Factors That Affect Survival Outcomes in Patients with Endometrial Clear Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations of Our Study
4.2. Conclusions and Implications for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ASCO. Uterine Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/uterine-cancer/statistics (accessed on 13 August 2022).
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Lorusso, D.; Coleman, R.; et al. Clear cell carcinoma of the endometrium. Gynecol. Oncol. 2022, 164, 658–666. [Google Scholar] [CrossRef]
- Guyon, F.; Stoeckle, E.; Thomas, L.; Petit, A.; Sire, M.; Floquet, A. Advanced endometrial carcinoma: Primary debulking surgery or neoadjuvant chemotherapy? Bull. Cancer 2012, 99, 43–49. [Google Scholar] [CrossRef]
- Albright, B.B.; Monuszko, K.A.; Kaplan, S.J.; Davidson, B.A.; Moss, H.A.; Huang, A.B.; Melamed, A.; Wright, J.D.; Havrilesky, L.J.; Previs, R.A. Primary cytoreductive surgery for advanced stage endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 225, e231–e237.e224. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Bradley, K.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines® Insights: Uterine Neoplasms, Version 3.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 888–895. [Google Scholar] [CrossRef]
- Loizzi, V.; Cicinelli, E.; Lepera, A.; Legge, F.; Memmola, M.; Chiarello, G.; Arezzo, F.; Del Vecchio, V.; Resta, L.; Cormio, G. Prognostic factors in clear cell carcinoma of endometrium: Analysis of 55 cases. Acta Biomed 2022, 92, e2021362. [Google Scholar]
- Abdulfatah, E.; Sakr, S.; Thomas, S.; Al-Wahab, Z.; Mutch, D.G.; Dowdy, S.; Bandyopadhyay, S.; Munkarah, A.; Elshaikh, M.; Morris, R.; et al. Clear Cell Carcinoma of the Endometrium: Evaluation of Prognostic Parameters in a Multi-institutional Cohort of 165 Cases. Int. J. Gynecol. Cancer 2017, 27, 1714–1721. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Cheung, M.K.; Osann, K.; Chen, L.; Teng, N.N.; Longacre, T.A.; Powell, M.A.; Hendrickson, M.R.; Kapp, D.S.; Chan, J.K. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer 2006, 94, 642–646. