A Natural History Study of RP2-Related Retinopathy
Abstract
1. Introduction
2. Material and Methods
2.1. Settings and Study Population
2.2. Retinal Imaging
2.3. Data Analysis
3. Results
3.1. Molecular Characteristics
3.2. Visual Acuity
3.3. Ocular Imaging
3.4. SD-OCT Quantitative Measures
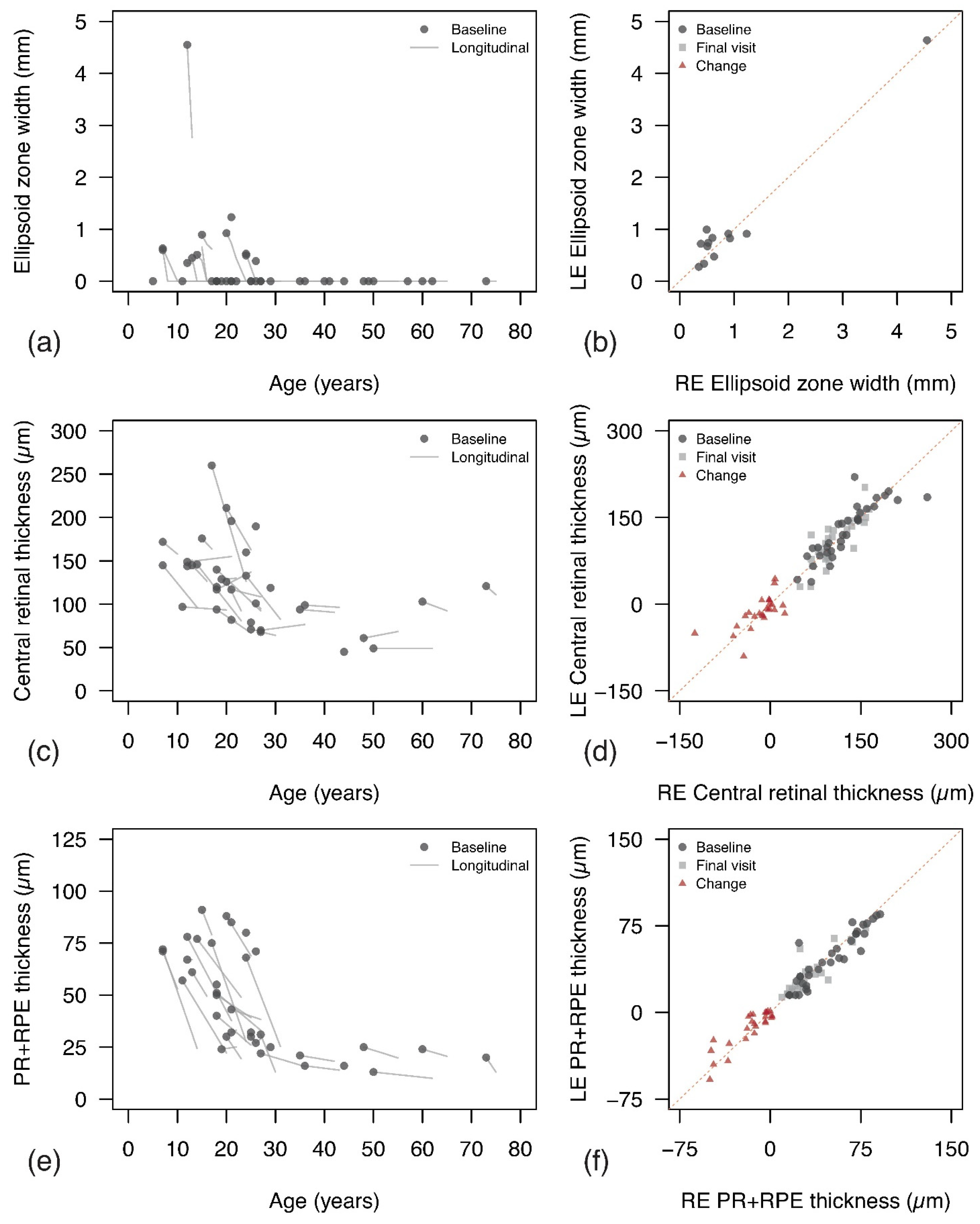
3.4.1. Ellipsoid Zone
3.4.2. Central Retinal Thickness and PR+RPE Complex
3.5. Additional Analyses
4. Discussion
Considerations for Future Clinical Trials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Solebo, A.L.; Teoh, L.; Rahi, J. Epidemiology of blindness in children. Arch. Dis. Child. 2017, 102, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Michaelides, M.; Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014, 4, e004015. [Google Scholar] [CrossRef]
- Bunker, C.H.; Berson, E.L.; Bromley, W.C.; Hayes, R.P.; Roderick, T.H. Prevalence of retinitis pigmentosa in Maine. Am. J. Ophthalmol. 1984, 97, 357–365. [Google Scholar] [CrossRef]
- Pontikos, N.; Arno, G.; Jurkute, N.; Schiff, E.; Ba-Abbad, R.; Malka, S.; Gimenez, A.; Georgiou, M.; Wright, G.; Armengol, M.; et al. Genetic Basis of Inherited Retinal Disease in a Molecularly Characterized Cohort of More Than 3000 Families from the United Kingdom. Ophthalmology 2020, 127, 1384–1394. [Google Scholar] [CrossRef]
- Birtel, J.; Gliem, M.; Mangold, E.; Müller, P.L.; Holz, F.G.; Neuhaus, C.; Lenzner, S.; Zahnleiter, D.; Betz, C.; Eisenberger, T.; et al. Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PLoS ONE 2018, 13, e0207958. [Google Scholar] [CrossRef]
- Fahim, A.T.; Daiger, S.P. The Role of X-Chromosome Inactivation in Retinal Development and Disease. Adv. Exp. Med. Biol. 2016, 854, 325–331. [Google Scholar]
- De Silva, S.R.; Arno, G.; Robson, A.G.; Fakin, A.; Pontikos, N.; Mohamed, M.D.; Bird, A.C.; Moore, A.T.; Michaelides, M.; Webster, A.R.; et al. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog. Retin. Eye Res. 2021, 82, 100898. [Google Scholar] [CrossRef]
- Breuer, D.K.; Yashar, B.M.; Filippova, E.; Hiriyanna, S.; Lyons, R.H.; Mears, A.J.; Asaye, B.; Acar, C.; Vervoort, R.; Wright, A.F.; et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am. J. Hum. Genet. 2002, 70, 1545–1554. [Google Scholar] [CrossRef]
- Pelletier, V.; Jambou, M.; Delphin, N.; Zinovieva, E.; Stum, M.; Gigarel, N.; Dollfus, H.; Hamel, C.; Toutain, A.; Dufier, J.-L.; et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: Genotype-phenotype correlations and impact on genetic counseling. Hum. Mutat. 2007, 28, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, U.; Lenzner, S.; Dong, J.; Feil, S.; Hinzmann, B.; Van Duijnhoven, G.; Kirschner-Schwabe, R.; Hemberger, M.; Bergen, A.A.; Rosenberg, T.; et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat. Genet. 