Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Nutritional Data
2.2. Patient Stratification According to Genotype
2.3. Biochemical Analyses
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Gelzo, M.; Iacotucci, P.; Caputo, M.; Cernera, G.; Comegna, M.; Carnovale, V.; Corso, G.; Castaldo, G. Lumacaftor/ivacaftor improves liver cholesterol metabolism but does not influence hypocholesterolemia in patients with cystic fibrosis. J. Cyst. Fibros. 2021, 20, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Gelzo, M.; Caputo, M.; Comegna, M.; Cernera, G.; Di Minno, A.; Scialo, F.; Castaldo, G.; Corso, G. Exploiting sterol profile analysis: Over ten years of experience. A narrative review. Acta Med. Mediterr. 2021, 37, 35–42. [Google Scholar] [CrossRef]
- Amato, F.; Castaldo, A.; Castaldo, G.; Cernera, G.; Corso, G.; Ferrari, E.; Gelzo, M.; Monzani, R.; Villella, V.R.; Raia, V. Impaired cholesterol metabolism in the mouse model of cystic fibrosis. A preliminary study. PLoS ONE 2021, 16, e0245302. [Google Scholar] [CrossRef]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef]
- Marshall, B.; Faro, A.; Fink, A.K. Cystic Fibrosis Patient Registry: 2017 Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2018. [Google Scholar]
- Dalemans, W.; Barbry, P.; Champigny, G.; Jallat, S.; Dott, K.; Dreyer, D.; Crystal, R.G.; Pavirani, A.; Lecocq, J.P.; Lazdunski, M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991, 354, 526–528. [Google Scholar] [CrossRef]
- Lukacs, G.; Chang, X.; Bear, C.; Kartner, N.; Mohamed, A.; Riordan, J.; Grinstein, S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J. Biol. Chem. 1993, 268, 21592–21598. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- National Institutes of Health. A Study to Evaluate the Efficacy and Safety of VX-661 in Combination with Ivacaftor in Subjects Aged 12 Years and Older with Cystic Fibrosis, Heterozygous for the F508del-CFTR Mutation: Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02516410 (accessed on 10 October 2022).
- Rowe, S.M.; McColley, S.A.; Rietschel, E.; Li, X.; Bell, S.C.; Konstan, M.W.; Marigowda, G.; Waltz, D.; Boyle, M.P. Lumacaftor/Ivacaftor Treatment of Patients with Cystic Fibrosis Heterozygous for F508del-CFTR. Ann. Am. Thorac. Soc. 2017, 14, 213–219. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, V.; Iacotucci, P.; Terlizzi, V.; Colangelo, C.; Ferrillo, L.; Pepe, A.; Francalanci, M.; Taccetti, G.; Buonaurio, S.; Celardo, A.; et al. Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508del Mutation and Advanced Lung Disease: A 48-Week Observational Study. J. Clin. Med. 