Combination of Bone-Modifying Agents with Immunotarget Therapy for Hepatocellular Carcinoma with Bone Metastases
Abstract
:1. Background
2. Methods
2.1. Study Design and Patients
2.2. Study Treatment and Basis for Grouping
2.3. Data Collection and Statistics
3. Results
3.1. Patient Baseline Characteristics
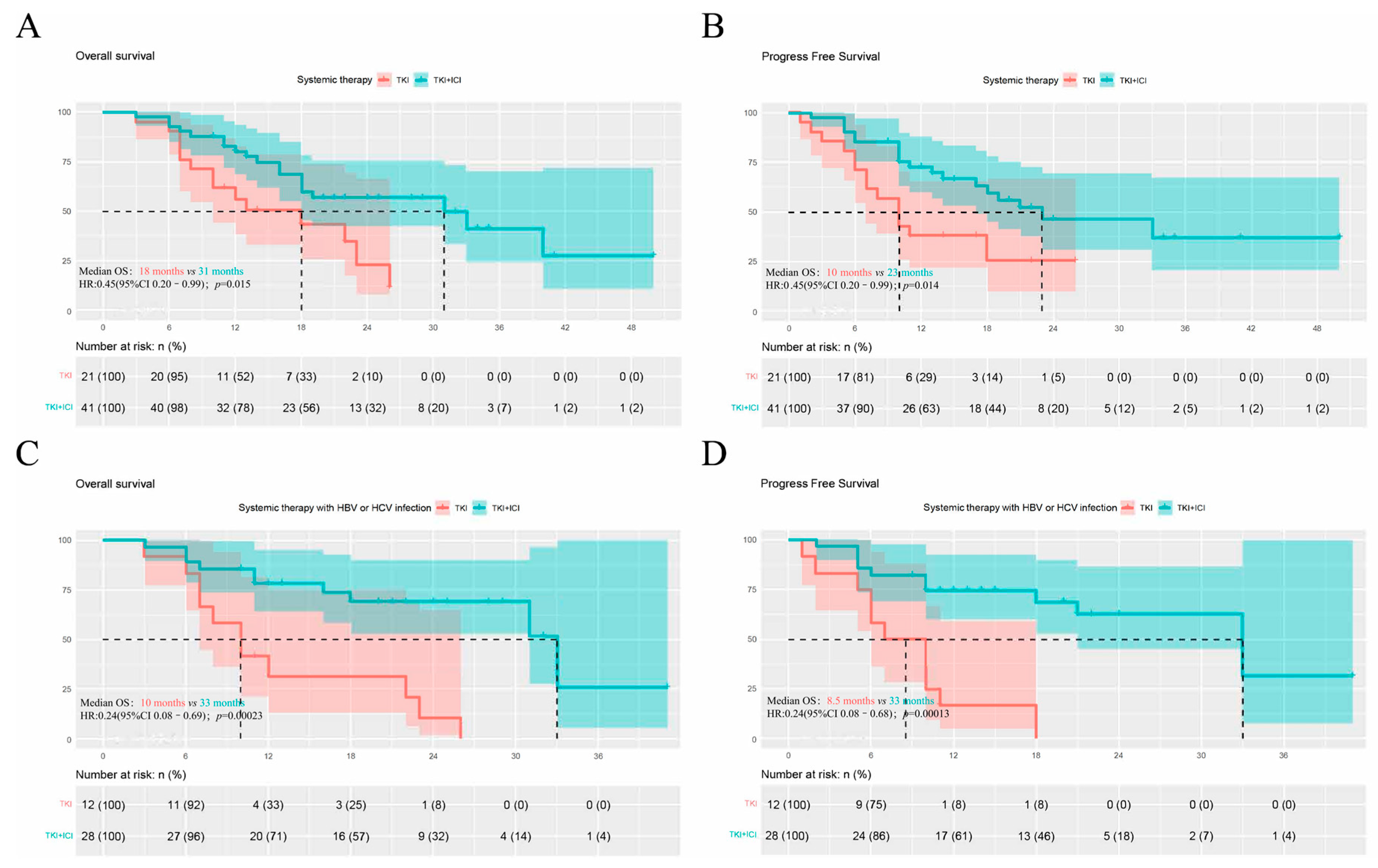
3.2. Outcomes of TKIs and TKIs Plus ICIs
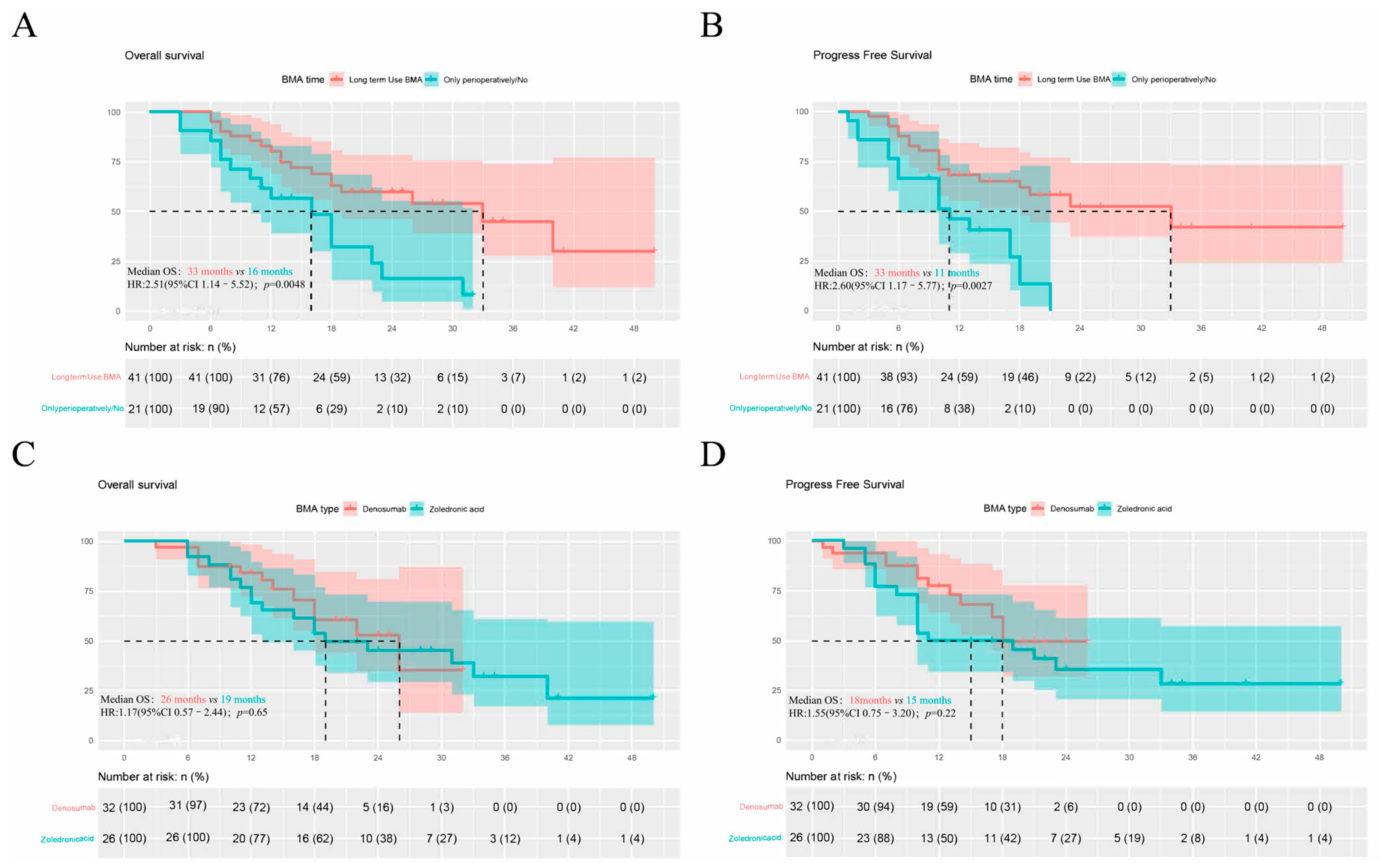
3.3. Outcomes of BMAs as Adjuvant Therapy in Systemic Regimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Pantano, F.; Riccardi, F.; Di Costanzo, G.G.; Addeo, R.; Guida, F.M.; Ceruso, M.S.; Barni, S.; Bertocchi, P.; Marinelli, S.; et al. Natural history of malignant bone disease in hepatocellular carcinoma: Final results of a multicenter bone metastasis survey. PLoS ONE 2014, 9, e105268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, J.J.; Abu-Zeinah, G.; Chou, J.F.; Owen, D.H.; Ly, M.; Lowery, M.A.; Capanu, M.; Do, R.; Kemeny, N.E.; O’Reilly, E.M.; et al. Frequency, Morbidity, and Mortality of Bone Metastases in Advanced Hepatocellular Carcinoma. J. Natl. Compr. Cancer Netw. JNCCN 2018, 16, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 541–565. [Google Scholar]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schiöth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nature reviews. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2022 update. Pharmacol. Res. 2022, 175, 106037. [Google Scholar]
- Zhao, L.; Chang, N.; Shi, L.; Li, F.; Meng, F.; Xie, X.; Xu, Z.; Wang, F. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: A retrospective, real-world study. Heliyon 2022, 8, e09538. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, P.; Wang, K.; Xiao, S.; Cheng, Y.; Li, X.; Wang, B.; Li, J.; Yu, W.; Cheng, Y. Efficacy and safety of lenvatinib monotreatment and lenvatinib-based combination therapy for patients with unresectable hepatocellular carcinoma: A retrospective, real-world study in China. Cancer Cell Int. 2021, 21, 503. [Google Scholar]