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Bao, Z.; Zeng, L.; Yao, J.; Chai, D.; Li, T. Clear Cell Carcinoma of the Endometrium: Evaluation of Prognostic Parameters in 27 Cases. Front. Oncol. 2021, 11, 732782. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shrestha, A.; Maskey, N.; Dong, X.; Zheng, Z.; Yang, F.; Wang, R.; Ma, W.; Liu, J.; Li, C.; et al. Recent Trends in the Incidence of Clear Cell Adenocarcinoma and Survival Outcomes: A SEER Analysis. Front. Endocrinol. 2022, 13, 762589. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hanna, R.K.; Elshaikh, M.A. Adjuvant therapy of uterine clear cell carcinoma: A review. Arch. Gynecol. Obstet. 2016, 293, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Nieto, K.; Adams, W.; Pham, N.; Block, A.M.; Grover, S.; Small, W., Jr.; Harkenrider, M.M. Adjuvant therapy in patients with clear cell endometrial carcinoma: An analysis of the National Cancer Database. Gynecol. Oncol. 2018, 148, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; English, D.P.; Kidd, E.A. National patterns of care and cancer-specific outcomes of adjuvant treatment in patients with serous and clear cell endometrial carcinoma. Gynecol. Oncol. 2019, 152, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.M.; Bouchard-Fortier, G.; Bernardini, M.Q.; Atenafu, E.G.; Han, G.; Vicus, D.; Ferguson, S.E.; Gien, L.T. Uterine Clear Cell Carcinoma: Does Adjuvant Chemotherapy Improve Outcomes? Int. J. Gynecol. Cancer 2017, 27, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Leath, C.A., 3rd. Contemporary management of uterine clear cell carcinoma: A Society of Gynecologic Oncology (SGO) review and recommendation. Gynecol. Oncol. 2019, 155, 365–373. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Santoro, A.; Raimondo, D.; Angelico, G.; Valente, M.; Arciuolo, D.; Scaglione, G.; D’Alessandris, N.; Casadio, P.; et al. Clear cell endometrial carcinomas with mismatch repair deficiency have a favorable prognosis: A systematic review and meta-analysis. Gynecol. Oncol. 2021, 162, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S.; Spirito, N.; Cionini, L. Clear cell carcinoma of the endometrium: A biological and clinical enigma. Anticancer Res. 2010, 30, 1327–1334. [Google Scholar] [PubMed]