1998, 19, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, A.; Thiselton, D.L.; Van Maldergem, L.; Saha, B.K.; Jay, M.; Plant, C.; Taylor, R.; Bird, A.C.; Bhattacharya, S. Mutations in the RP2 gene cause disease in 10% of families with familial X-linked retinitis pigmentosa assessed in this study. Am. J. Hum. Genet. 1999, 64, 1210–1215. [Google Scholar] [CrossRef]
- Fujinami, K.; Liu, X.; Ueno, S.; Mizota, A.; Shinoda, K.; Kuniyoshi, K.; Fujinami-Yokokawa, Y.; Yang, L.; Arno, G.; Pontikos, N.; et al. RP2-associated retinal disorder in a Japanese cohort: Report of novel variants and a literature review, identifying a genotype-phenotype association. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 675–693. [Google Scholar] [CrossRef]
- Zhang, H.; Hanke-Gogokhia, C.; Jiang, L.; Li, X.; Wang, P.; Gerstner, C.D.; Frederick, J.M.; Yang, Z.; Baehr, W. Mistrafficking of prenylated proteins causes retinitis pigmentosa 2. FASEB J. 2015, 29, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Sharon, D.; Sandberg, M.A.; Rabe, V.W.; Stillberger, M.; Dryja, T.P.; Berson, E.L. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am. J. Hum. Genet. 2003, 73, 1131–1146. [Google Scholar] [CrossRef]
- Jayasundera, T.; Branham, K.E.; Othman, M.; Rhoades, W.R.; Karoukis, A.J.; Khanna, H.; Swaroop, A.; Heckenlively, J.R. RP2 phenotype and pathogenetic correlations in X-linked retinitis pigmentosa. Arch. Ophthalmol. 2010, 128, 915–923. [Google Scholar] [CrossRef]
- Zada, M.; Cornish, E.E.; Fraser, C.L.; Jamieson, R.V.; Grigg, J.R. Natural history and clinical biomarkers of progression in X-linked retinitis pigmentosa: A systematic review. Acta. Ophthalmol. 2021, 99, 499–510. [Google Scholar] [CrossRef]
- Zada, M.; Cornish, E.E.; Fraser, C.L.; Jamieson, R.V.; Grigg, J.R. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar]
- Pawlyk, B.S.; Bulgakov, O.V.; Sun, X.; Adamian, M.; Shu, X.; Smith, A.J.; Berson, E.L.; Ali, R.; Khani, S.; Wright, A.F.; et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Ther. 2016, 23, 196–204. [Google Scholar] [CrossRef]
- Beltran, W.A.; Cideciyan, A.V.; Lewin, A.S.; Iwabe, S.; Khanna, H.; Sumaroka, A.; Chiodo, V.A.; Fajardo, D.S.; Román, A.J.; Deng, W.-T.; et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2012, 109, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, S.; Hiriyanna, S.; Kaneshiro, K.; Li, L.; Li, Y.; Li, W.; Qian, H.; Li, T.; Khanna, H.; Colosi, P.; et al. Long-term rescue of cone photoreceptor degeneration in retinitis pigmentosa 2 (RP2)-knockout mice by gene replacement therapy. Hum. Mol. Genet. 2015, 24, 6446–6458. [Google Scholar] [CrossRef]