- Wu, Y.; Lin, H.; You, X.; Guo, T.; Sun, T.; Xu, H.; Fu, X. Immune Checkpoint Blockade in Chinese Patients With Hepatocellular Carcinoma: Characteristics and Particularity. Front. Oncol. 2022, 12, 764923. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liede, A.; Hernandez, R.K.; Wade, S.W.; Bo, R.; Nussbaum, N.C.; Ahern, E.; Dougall, W.C.; Smyth, M.J. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology 2018, 7, e1480301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Sigl, V.; Wimmer, R.A.; Novatchkova, M.; Jais, A.; Wagner, G.; Handschuh, S.; Uribesalgo, I.; Hagelkruys, A.; Kozieradzki, I.; et al. RANK rewires energy homeostasis in lung cancer cells and drives primary lung cancer. Genes Dev. 2017, 31, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- van Dam, P.A.; Verhoeven, Y.; Trinh, X.B. The Non-Bone-Related Role of RANK/RANKL Signaling in Cancer. Adv. Exp. Med. Biol. 2020, 1277, 53–62. [Google Scholar]
- Lv, J.; Chen, F.K.; Liu, C.; Liu, P.J.; Feng, Z.P.; Jia, L.; Yang, Z.X.; Hou, F.; Deng, Z.Y. Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway. Life Sci. 2020, 256, 117925. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell BC, V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, Y.; Sun, T.; Xue, H.; Jin, Z.; Tian, J. PD-1 blockade in combination with zoledronic acid to enhance the antitumor efficacy in the breast cancer mouse model. BMC Cancer 2018, 18, 669. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.; Walsh, J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969, 7, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Extermann, M.; Overcash, J.; Lyman, G.H.; Parr, J.; Balducci, L. Comorbidity and functional status are independent in older cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef] [Green Version]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE-Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nature reviews. Clin. Oncol. 2019, 16, 563–580. [Google Scholar]
- Rimassa, L.; Danesi, R.; Pressiani, T.; Merle, P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 2019, 77, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Yi, J.Z.; Zhao, M. Immunotherapy in older patients with hepatocellular carcinoma. Eur. J. Cancer 2022, 162, 76–98. [Google Scholar] [CrossRef]