- Kim, S.R.; Cloutier, B.T.; Leung, S.; Cochrane, D.; Britton, H.; Pina, A.; Storness-Bliss, C.; Farnell, D.; Huang, L.; Shum, K.; et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol. Oncol. 2020, 158, 3–11. [Google Scholar] [CrossRef] [PubMed]

| Patient, Tumor and Operative Characteristics | |
|---|---|
| Age | 68 (60–76) |
| BMI | 31 (25–35) |
| Stage | |
| I | 18 (33%) |
| II | 8 (14.5%) |
| III | 20 (36%) |
| IV | 9 (16.5%) |
| Malignant ascites | 17 (31%) |
| Intrauterine tumor diameter (cm) | 4 (3–7) |
| LVSI | 39 |
| Upper abdominal surgery | 9 |
| Chemotherapy | 48 |
| Radiotherapy | 47 |
| Bowel excision | 5 |
| Partial peritonectomy | 8 |
| Residual tumor | 7 |
| Declined post-recurrence chemotherapy | 9 |
| Parameter | Progression Free Survival (95% CI) | p -Value | Overall Survival Months (95% CI) | p -Value | Hazard Ratio 95% CI PFS | p -Value | Hazard Ratio 95% CI OS | p -Value |
|---|---|---|---|---|---|---|---|---|
| Stage | ||||||||
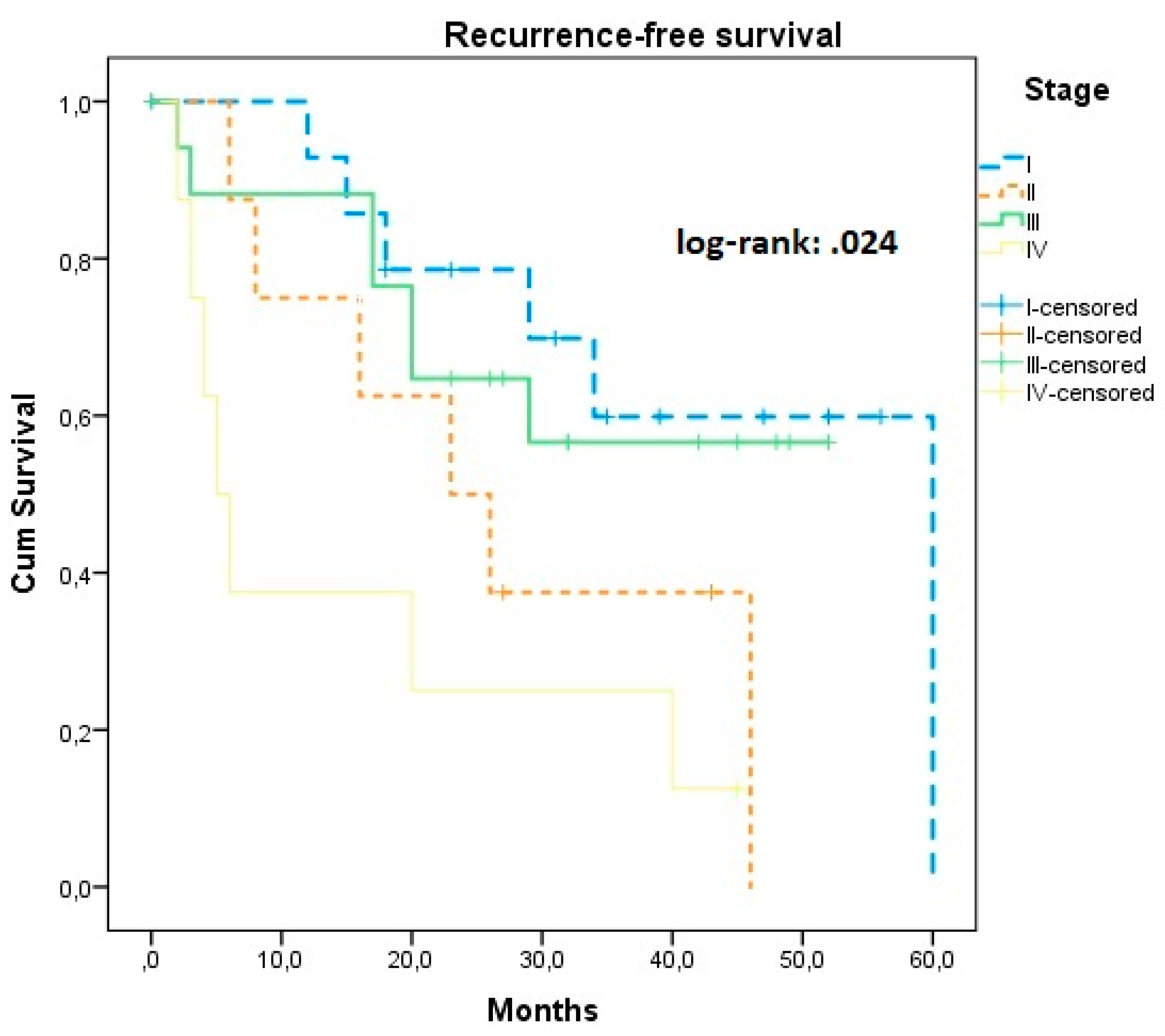
| I | 45.05 (33.59, 55.52) | 56.15 (44.14, 68.16) | Ref | Ref | ||||
| II | 30.04 (18.32, 42.47) | 0.024 | 47.00 (25.71, 68.28) | 0.048 | 2.42 (0.29, 20.23) | 0.065 | 1.07 (0.12, 9.83) | 0.079 |
| III | 36.43 (27.32, 45.55) | 39.97 (31.78, 48.16) | 1.16 (0.14, 9.51) | 0.89 (0.11, 7.41) | ||||
| IV | 15.62 (4.23, 27.02) * | 0.007 * | 32.56 (15.11, 40.02) | 4.34 (1.52, 35.69) * | 0.037 | 3.54 (1.81, 7.25) | 0.033 * | |
| Malignant ascites | ||||||||
| Absent | 34.68 (27.59, 41.76) | 0.248 | 43.82 (36.02, 51.62) | 0.487 | Ref | 0.257 | Ref | 0.490 |
| Present | 30.43 (19.38, 4.48) | 44.61 (33.67, 55.54) | 1.58 (0.72, 3.49) | 1.38 (0.55, 3.44) | ||||
| Tumor diameter | - | - | 1.08 (1.02, 1.19) | 1.10 (0.99, 1.21) | ||||
| LVSI | ||||||||
| Negative | 39.40 (26.73, 52.08) | 0.642 | 47.45 (34.60, 60.29) | 0.744 | Ref | Ref | ||
