The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Demographic and Clinical Data Collection
2.3. AEFIs Data Collection
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scavone, C.; Brusco, S.; Bertini, M.; Sportiello, L.; Rafaniello, C.; Zoccoli, A.; Berrino, L.; Racagni, G.; Rossi, F.; Capuano, A. Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 2020, 177, 4813–4824. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, G.; Scavone, C.; Rafaniello, C.; Rossi, F.; Capuano, A. SARS-Cov-2 infection: Response of human immune system and possible implications for the rapid test and treatment. Int. Immunopharmacol. 2020, 84, 106519. [Google Scholar] [CrossRef] [PubMed]
- Capuano, A.; Scavone, C.; Racagni, G.; Scaglione, F.; Italian Society of Pharmacology. NSAIDs in patients with viral infections, including COVID-19: Victims or perpetrators? Pharmacol. Res. 2020, 157, 104849. [Google Scholar] [CrossRef] [PubMed]
- Scavone, C.; Mascolo, A.; Rafaniello, C.; Sportiello, L.; Trama, U.; Zoccoli, A.; Bernardi, F.F.; Racagni, G.; Berrino, L.; Castaldo, G.; et al. Therapeutic strategies to fight COVID-19: Which is the status artis? Br. J. Pharmacol. 2021, 179, 2128–2148. [Google Scholar] [CrossRef]
- European Medicine Agency. Comirnaty (COVID-19 mRNA Vaccine [Nucleoside Modified]). An Overview of Comirnaty and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/comirnaty-epar-medicine-overview_en.pdf (accessed on 16 September 2021).
- European Medicine Agency. Spikevax1 (COVID-19 mRNA Vaccine [Nucleoside Modified]). An Overview of Spikevax and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/spikevax-previously-covid-19-vaccine-moderna-epar-medicine-overview_en.pdf (accessed on 16 September 2021).
- CDC. Possible Side Effects after Getting a COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (accessed on 15 June 2021).
- Raccomandazioni Aggiornate sul COVID-19 per le Persone con Sclerosi Multipla (SM)—12 Gennaio 2021 SIN ed AISM 12 Gennaio 2021 Ministero della Salute: Raccomandazioni ad Interim sui Gruppi Target della Vaccinazione Anti-SARS-CoV-2/COVID-19 8 Febbraio 2021. Available online: https://www.aism.it/sites/default/files/Raccomandazioni_COVID_SM_AISM_SIN.pdf (accessed on 16 November 2022).
- Auricchio, F.; Scavone, C.; Cimmaruta, D.; Di Mauro, G.; Capuano, A.; Sportiello, L.; Rafaniello, C. Drugs approved for the treatment of multiple sclerosis: Review of their safety profile. Expert Opin. Drug Saf. 2017, 16, 1359–1371. [Google Scholar] [CrossRef]
- Faissner, S.; Gold, R. Efficacy and Safety of the Newer Multiple Sclerosis Drugs Approved Since 2010. CNS Drugs 2018, 32, 269–287. [Google Scholar] [CrossRef]
- Tullman, M.J. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am. J. Manag. Care 2013, 19 (Suppl. S2), S15–S20. [Google Scholar]
- Chilimuri, S.; Mantri, N.; Gongati, S.; Zahid, M.; Sun, H. COVID-19 vaccine failure in a patient with multiple sclerosis on ocrelizumab. Vaccines 2021, 9, 219. [Google Scholar] [CrossRef]
- Mazzitello, C.; Esposito, S.; De Francesco, A.E.; Capuano, A.; Russo, E.; De Sarro, G. Pharmacovigilance in Italy: An overview. J. Pharmacol. Pharmacother. 2013, 4 (Suppl. S1), S20–S28. [Google Scholar] [CrossRef]
- ICH E2D Post-Approval Safety Data Management|European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-12.pdf (accessed on 16 September 2021).
- World Health Organization. Adverse Events Following Immunization (AEFI). Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi (accessed on 15 September 2021).
- WHO. Casuality Assessment of an Adverse Event Following Immunizaiton (AEFI); WHO: Geneva, Switzerland, 2013; pp. 1–56.
- Scavone, C.; Di Mauro, C.; Brusco, S.; Bertini, M.; di Mauro, G.; Rafaniello, C.; Sportiello, L.; Rossi, F.; Capuano, A. Surveillance of adverse events following immunization related to human papillomavirus vaccines: 12 years of vaccinovigilance in Southern Italy. Expert Opin. Drug Saf. 2019, 18, 427–433. [Google Scholar] [CrossRef]
- Scavone, C.; Rafaniello, C.; Brusco, S.; Bertini, M.; Menditto, E.; Orlando, V.; Trama, U.; Sportiello, L.; Rossi, F.; Capuano, A. Did the New Italian Law on Mandatory Vaccines Affect Adverse Event Following Immunization’s Reporting? A Pharmacovigilance Study in Southern Italy. Front. Pharmacol. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. FAQ Vaccini a mRNA. Available online: https://www.aifa.gov.it/domande-e-risposte-su-vaccini-mrna (accessed on 21 December 2021).