- Razavi, H. Global Epidemiology of Viral Hepatitis. Gastroenterol. Clin. N. Am. 2020, 49, 179–189. [Google Scholar] [CrossRef]
- Wei, F.; Zheng, Q.; Li, M.; Wu, M. The association between hepatitis B mutants and hepatocellular carcinoma: A meta-analysis. Medicine 2017, 96, e6835. [Google Scholar] [CrossRef]
- Vandeven, N.; Nghiem, P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol. Res. 2014, 2, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Yan, C.; Zhu, J.; Chen, X.; Fu, Q.; Zhang, H.; Tong, Z.; Liu, L.; Zheng, Y.; Zhao, P.; et al. Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review. Front. Immunol. 2020, 11, 1037. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, H.; Lei, Y.; Shen, Y.; Li, J.; Zhong, H.; Zhong, R.; Zhang, X.; Chang, Q.; Lu, J.; Feng, H.; et al. Pembrolizumab monotherapy or combination therapy for bone metastases in advanced non-small cell lung cancer: A real-world retrospective study. Transl. Lung Cancer Res. 2022, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ji, M.; Jiang, Y.; Yin, R.; Wang, Z.; Li, H.; Wang, S.; He, K.; Ma, Y.; Wang, Z.; et al. A cohort study of the efficacy and safety of immune checkpoint inhibitors plus anlotinib versus immune checkpoint inhibitors alone as the treatment of advanced non-small cell lung cancer in the real world. Transl. Lung Cancer Res. 2022, 11, 1051–1068. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef] [Green Version]
- DA approves ZOMETA for treatment of cancer-related bone complications. Expert Rev. Anticancer Ther. 2002, 2, 137–138.
- Mullard, A. 2010 FDA drug approvals. Nature reviews. Drug Discov. 2011, 10, 82–85. [Google Scholar] [CrossRef]
- Turpin, A.; Duterque-Coquillaud, M.; Vieillard, M.H. Bone Metastasis: Current State of Play. Transl. Oncol. 2020, 13, 308–320. [Google Scholar] [CrossRef]
- Qin, A.; Zhao, S.; Miah, A.; Wei, L.A.-O.; Patel, S.; Johns, A.A.-O.; Grogan, M.; Bertino, E.M.; He, K.; Shields, P.A.-O.X.; et al. Bone Metastases, Skeletal-Related Events, and Survival in Patients With Metastatic Non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. J. Natl. Compr. Cancer Netw. 2021, 19, 915–921. [Google Scholar] [CrossRef]
