ad5-UPII-E1A | An adenovirus of serotype 5 (Ad5) with the expression of E1A regulated by the uroplakin II (UPII) promoter | [43] | |
| Ad5/F11p-PSCAE-UPII-E1A | This adenovirus targets BC with its PSCAE and UPII promoters and possesses a chimeric fiber gene that codes for the Ad11p fiber shaft and knob domains, as well as the Ad5 fiber tail domain. | [35] | |
| XVir-N-31 | Also known as Ad-Delo3-RGD, the virus possesses a deletion in the E1A CR3-region, as well as one in the E1B19k gene and the E3 area. There is an RGD motif on the fiber knob. | [44] | |
| Ad/PSCAE/UPII/E1A-AR | An adenovirus that targets BC and carries E1A-AR controlled by UPII and PSCAE promoters | [47] | |
| Alphavirus | M1 | A Getah-like virus with a positive single-strand RNA genome | [39,45] |
| Coxsackievirus | CVA21 | A novel ICAM-1 targeted immunotherapeutic virus | [34] |
| HSV-1 | NV1066 | A mutant form of the HSV-1 that has been attenuated, produces green fluorescent protein, and lacks the viral genes ICP0 and ICP4. | [48] |
| HF10 | A significantly attenuated, replication-capable variant of HSV-1 | [42] | |
| oHSV-1 | A HSV-1 mutant that expresses endogenous microR124 and microR143 | [65] | |
| HSV-2 | FusOn-H2 | A HSV-2 mutant that has had the protein kinase domain removed and specifically targets BC cells by activating the Ras signaling system | [40] |
| Pseudorabies virus | YP2 | A mutant of the pseudorabies virus with the HSV-1 thymidine kinase and glycoprotein D genes | [54] |
| Newcastle disease virus | 73-T strain | 73-T strain | [53] |
| LaSota strain | Recombinant lentogenic NDV LaSota strain | [49] | |
| Vaccinia virus | ∆F4L VACV | F4L-deleted vaccinia virus | [50] |
| Reovirus | Reovirus | A double-stranded RNA virus with an icosahedral capsid and no envelope | [38,41] |
| Vesicular stomatitis virus | AV3 | A mutant Delta51M variant | [37] |
| AV3 | A mutant Delta51M variant encoding a green fluorescent protein | [66] | |
| VSVd51-hGM-CSF | VSV carring the human GM-CSF transgene | [52] |
| Tumor Type | Virus (Alias) | Parent Virus | Phase | Delivery | Therapy | Status | Results | Trial Identifier |
|---|---|---|---|---|---|---|---|---|
| NMIBC | CAVATAK | Adenovirus | I | IVT | monotherapy/combined MMC before TURBT | Completed | Caused noticeable inflammatory alterations in tissue biopsies of NMIBC; no patient reported experiencing any significant toxicities; CR: 1/15 | NCT02316171 |
| BCG unresponsive NMIBC | CG0070 | Adenovirus | I | IVT | monotherapy | Completed | 35 pts included; CR: 48.6%; median duration of CR: 10.4 months. Grade 1–2 bladder toxicities were the most common adverse events observed. | NCT00109655 |
| BCG unresponsive NMIBC | CG0070 | Adenovirus | II | IVT | monotherapy | Completed | 6-month overall CR: 47%; patients with CIS CR: 50%; acceptable level of toxicity | NCT02365818 |
| BCG unresponsive NMIBC | CG0070 | Adenovirus | II | IVT | combining pembrolizumab | Recruiting | NA | NCT04387461 |
| BCG unresponsive NMIBC | CG0070 | Adenovirus | III | IVT | monotherapy | Recruiting | NA | NCT04452591 |
| HG NMIBC failed first-line prophylactic intravesical therapy. | OH2 | HSV-2 | I/II | IVT | monotherapy | Recruiting | NA | NCT05232136 |
| BCG-refractory NMIBC or MIBC undergoes radical cystectomy | MV-NIS | Measles virus | I | IVT | monotherapy as neoadjuvant therapy | Recruiting | NA | NCT03171493 |
| Resectable MIBC | Enadenotucirev | Adenovirus | I | IV | monotherapy as neoadjuvant therapy | Completed | Following IV administration, enadenotucirev was found in the majority of tumor samples and caused significant local CD8+ cell infiltration in 80% of the tumor samples evaluated. | NCT02053220 |
| Advanced BC | Enadenotucirev | Adenovirus | I/II | IV | monotherapy | Completed | Enadenotucirev can be administered in single/repeated cycles with manageable tolerability. | NCT02028442 |
| Advanced BC | CVA21 | Coxsackievirus | I | IV | monotherapy/combining pembrolizumab | Completed | Combination therapy was generally well tolerated; median OS: 11.2 mos; ORR: 31% (8/26, 3CR + 5PR); notable increase in PD-L1+ tumor cells: 62% (8/13). | NCT02043665 |
| Advanced BC | CAdVEC | Adenovirus | I | IT | combining chimeric antigen receptor T cells | Recruiting | NA | NCT03740256 |
| Advanced BC | OH2 | HSV-2 | II | IT | monotherapy | Recruiting | NA | NCT05248789 |
| Advanced BC | PF-07263689 | Vaccinia virus | I | IV | monotherapy/combining sasanlimab | Recruiting | NA | NCT05061537 |
| Advanced BC | YSCH-01 | Adenovirus | I | IT | monotherapy | Recruiting | NA | NCT05180851 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Xia, Q.; Hu, J.; Wang, S. Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects. J. Clin. Med. 2022, 11, 6997. https://doi.org/10.3390/jcm11236997
Hu H, Xia Q, Hu J, Wang S. Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects. Journal of Clinical Medicine. 2022; 11(23):6997. https://doi.org/10.3390/jcm11236997
Chicago/Turabian StyleHu, Henglong, Qidong Xia, Jia Hu, and Shaogang Wang. 2022. "Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects" Journal of Clinical Medicine 11, no. 23: 6997. https://doi.org/10.3390/jcm11236997
APA StyleHu, H., Xia, Q., Hu, J., & Wang, S. (2022). Oncolytic Viruses for the Treatment of Bladder Cancer: Advances, Challenges, and Prospects. Journal of Clinical Medicine, 11(23), 6997. https://doi.org/10.3390/jcm11236997