- Lane, A.; Jovanovic, K.; Shortall, C.; Ottaviani, D.; Panes, A.B.; Schwarz, N.; Guarascio, R.; Hayes, M.J.; Palfi, A.; Chadderton, N.; et al. Modeling and Rescue of RP2 Retinitis Pigmentosa Using iPSC-Derived Retinal Organoids. Stem Cell Rep. 2020, 15, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Tee, J.J.; Yang, Y.; Kalitzeos, A.; Webster, A.; Bainbridge, J.; Michaelides, M. Natural history study of retinal structure, progression, and symmetry using ellipzoid zone metrics in RPGR-associated retinopathy. Am. J. Ophthalmol. 2019, 198, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Van Schooneveld, M.J.; Thiadens, A.A.; Fiocco, M.; Wijnholds, J.; Florijn, R.J.; Schalij-Delfos, N.E.; Van Genderen, M.M.; Putter, H.; Cremers, F.P.; et al. Clinical and Genetic Characteristics of Male Patients with RPGR-associated Retinal Dystrophies: A Long-Term Follow-up Study. Retina 2019, 39, 1186–1199. [Google Scholar] [CrossRef]
- Saeed, O.B.; Traboulsi, E.I.; Coussa, R.G. Profiling of visual acuity and genotype correlations in RP2 patients: A cross-sectional comparative meta-analysis between carrier females and affected males. Eye 2022. [Google Scholar] [CrossRef]
- Méjécase, C.; Malka, S.; Guan, Z.; Slater, A.; Arno, G.; Moosajee, M. Practical guide to genetic screening for inherited eye diseases. Ther. Adv. Ophthalmol. 2020, 12, 2515841420954592. [Google Scholar] [CrossRef]
- Harding, P.; Gore, S.; Malka, S.; Rajkumar, J.; Oluonye, N.; Moosajee, M. Real-world clinical and molecular management of 50 prospective patients with microphthalmia, anophthalmia and/or ocular coloboma. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Bouzia, Z.; Georgiou, M.; Hull, S.; Robson, A.G.; Fujinami, K.; Rotsos, T.; Pontikos, N.; Arno, G.; Webster, A.R.; Hardcastle, A.J.; et al. GUCY2D-Associated Leber Congenital Amaurosis: A Retrospective Natural History Study in Preparation for Trials of Novel Therapies. Am. J. Ophthalmol. 2020, 210, 59–70. [Google Scholar] [CrossRef]
- Ctori, I.; Huntjens, B. Repeatability of Foveal Measurements Using Spectralis Optical Coherence Tomography Segmentation Software. PLoS ONE 2015, 10, e0129005. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; van Cauwenbergh, C.; van Schooneveld, M.J.; Fiocco, M.; Wijnholds, J.; Jacoline, B.; Florijn, R.J.; Schalij-Delfos, N.E.; Dagnelie, G.; van Genderen, M.M.; et al. Clinical Characteristics and Natural History of RHO-associated Retinitis pigmentosa: A Long-Term Follow-Up Study. Retina 2021, 41, 213–223. [Google Scholar]