- Schmid, S.; Diem, S.; Li, Q.; Krapf, M.; Flatz, L.; Leschka, S.; Desbiolles, L.; Klingbiel, D.; Jochum, W.; Früh, M. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol. Immunother. 2018, 67, 1825–1832. [Google Scholar] [CrossRef]
- Osorio, J.C.; Arbour, K.C.; Le, D.T.; Durham, J.N.; Plodkowski, A.J.; Halpenny, D.F.; Ginsberg, M.S.; Sawan, P.; Crompton, J.G.; Yu, H.A.; et al. Lesion-Level Response Dynamics to Programmed Cell Death Protein (PD-1) Blockade. J. Clin. Oncol. 2019, 37, 3546. [Google Scholar] [CrossRef]
- Wang, L.; Fang, D.; Xu, J.; Luo, R. Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: A brief review. BMC Cancer 2020, 20, 1059. [Google Scholar] [CrossRef]
- He, G.; Karin, M. NF-κB and STAT3-key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Tian, W.; Jiang, Z.; Huang, T.; Ge, C.; Liu, T.; Zhao, F.; Chen, T.; Cui, Y.; Li, H.; et al. A Positive Feedback Loop of AKR1C3-Mediated Activation of NF-κB and STAT3 Facilitates Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2021, 81, 1361–1374. [Google Scholar] [CrossRef]
- Honda, Y.; Aikata, H.; Honda, F.; Nakano, N.; Nakamura, Y.; Hatooka, M.; Morio, K.; Kobayashi, T.; Fukuhara, T.; Nagaoki, Y.; et al. Clinical outcome and prognostic factors in hepatocellular carcinoma patients with bone metastases medicated with zoledronic acid. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 1053–1060. [Google Scholar] [CrossRef]
- Katamura, Y.; Aikata, H.; Hashimoto, Y.; Kimura, Y.; Kawaoka, T.; Takaki, S.; Waki, K.; Hiramatsu, A.; Kawakami, Y.; Takahashi, S.; et al. Zoledronic acid delays disease progression of bone metastases from hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2010, 40, 1195–1203. [Google Scholar] [CrossRef]
- Montella, L.; Addeo, R.; Palmieri, G.; Caraglia, M.; Cennamo, G.; Vincenzi, B.; Guarrasi, R.; Mamone, R.; Faiola, V.; Frega, N.; et al. Zoledronic acid in the treatment of bone metastases by hepatocellular carcinoma: A case series. Cancer Chemother. Pharmacol. 2010, 65, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Afzal, M.Z.; Shirai, K. Does denosumab offer survival benefits? -Our experience with denosumab in metastatic non-small cell lung cancer patients treated with immune-checkpoint inhibitors. J. Thorac. Dis. 2021, 13, 4668–4677. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Foca, F.; Menis, J.; Stucci, S.L.; Artioli, F.; Guadalupi, V.; Forcignanò, M.R.; Fantini, M.; Recine, F.; Mercatali, L.; et al. Immune Checkpoint Inhibitors With or Without Bone-Targeted Therapy in NSCLC Patients With Bone Metastases and Prognostic Significance of Neutrophil-to-Lymphocyte Ratio. Front. Immunol. 2021, 12, 697298. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
| TKIs Monotreatment, N = 21 | TKIs + ICIs Combination Therapy, N = 41 | Total, N = 62 | p Value | |
|---|---|---|---|---|
| General Information | ||||
| Age: | 0.15 | |||
| Mean (SD) | 57.5 (11.2) | 53.3 (9.70) | 54.7 (10.3) | |
| Median [IQR: Q1-Q3] | 56.0 [47.0–62.25] | 53.0 [46.5–58.5] | 53.0 [47.0–61.75] | |
| Gender: | 0.041 | |||
| Male/Female | 17 (81.0%)/4 (19.0%) | 40 (97.6%)/1 (2.4%) | 57 (91.9%)/5 (8.1%) | |
| ECOG-PS: | 0.58 | |||
| 0–1/≥2 | 15 (71.4%)/6 (28.6%) | 25 (61.0%)/16 (39.0%) | 40 (64.5%)/22 (35.5%) | |
| Preoperational Data | ||||
| Metastatic bone location: | 0.090 | |||
| Cervical/Thoracic/Lumber Sacrum/Limb bone | 5 (23.8%)/5 (23.8%)/ 6 (28.6%) 4 (19.0%)/1 (4.8%) | 14 (34.1%)/18 (43.9%)/ 7 (17.1%) 2 (4.9%)/0 (0%) | 19 (30.6%)/23 (37.1%)/ 13 (21.0%) 6 (9.7%)/1 (1.6%) | |
| HBV/HCV infection: | 0.41 | |||
| Yes/No | 12 (57.1%)/9 (42.9%) | 28 (68.3%)/13 (31.7%) | 40 (64.5%)/22 (35.5%) | |
| Child-Pugh: | 0.14 | |||
| A/B | 20 (95.2%)/1 (4.8%) | 32 (78.0%)/9 (22.0%) | 52 (83.9%)/10 (16.1%) | |
| Surgery on the primary lesion: | 1.00 | |||
| Yes/No | 16 (76.2%)/5 (23.8%) | 31 (75.6%)/10 (24.4%) | 47 (75.8%)/15 (24.2%) | |
| AFP: | 0.23 | |||
| <400 ng/mL/≥400 ng/mL | 18 (85.7%)/3 (14.3%) | 29 (70.7%)/12 (29.3%) | 47 (75.8%)/15 (24.2%) | |
| Preoperative Frankel grade: | 0.76 | |||
| A/B | 0 (0%)/0 (0%) | 2 (4.9%)/2 (4.9%) | 2 (3.2%)/2 (3.2%) | |
| C/D | 1 (4.8%)/20 (95.2%) | 3 (7.3%)/34 (82.9%) | 4 (6.5%)/54 (87.1%) | |
| Preoperative Tomita score: | 1.00 | |||
| 5–6/7–8 | 19 (90.5%)/2 (9.5%) | 36 (87.8%)/5 (12.2%) | 55 (88.7%)/7 (11.3%) | |
| Sequential Treatment | ||||
| Visceral Metastases: | 0.90 | |||