- Ziello, A.; Scavone, C.; Di Battista, M.E.; Salvatore, S.; Di Giulio Cesare, D.; Moreggia, O.; Allegorico, L.; Sagnelli, A.; Barbato, S.; Manzo, V.; et al. Influenza Vaccine Hesitancy in Patients with Multiple Sclerosis: A Monocentric Observational Study. Brain Sci. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Brescia Morra, V.; Florio, C.; Lus, G.; Tedeschi, G.; Cianfrani, M.; Docimo, R.; Miniello, S.; Romano, F.; Sinisi, L.; et al. Preliminary Results of the FASM Study, an On-Going Italian Active Pharmacovigilance Project. Pharmaceuticals 2020, 13, 466. [Google Scholar] [CrossRef]
- Koutsouraki, E.; Costa, V.; Baloyannis, S.J.; Koutsouraki, E. Epidemiology of multiple sclerosis in Europe: A Review. Int. Rev. Psychiatry 2010, 22, 2–13. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Wilf-Yarkoni, A.; Friedman, Y.; Stiebel-Kalish, H.; Steiner, I.; Hellmann, M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021, 28, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. 2021, 27, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Sibley, W.; Bamford, C.; Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1985, 325, 1313–1315. [Google Scholar] [CrossRef]
- Andersen, O.; Lygner, P.E.; Bergstr∂m, T.; Andersson, M.; Vablne, A. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef]
- Tremlett, H.; Van Der Mei, I.A.; Pittas, F.; Blizzard, L.; Paley, G.; Mesaros, D.; Woodbaker, R.; Nunez, M.; Dwyer, T.; Taylor, B.V.; et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology 2008, 31, 271–279. [Google Scholar] [CrossRef]