- Chung, D.C.; Bertelsen, M.; Lorenz, B.; Pennesi, M.E.; Leroy, B.P.; Hamel, C.P.; Pierce, E.; Sallum, J.; Larsen, M.; Stieger, K.; et al. The Natural History of Inherited Retinal Dystrophy Due to Biallelic Mutations in the RPE65 Gene. Am. J. Ophthalmol. 2019, 199, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F.; Fishman, G.A.; Roberts, D.K.; Heckenlively, J.R.; Weleber, R.G.; Anderson, R.J.; Grover, S. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br. J. Ophthalmol. 2002, 86, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Csaky, K.; Ferris, F.; Chew, E.Y.; Nair, P.; Cheetham, J.K.; Duncan, J.L. Report From the NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3456–3463. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-152. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 17 November 2022).
- Clarke, G.; Collins, R.A.; Leavitt, B.R.; Andrews, D.F.; Hayden, M.R.; Lumsden, C.J.; McInnes, R.R. A one-hit model of cell death in inherited neuronal degenerations. Nature 2000, 406, 195–199. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000; pp. 39–77. [Google Scholar]
- Wang, Q.; Bin Wei, W.; Wang, Y.X.; Ni Yan, Y.; Yang, J.Y.; Zhou, W.J.; Chan, S.Y.; Xu, L.; Jonas, J.B. Thickness of individual layers at the macula and associated factors: The Beijing Eye Study 2011. BMC Ophthalmol. 2020, 20, 49. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Prokisch, H.; Hartig, M.; Hellinger, R.; Meitinger, T.; Rosenberg, T. A population-based epidemiological and genetic study of X-linked retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4012–4018. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Rosner, B.; Weigel-DiFranco, C.; Dryja, T.P.; Berson, E.L. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1298–1304. [Google Scholar] [CrossRef]
- Berson, E.L.; Sandberg, M.A.; Rosner, B.; Birch, D.G.; Hanson, A.H. Natural course of retinitis pigmentosa over a three-year interval. Am. J. Ophthalmol. 1985, 99, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.M.; Jolly, J.K.; Josan, A.S.; Wood, L.J.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Clinical applications of microperimetry in RPGR-related retinitis pigmentosa: A review. Acta Ophthalmol. 2021, 99, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Kherani, S.; Kaur, R.; Lemus, M.; Nefalar, A.; Usmani, B.; Junaid, N.; Campochiaro, P.A.; Scholl, H.P.; Shah, S.M. Progression of Retinitis Pigmentosa as Measured on Microperimetry: The PREP-1 Study. Ophthalmol. Retin. 2018, 2, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Locke, K.G.; Wen, Y.; Locke, K.I.; Hoffman, D.R.; Hood, D.C. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013, 131, 1143–1150. [Google Scholar] [CrossRef]