| Positive | 32.04 (25.59, 38.48) | 46.81 (37.18, 56.44) | 0.82 (0.36, 1.90) | 0.641 | 1.15 (0.48, 2.79) | 0.752 | ||
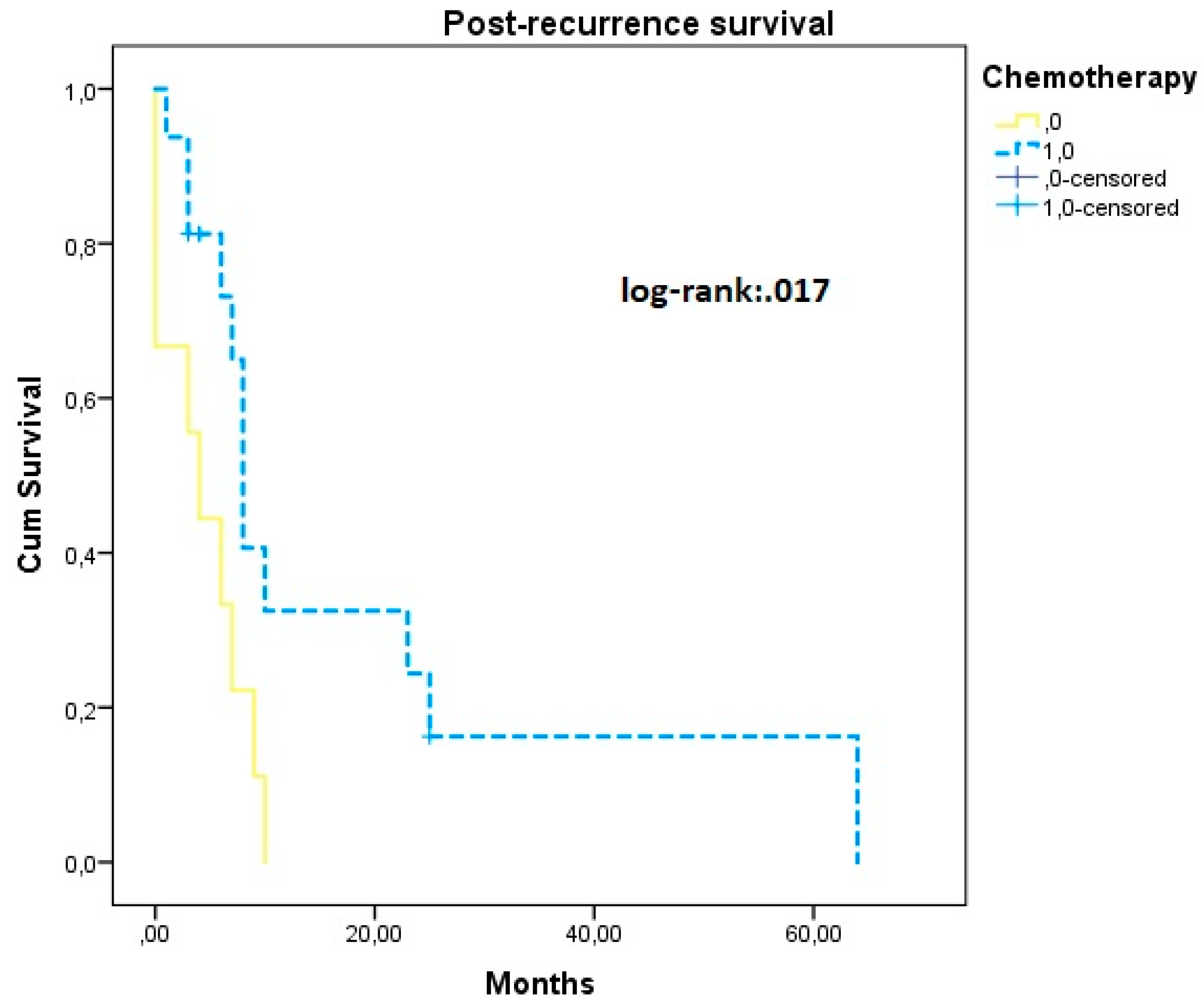
| Chemotherapy | ||||||||
| Yes | 36.22 (28.94, 32.50) | 0.825 | 47.36 (39.22, 55.52) | 0.662 | Ref | Ref | ||
| No | 33.75 (18.72, 45.77) | 45.56 (28.40, 57.71 | 1.53 (1.02, 3.30) * | 0.048 | 1.31 (0.38, 4.57) | 0.654 | ||
| Radiotherapy | ||||||||
| Yes | 39.54 (31.85, 47.25) | 0.095 | 43.84 (34.16, 53.51) | 0.637 | Ref | Ref | ||
| No | 32.14 (23.54, 40.73) | 44.01 (37.99, 50.04) | 0.89 (0.41, 1.16) | 0.090 | 0.54 (0.19, 1.52) | 0.227 | ||
| Residual tumor | ||||||||
| Yes | 15.56 (5.70, 25.41) | <0.001 | 40.56 (26.63, 57.48) | 0.521 | Ref | Ref | ||
| No | 40.14 (32.99, 47.29) * | 47.43 (38.61, 56.26) | 4.04 (1.70, 9.57) * | 0.004 | 1.38 (0.50, 3.81) | 0.547 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergialiotis, V.; Haidopoulos, D.; Christodoulou, T.; Rodolakis, I.; Prokopakis, I.; Liontos, M.; Rodolakis, A.; Thomakos, N. Factors That Affect Survival Outcomes in Patients with Endometrial Clear Cell Carcinoma. J. Clin. Med. 2022, 11, 6931. https://doi.org/10.3390/jcm11236931
Pergialiotis V, Haidopoulos D, Christodoulou T, Rodolakis I, Prokopakis I, Liontos M, Rodolakis A, Thomakos N. Factors That Affect Survival Outcomes in Patients with Endometrial Clear Cell Carcinoma. Journal of Clinical Medicine. 2022; 11(23):6931. https://doi.org/10.3390/jcm11236931
Chicago/Turabian StylePergialiotis, Vasilios, Dimitrios Haidopoulos, Theano Christodoulou, Ioannis Rodolakis, Ioannis Prokopakis, Michalis Liontos, Alexandros Rodolakis, and Nikolaos Thomakos. 2022. "Factors That Affect Survival Outcomes in Patients with Endometrial Clear Cell Carcinoma" Journal of Clinical Medicine 11, no. 23: 6931. https://doi.org/10.3390/jcm11236931
APA StylePergialiotis, V., Haidopoulos, D., Christodoulou, T., Rodolakis, I., Prokopakis, I., Liontos, M., Rodolakis, A., & Thomakos, N. (2022). Factors That Affect Survival Outcomes in Patients with Endometrial Clear Cell Carcinoma. Journal of Clinical Medicine, 11(23), 6931. https://doi.org/10.3390/jcm11236931