| None/Lung/Multiple | 13 (61.9%)/2 (9.5%)/ 1 (4.8%) | 26 (63.4%)/4 (9.8%)/ 4 (9.8%) | 39 (62.9%)/6 (9.7%)/ 5 (8.1%) | |
| Intrahepatic metastasis/Other locations | 5 (23.8%)/0 (0%) | 6 (14.6%)/1 (2.4%) | 11 (17.7%)/1 (1.6%) | |
| Chemotherapy: | 1.00 | |||
| Yes/No | 7 (33.3%)/14 (66.7%) | 14 (34.1%)/27 (65.9%) | 21 (33.9%)/41 (66.1%) | |
| Radiotherapy: | 1.00 | |||
| Yes/No | 8 (38.1%)/13 (61.9%) | 16 (39.0%)/25 (61.0%) | 24 (38.7%)/38 (61.3%) | |
| The type of BMAs: | 0.21 | |||
| No/Zoledronic acid | 3 (14.3%)/8 (38.1%) | 1 (2.4%)/18 (43.9%) | 4 (6.5%)/26 (41.9%) | |
| Denosumab | 10 (47.6%) | 22 (53.7%) | 32 (51.6%) | |
| The time of BMAs use: | ||||
| Long term use/Only perioperatively or No | 10 (47.6%)/11 (52.4%) | 31 (75.6%)/10 (24.4%) | 41 (66.1%)/21 (33.9%) | 0.046 |
| TKIs, N = 21 | TKIs + ICIs, N = 41 | Total, N = 62 | p Value | |
|---|---|---|---|---|
| mRECIST: | ||||
| Complete response | 1 (4.7%) | 0 (0%) | 1 (1.6%) | 0.34 |
| Partial response | 0 (0%) | 9 (22.0%) | 9 (14.5%) | 0.022 |
| Stable disease | 7 (33.3%) | 12 (29.3%) | 21 (33.9%) | 0.78 |
| Progressive disease | 13 (61.9%) | 20 (48.8%) | 33 (53.2%) | 0.42 |
| Objective Response Rate (ORR) | 1 (4.7%) | 9 (22.0%) | 10 (16.1%) | 0.14 |
| Disease Control Rate (DCR) | 8 (38.1%) | 21 (51.2%) | 29 (46.8%) | 0.42 |
| TKIs Mono-Treatment, N = 15 | TKIs + ICIs Combination, N = 34 | p Value | ||||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Nausea | 6 (40.0%) | 0 (0%) | 15 (44.1%) | 0 (0%) | 1.00 | 1.00 |
| Fatigue | 5 (33.3%) | 0 (0%) | 6 (17.6%) | 0 (0%) | 0.28 | 1.00 |
| Pruritus | 2 (13.3%) | 0 (0%) | 6 (17.6%) | 1 (2.9%) | 1.00 | 1.00 |
| Rash | 3 (20.0%) | 0 (0%) | 9 (26.5%) | 6 (17.6%) | 0.73 | 0.16 |
| Myasthenia | 2 (13.3%) | 0 (0%) | 3 (8.8%) | 0 (0%) | 0.64 | 1.00 |
| Decreased Appetite | 6 (40.0%) | 1 (6.67%) | 11 (32.4%) | 0 (0%) | 0.75 | 0.31 |
| Colitis | 1 (6.7%) | 0 (0%) | 2 (5.9%) | 0 (0%) | 1.00 | 1.00 |
| Hemorrhagic Tendency | 1 (6.7%) | 0 (0%) | 2 (5.9%) | 0 (0%) | 1.00 | 1.00 |
| Dyspnea | 0 (0%) | 0 (0%) | 2 (5.9%) | 0 (0%) | 1.00 | 1.00 |
| Pyrexia | 1 (6.7%) | 0 (0%) | 2 (5.9%) | 0 (0%) | 1.00 | 1.00 |