- Eliwa, T.F.; Hussein, M.A.; Zaki, M.A.; Raslan, O.A. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina 2018, 38, 805–811. [Google Scholar] [CrossRef]
- Anikina, E.; Georgiou, M.; Tee, J.; Webster, A.R.; Weleber, R.G.; Michaelides, M. Characterization of Retinal Function Using Microperimetry-Derived Metrics in Both Adults and Children With RPGR-Associated Retinopathy. Am. J. Ophthalmol. 2022, 234, 81–90. [Google Scholar] [CrossRef]



| n or Median | % or IQR | |
|---|---|---|
| Age at first visit (n = 47) Median (IQR), years | 20 | 12.5–36.5 |
| Age at last visit (n = 41) Median (IQR), years | 27 | 22.08–41.5 |
| Age at first symptoms (n = 14) Median (IQR), years | 7 | 2.25–12 |
| Presenting symptoms (n = 23) | ||
| Nyctalopia, n (%) | 16 | 69.6% |
| Reduced vision, n (%) | 3 | 13.0% |
| Reduced vision and nyctalopia, n (%) | 2 | 8.7% |
| Nystagmus, n (%) | 1 | 4.4% |
| Asymptomatic, n (%) | 1 | 4.4% |
| BCVA baseline, RE (n = 47) Median (IQR), LogMAR | 0.66 | 0.35–1.4 |
| BCVA last visit, RE (n = 41) Median (IQR), LogMAR | 1.3 | 0.6–1.4 |
| Lens status baseline, RE (n = 22) | ||
| Phakic clear, n (%) | 9 | 40.9% |
| Cataract, n (%) | 8 | 36.4% |
| Pseudophakia, n (%) | 5 | 22.7% |
| Fundus photo baseline, RE (n = 37) | ||
| Intra-retinal pigmentation, n (%) | 33 | 89.2% |
| Attenuated blood vessels, n (%) | 35 | 94.6% |
| Optic disc waxy pallor, n (%) | 10 | 27.0% |
| Macular changes, n (%) | 22 | 58.5% |
| FAF baseline, RE (n = 38) | ||
| Hyper autofluorescent ring, n (%) | 9 | 23.8% |
| Central hyper autofluorescence, n (%) | 6 | 15.8% |
| Severe loss of autofluorescence, n (%) | 21 | 55.3% |
| OCT metrics baseline, RE (n = 35) | ||
| EZ intact, n (%) | 12 | 34.3% |
| Severely disrupted EZ, n (%) | 23 | 65.7% |
| EZ width (n = 12) | 564.5 | 484.8–903.3 |
| CRT (n = 35) | 119 | 94–145.5 |
| PR+RPE thickness (n = 35) | 43 | 25–71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheloni, R.; Jackson, D.; Moosajee, M. A Natural History Study of RP2-Related Retinopathy. J. Clin. Med. 2022, 11, 6877. https://doi.org/10.3390/jcm11236877
Cheloni R, Jackson D, Moosajee M. A Natural History Study of RP2-Related Retinopathy. Journal of Clinical Medicine. 2022; 11(23):6877. https://doi.org/10.3390/jcm11236877
Chicago/Turabian StyleCheloni, Riccardo, Daniel Jackson, and Mariya Moosajee. 2022. "A Natural History Study of RP2-Related Retinopathy" Journal of Clinical Medicine 11, no. 23: 6877. https://doi.org/10.3390/jcm11236877
APA StyleCheloni, R., Jackson, D., & Moosajee, M. (2022). A Natural History Study of RP2-Related Retinopathy. Journal of Clinical Medicine, 11(23), 6877. https://doi.org/10.3390/jcm11236877