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, V.; Iacotucci, P.; Terlizzi, V.; Colangelo, C.; Medio, P.; Ferrillo, L.; De Gregorio, F.; Francalanci, M.; Taccetti, G.; Buonaurio, S.; et al. Effectiveness and safety of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease with the Phe508del/minimal function genotype. Respir. Med. 2021, 189, 106646. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, R.; Sangiuolo, F.; Bombieri, C.; Bonizzato, A.; Cardillo, G.; Raia, V.; D’Apice, M.; Bettin, M.; Pignatti, P.; Castaldo, G.; et al. Epidemiology and a novel procedure for large scale analysis of CFTR rearrangements in classic and atypical CF patients: A multicentric Italian study. J. Cyst. Fibros. 2008, 7, 347–351. [Google Scholar] [CrossRef]
- Amato, F.; Bellia, C.; Cardillo, G.; Castaldo, G.; Ciaccio, M.; Elce, A.; Lembo, F.; Tomaiuolo, R. Extensive molecular analysis of patients bearing CFTR-related disorders. J. Mol. Diagn. 2012, 14, 81–89. [Google Scholar] [CrossRef]
- Bergougnoux, A.; D’Argenio, V.; Sollfrank, S.; Verneau, F.; Telese, A.; Postiglione, I.; Lackner, K.J.; Claustres, M.; Castaldo, G.; Rossmann, H.; et al. Multicenter validation study for the certification of a CFTR gene scanning method using next generation sequencing technology. Clin. Chem. Lab. Med. 2018, 56, 1046–1053. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Di Dato, F.; Spadarella, S.; Puoti, M.G.; Caprio, M.G.; Pagliardini, S.; Zuppaldi, C.; Vallone, G.; Fecarotta, S.; Esposito, G.; Iorio, R.; et al. Daily Fructose Traces Intake and Liver Injury in Children with Hereditary Fructose Intolerance. Nutrients 2019, 11, 2397. [Google Scholar] [CrossRef]
- Jørgensen, L.M.; Isaksson, B.; Schroll, M. Reproducibility and validity of 7-day food records. Eur. J. Clin. Nutr. 1992, 46, 729–734. [Google Scholar] [PubMed]
- Munck, A.; Kerem, E.; Ellemunter, H.; Campbell, D.; Wang, L.T.; Ahluwalia, N.; Owen, C.A.; Wainwright, C. Tezacaftor/ivacaftor in people with cystic fibrosis heterozygous for minimal function CFTR mutations. J. Cyst. Fibros. 2020, 19, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Elce, A.; Nigro, E.; Gelzo, M.; Iacotucci, P.; Carnovale, V.; Liguori, R.; Izzo, V.; Corso, G.; Castaldo, G.; Daniele, A.; et al. Supervised physical exercise improves clinical, anthropometric and biochemical parameters in adult cystic fibrosis patients: A 2-year evaluation. Clin. Respir. J. 2018, 12, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Polito, R.; Nigro, E.; Elce, A.; Monaco, M.L.; Iacotucci, P.; Carnovale, V.; Comegna, M.; Gelzo, M.; Zarrilli, F.; Corso, G.; et al. Adiponectin Expression Is Modulated by Long-Term Physical Activity in Adult Patients Affected by Cystic Fibrosis. Mediators Inflamm. 2019, 2019, 2153934. [Google Scholar] [CrossRef] [PubMed]