- Correale, J.; Fiol, M.; Gilmore, W. The risk of relapses in multiple sclerosis during systemic infections. Neurology 2006, 67, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J. The role of infections in multiple sclerosis. Mult. Scler. J. 2019, 25, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Landi, D.; Ponzano, M.; Nicoletti, C.G.; Cecchi, G.; Cola, G.; Mataluni, G.; Mercuri, N.B.; Sormani, M.P.; Marfia, G.A. Adherence to social distancing and use of personal protective equipment and the risk of SARS-CoV-2 infection in a cohort of patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102359. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum. Vaccin. Immunother. 2021, 20, 1–3. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Nistri, R.; Barbuti, E.; Rinaldi, V.; Tufano, L.; Pozzilli, V.; Ianniello, A.; Marinelli, F.; De Luca, G.; Prosperini, L.; Tomassini, V.; et al. Case Report: Multiple Sclerosis Relapses After Vaccination Against SARS-CoV2: A Series of Clinical Cases. Front. Neurol. 2021, 12, 765954. [Google Scholar] [CrossRef]
- Di Filippo, M.; Cordioli, C.; Malucchi, S.; Annovazzi, P.; Cavalla, P.; Clerici, V.T.; Ragonese, P.; Nociti, V.; Radaelli, M.; Laroni, A.; et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2021, 93, 448–450. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Petersone, L.; Edner, N.M.; Ovcinnikovs, V.; Heuts, F.; Ross, E.M.; Ntavli, E.; Wang, C.J.; Walker, L.C.K. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Front. Immunol. 2018, 9, 1941. [Google Scholar] [CrossRef]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Ferrara, A.L.; Perrella, A.; Di Battista, M.; Salvatore, S.; Graziano, D.; Viola, A.; Amato, G.; Moreggia, O.; et al. Interferon Beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects. Mult. Scler. Relat. Disord. 2021, 58, 103455. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef]