| Increased Transaminase | 4 (26.7%) | 2 (13.3%) | 2 (5.9%) | 1 (2.9%) | 0.062 | 0.22 |
| Hypertension | 0 (0%) | 0 (0%) | 3 (8.8%) | 0 (0%) | 0.54 | 1.00 |
| Hand-foot syndrome | 2 (13.3%) | 0 (0%) | 2 (5.9%) | 0 (0%) | 0.58 | 1.00 |
| Hypothyroidism | 0 (0%) | 0 (0%) | 2 (5.9%) | 1 (2.9%) | 1.00 | 1.00 |
| Hypocalcemia | 1 (6.7%) | 0 (0%) | 5 (14.7%) | 0 (0%) | 0.65 | 1.00 |
| Vitiligo | 0 (0%) | 0 (0%) | 1 (2.9%) | 0 (0%) | 1.00 | 1.00 |
| Only Used BMAs Perioperatively/No Treatment (N = 21) | Long Term Use of BMAs (N = 41) | Total (N = 62) | p Value | |
|---|---|---|---|---|
| mRECIST: | ||||
| Complete response | 1 (4.7%) | 0 (0%) | 1 (1.6%) | 0.34 |
| Partial response | 1 (4.7%) | 8 (19.5%) | 9 (14.5%) | 0.15 |
| Stable disease | 3 (14.3%) | 16 (39.0%) | 19 (30.6%) | 0.079 |
| Progressive disease | 16 (76.2%) | 17 (41.5%) | 33 (53.2%) | 0.015 |
| Objective Response Rate | 2 (9.5%) | 8 (19.5%) | 10 (16.1%) | 0.47 |
| Disease Control Rate | 5 (23.8%) | 24 (58.5%) | 29 (46.8%) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shen, Z.; Wang, X.; Wang, P.; Zhu, X.; Fan, J.; Li, B.; Xu, W.; Xiao, J. Combination of Bone-Modifying Agents with Immunotarget Therapy for Hepatocellular Carcinoma with Bone Metastases. J. Clin. Med. 2022, 11, 6901. https://doi.org/10.3390/jcm11236901
Chen Z, Shen Z, Wang X, Wang P, Zhu X, Fan J, Li B, Xu W, Xiao J. Combination of Bone-Modifying Agents with Immunotarget Therapy for Hepatocellular Carcinoma with Bone Metastases. Journal of Clinical Medicine. 2022; 11(23):6901. https://doi.org/10.3390/jcm11236901
Chicago/Turabian StyleChen, Zhaoyu, Zhilong Shen, Xiang Wang, Pengru Wang, Xiaofei Zhu, Jiefu Fan, Bo Li, Wei Xu, and Jianru Xiao. 2022. "Combination of Bone-Modifying Agents with Immunotarget Therapy for Hepatocellular Carcinoma with Bone Metastases" Journal of Clinical Medicine 11, no. 23: 6901. https://doi.org/10.3390/jcm11236901
APA StyleChen, Z., Shen, Z., Wang, X., Wang, P., Zhu, X., Fan, J., Li, B., Xu, W., & Xiao, J. (2022). Combination of Bone-Modifying Agents with Immunotarget Therapy for Hepatocellular Carcinoma with Bone Metastases. Journal of Clinical Medicine, 11(23), 6901. https://doi.org/10.3390/jcm11236901