- Bear, C.E. A Therapy for Most with Cystic Fibrosis. Cell 2020, 180, 211. [Google Scholar] [CrossRef] [PubMed]
- Meoli, A.; Fainardi, V.; Deolmi, M.; Chiopris, G.; Marinelli, F.; Caminiti, C.; Esposito, S.; Pisi, G. State of the Art on Approved Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators and Triple-Combination Therapy. Pharmaceuticals 2021, 14, 928. [Google Scholar] [CrossRef]
- Cottrill, K.A.; Farinha, C.M.; McCarty, N.A. The bidirectional relationship between CFTR and lipids. Commun. Biol. 2020, 3, 179. [Google Scholar] [CrossRef]
- Sommerburg, O.; Hämmerling, S.; Schneider, S.; Okun, J.; Langhans, C.-D.; Leutz-Schmidt, P.; Wielpütz, M.; Siems, W.; Gräber, S.; Mall, M.; et al. CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis. Antioxidants 2021, 10, 483. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Ferreira, T. Glued in lipids: Lipointoxication in cystic fibrosis. EBioMedicine 2020, 61, 103038. [Google Scholar] [CrossRef]
- Mayer, M. Lumacaftor-ivacaftor (Orkambi) for cystic fibrosis: Behind the ‘breakthrough’. Evid. Based Med. 2016, 21, 83–86. [Google Scholar] [CrossRef][Green Version]
- Zaher, A.; ElSaygh, J.; Elsori, D.; ElSaygh, H.; Sanni, A. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy. Cureus 2021, 13, e16144. [Google Scholar] [CrossRef]
- Pagani, F.; Panteghini, M. Significance of various parameters derived from biological variability for lipid and lipoprotein analyses. Clin. Biochem. 1993, 26, 415–420. [Google Scholar] [CrossRef] [PubMed]
- United States General Accounting Office (GAO). The Potential Effect of Measurement Uncertainty and Agency Comments and Our Response. In Cholesterol Measurement: Test Accuracy and Factors That Influence Cholesterol Levels; GAO/PMED-95-8; GAO: Washington, DC, USA, 1994; p. 59. [Google Scholar]
- Kim, M.H.; Ahn, J.Y.; Song, J.E.; Choi, H.; Ann, H.W.; Kim, J.K.; Kim, J.H.; Jeon, Y.D.; Kim, S.B.; Jeong, S.J.; et al. The C-Reactive Protein/Albumin Ratio as an Independent Predictor of Mortality in Patients with Severe Sepsis or Septic Shock Treated with Early Goal-Directed Therapy. PLoS ONE 2019, 14, e0225620. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.C.; Hauschild, D.B.; Moreira, E.A.M.; Pereira, L.C.R.; Rosa, A.F.; Barbosa, E.; Ludwig-Neto, N.; da Rosa, J.S.; Fröde, T.S.; Moreno, Y.M.F. C-reactive protein/albumin ratio is associated with lung function among children/adolescents with cystic fibrosis: A three-year longitudinal study. Sao Paulo Med. J. 2018, 136, 29–36. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Values |
|---|---|
| Age, y. (SD) | 31.9 (7.7) |
| Male, n (%) | 8 (40) |
| Genotype group, n (%): | |
| First group | |
| Phe508del/G542X | 3 (15) |
| Phe508del/R553X | 1 (5) |
| Phe508del/G673X | 1 (5) |
| Second group | |
| Phe508del/2183AA>G | 2 (10) |
| Phe508del/R1158X | 1 (5) |
| Phe508del/W1282X | 1 (5) |
| Phe508del/4016insT | 1 (5) |
| Phe508del/17a-17b-18del 9T/9T | 1 (5) |
| Phe508del/CFTRdele14b17b | 1 (5) |
| Phe508del/macro22-23del | 1 (5) |
| Phe508del/1717-1G>A | 1 (5) |
| Third group | |
| Phe508del/N1303K | 5 (25) |
| Phe508del/G85E | 1 (5) |
| P. aeruginosa colonization, n (%) | 14 (70) |
| FEV1 (%) | 31.4 (8.2) |
| CFLD, n (%) | 12 (60) |
| CFRD, n (%) | 12 (60) |
| n.v. | Baseline | 3 Months | 12 Months | p Value | |
|---|---|---|---|---|---|
| Weight (kg) | 53 (50–58.25) | 56.6 (54.5–59.57) a | 58.2 (56.6–62.9) a,b | 0.021 | |
| BMI (kg/m2) | 20 (19.20–21.75) | 22 (20.62–23.75) a | 23.1 (21–24.8) a,b | 0.002 | |
| Glucose (mg/dL) | 70–110 | 89 (79–101.2) | 89.5 (71.25–102.5) | 88 (86–109.7) | n.s. |
| HbA1C (%) | 6.2 (5.92–7) | 5.8 (5.35–6.97) a | 5.8 (5.2–6.2) a | n.s. | |
| Insulin | 7.25 (4.10–16.53) | 7.90 (5–38) | 12.3 (5–31.8) a | n.s. | |
| Steatocrit (%) | 4.1 (2.05–6.22) | 3.1 (2.1–7.9) | 2.25 (1.7–3.45) | n.s. | |
| CRP (mg/dL) | 0–0.5 | 0.71 (0.33–2.79) | 0.33 (0.33–0.41) a | 0.30 (0.20–0.30) a | <0.0001 |
| CRP/Albumin ratio | 0.17 (0.08–0.81) | 0.08 (0.08–0.10) a | 0.07 (0.07–0.09) a,b | 0.0001 | |
| Liver Parameters | |||||
| AP (U/L) | 40–150 | 109.5 (84–146) | 133.5 (101.3–172.8) | 119 (95–172) | n.s. |
| 119 (95–172) | |||||
| Dir Bil (mg/mL) | 0–0.40 | 0.24 (0.16–0.34) | 0.36 (0.26–0.44) a | 0.33 (0.26–0.62) a | 0.007 |
| Total Bil (mg/mL) | 0.20–1.20 | 0.50 (0.31–0.66) | 0.78 (0.56–1.40) a | 0.95 (0.57–1.73) a,b | 0.003 |
| AST (U/L) | 0–34 | 20 (15–26.75) | 25.50 (20.25–34.50) a | 27.5 (17.5–40.5) a | 0.047 |
| ALT (U/L) | 0–55 | 22.5 (11.7–28.7) | 28 (22.50–53.25) a | 31.5 (20.25–47.75) a | n.s. |
| yGT (U/L) | 12–64 | 14.50 (10–20.75) | 11.50 (9.25–45.25) | 13 (10–29.75) | n.s. |
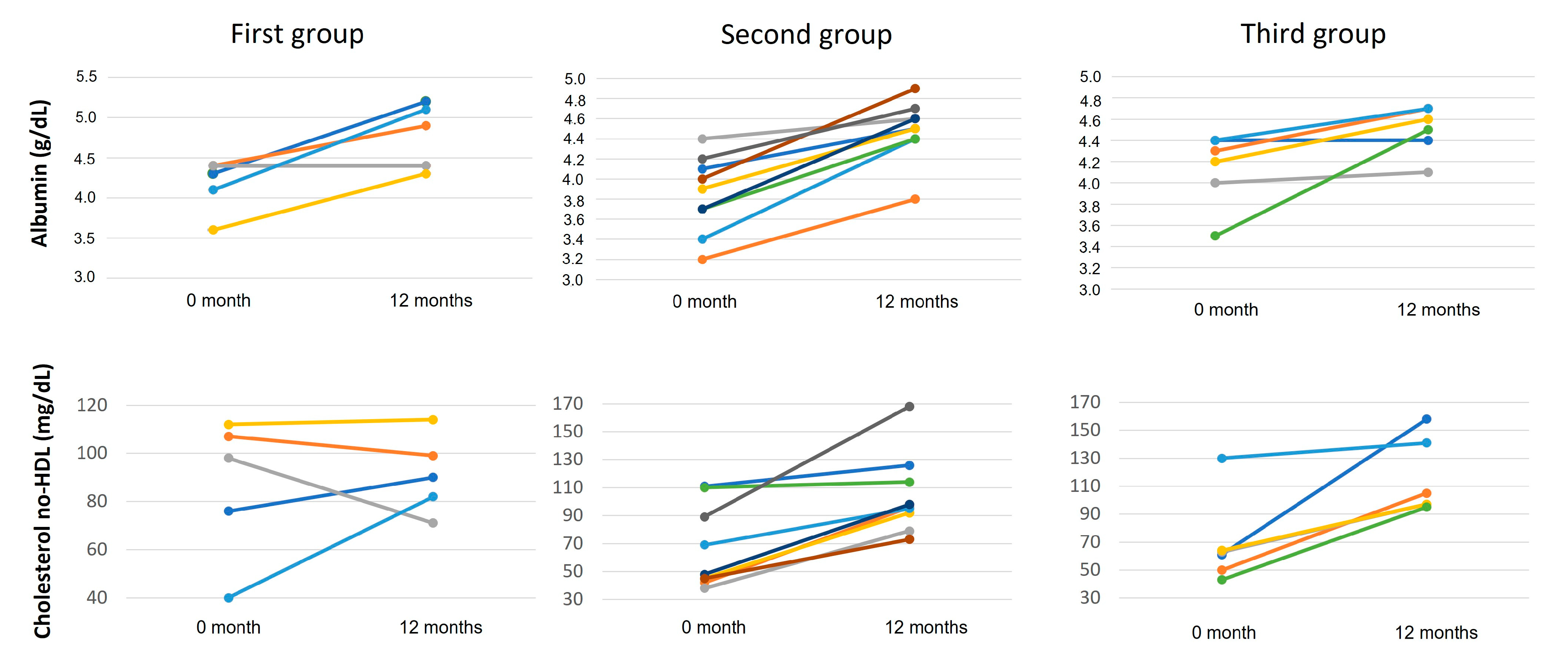
| Albumin (g/dL) | 3.5–5.2 | 4.1 (3.7–4.4) | 4.1 (4.0–4.3) a | 4.5 (4.4–4.7) a,b | <0.0001 |
| Lipids | |||||
| Total CHOL (mg/dl) | 121–232 | 134 (101–145) | 144 (114.8–152.8) a | 157.5 (135–167) a,b | 0.035 |
| HDL CHOL (mg/dL) | >40 | 56 (45.50–70) | 43 (41–53.75) a | 49 (44–66) b | n.s. |
| LDL CHOL (mg/dL) | <115 | 47 (34–84) | 79 (67–90) a | 91 (81–99) a,b | 0.0001 |
| Trig (mg/dL) | <150 | 80.50 (69.25–106.5) | 75 (66–82.50) a | 79 (62–103) b | n.s. |
| Vit. A (μg/dL) | 20–80 | 28.10 (21–36.15) | 39.75 (27.45–57.38) | 47.8 (39.2–61.8) | 0.002 |
| Vit. D (ng/mL) | 30–100 | 29.40 (22–34.80) | 25.25 (16.33–33.03) | 27.6 (22.9–34.17) | n.s. |
| Vit. E (μg/dL) | 500–1800 | 1181 (840.8–1481) | 1193 (730.4–1495) | 1316 (856.8–1803) | n.s. |
| non-HDL CHOL | <145 | 63.5 (45–104.8) | 90.5 (76–105.8) a | 97 (90–114) a,b | 0.004 |
| LDL/HDL ratio | 0.69 (0.53–2.02) | 1.79 (1.46–2.04) a | 1.93 (1.4–2.04) a | 0.005 | |
| ↑ CRP | ↓ Albumin | ↑ AP | ↑ Dir. Bil. | ↑ Total Bil. | ↑ AST | ↑ ALT | ↑ yGT | |
|---|---|---|---|---|---|---|---|---|
| CFLD (n = 12) | (Number of patients) | |||||||
| Baseline | 7 | 2 | 3 | 2 | 1 | 1 | 1 | 1 |
| After 1 year | 4 | - | 4 | 5 | 5 | 2 | 1 | 2 |
| No CFLD (n = 8) | ||||||||
| Baseline | 5 | - | 1 | - | - | 1 | - | 1 |
| After 1 year | - | - | 3 | 4 | 3 | 3 | 3 | 1 |
| Components | Baseline | 12 Months | p Value |
|---|---|---|---|
| Kcal | 2167 (472) | 2131 (496) | 0.66 |
| Proteins (g) | 102 (16) | 97 (22) | 0.56 |
| Proteins (%) | 19 (3) | 18 (3) | 0.53 |
| Lipids (g) | 80 (30) | 85 (22) | 0.42 |
| Lipids (%) | 33 (6) | 37 (9) | 0.29 |
| Carb (g) | 272 (61) | 255 (70) | 0.33 |
| Carb (%) | 50 (6) | 48 (12) | 0.33 |
| Fibers (g) | 18 (3) | 20 (6) | 0.10 |
| Calcium (mg) | 771 (245) | 798 (219) | 0.93 |
| Chol (mg) | 224 (59) | 237 (67) | 0.92 |
| SFA (g) | 10 (7) | 10 (4) | 0.72 |
| SFA (%) | 4.1 (1.8) | 4.2 (1.6) | 0.86 |
| MuFA (g) | 35 (11) | 31 (7) | 0.13 |
| MuFA (%) | 14 (3) | 13 (3) | 0.11 |
| PuFA (g) | 6.0 (2.8) | 5.7 (1.8) | 0.76 |
| PuFA % | 2.5 (0.7) | 2.4 (0.7) | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovale, V.; Scialò, F.; Gelzo, M.; Iacotucci, P.; Amato, F.; Zarrilli, F.; Celardo, A.; Castaldo, G.; Corso, G. Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment. J. Clin. Med. 2022, 11, 6900. https://doi.org/10.3390/jcm11236900
Carnovale V, Scialò F, Gelzo M, Iacotucci P, Amato F, Zarrilli F, Celardo A, Castaldo G, Corso G. Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment. Journal of Clinical Medicine. 2022; 11(23):6900. https://doi.org/10.3390/jcm11236900
Chicago/Turabian StyleCarnovale, Vincenzo, Filippo Scialò, Monica Gelzo, Paola Iacotucci, Felice Amato, Federica Zarrilli, Assunta Celardo, Giuseppe Castaldo, and Gaetano Corso. 2022. "Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment" Journal of Clinical Medicine 11, no. 23: 6900. https://doi.org/10.3390/jcm11236900
APA StyleCarnovale, V., Scialò, F., Gelzo, M., Iacotucci, P., Amato, F., Zarrilli, F., Celardo, A., Castaldo, G., & Corso, G. (2022). Cystic Fibrosis Patients with F508del/Minimal Function Genotype: Laboratory and Nutritional Evaluations after One Year of Elexacaftor/Tezacaftor/Ivacaftor Treatment. Journal of Clinical Medicine, 11(23), 6900. https://doi.org/10.3390/jcm11236900