- Sormani, M.P.; Inglese, M.; Schiavetti, I.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Da Rin, G.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021, 72, 103581. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Cárdenas-Robledo, S.; Tagliani, P.; Arrambide, G.; Otero-Romero, S.; Carbonell-Mirabent, P.; Rodriguez-Barranco, M.; Rodríguez-Acevedo, B.; Restrepo Vera, J.L.; Resina-Salles, M.; et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021, 28, 3384–3395. [Google Scholar] [CrossRef]
- Moccia, M.; Erro, R.; Picillo, M.; Vassallo, E.; Vitale, C.; Longo, K.; Amboni, M.; Santangelo, G.; Palladino, R.; Nardone, A.; et al. Quitting smoking: An early non-motor feature of Parkinson’s disease? Parkinsonism Relat. Disord. 2015, 21, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Naik, B.; Pandey, S.; Biswas, B.; Pati, B.; Verma, M.; Singh, P. Effectiveness of COVID-19 vaccine in preventing infection and disease severity: A case-control study from an Eastern State of India. Epidemiol. Infect. 2021, 149, e224. [Google Scholar] [CrossRef]
- Kampf, G. The epidemiological relevance of the COVID-19-vaccinated population is increasing. Lancet Reg. Health Eur. 2021, 11, 100272. [Google Scholar] [CrossRef]
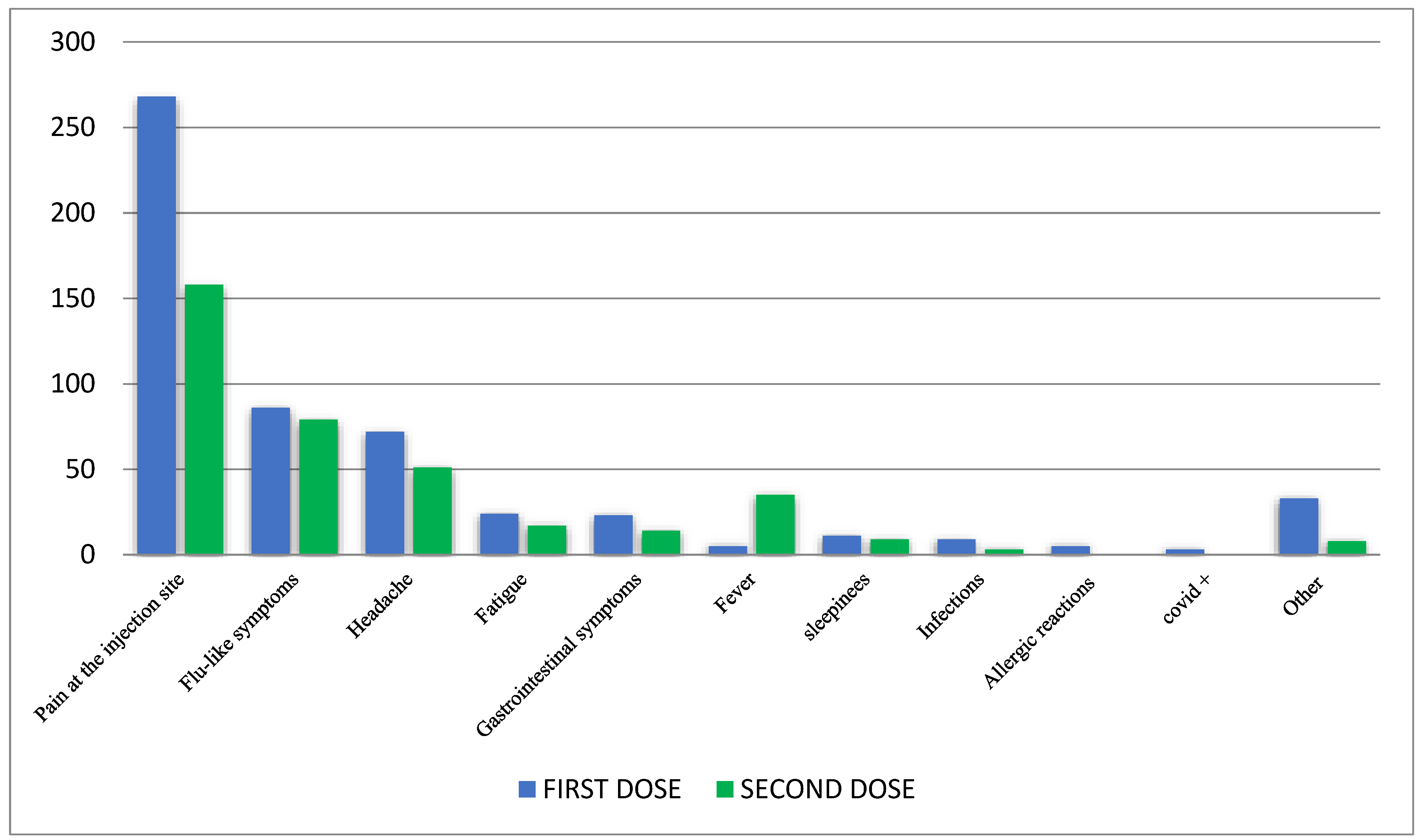
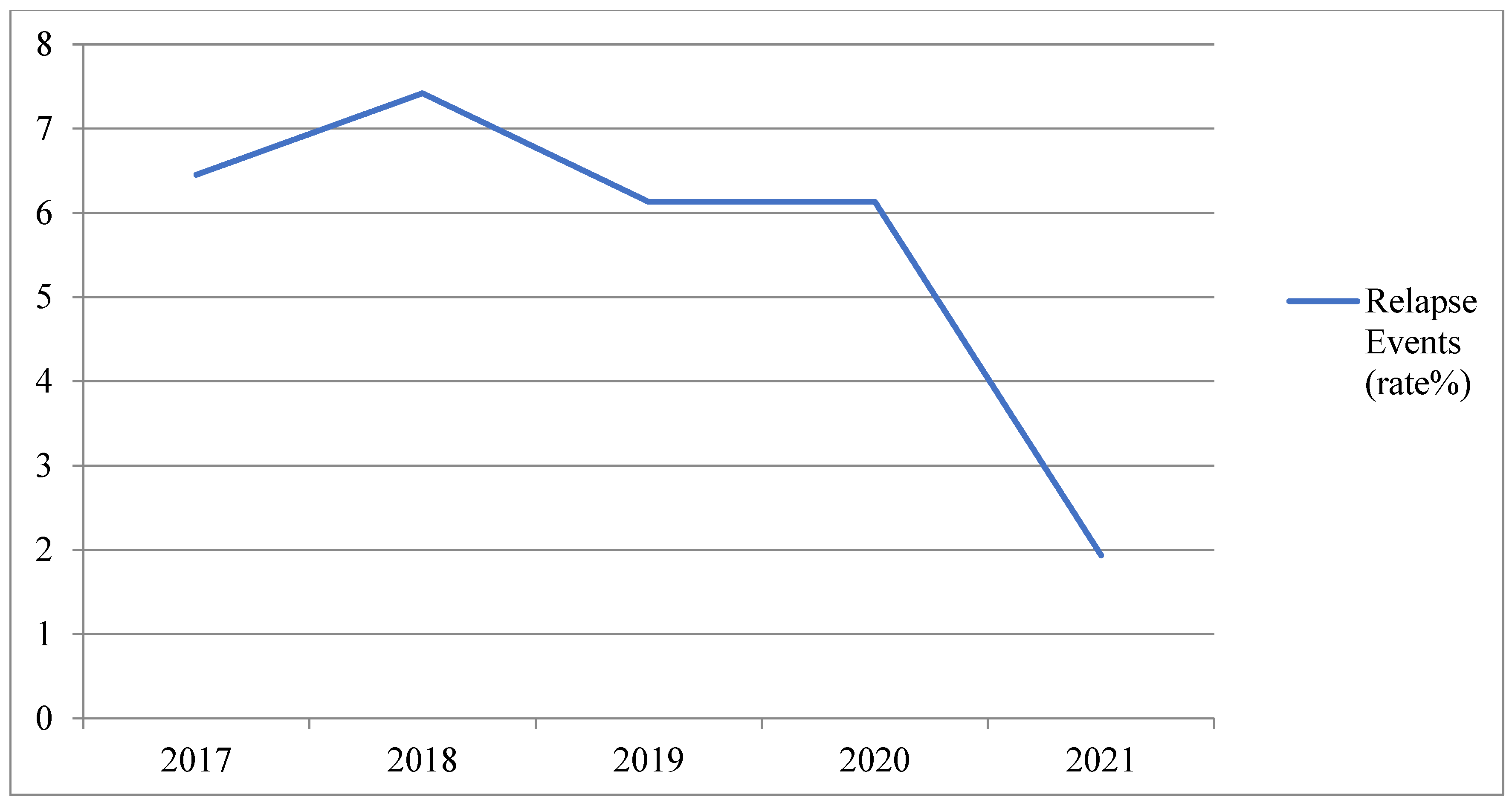
| Study Cohort | First Vaccine Dose | Second Vaccine Dose |
|---|---|---|
| Total patients | 310 | 288 |
| Gender, n (%) | ||
| Female | 195 (62.9) | 179 (62.2) |
| Male | 115 (37.1) | 109 (37.8) |
| Mean age (years) | 45.9 | 45.8 |
| Disease duration, years (mean) | 11.8 | 11.6 |
| Median (years) | 10.1 | 10.0 |
| MS type, n (%) | ||
| RRMS | 270 (87.1) | 251 (87.1) |
| SPMS | 32 (10.3) | 29 (10.1) |
| PPMS | 8 (2.6) | 8 (2.8) |
| Disability by EDSS, n (%) | ||
| ≤3.0 | 219 (70.6) | 209 (72.6) |
| 3.5–5.5 | 69 (22.3) | 59 (20.5) |
| ≥6.0 | 22 (7.1) | 20 (6.9) |
| SARS-CoV-2 infection before vaccination, n (%) | 34 (10.7) | 19 (6.6) |
| DMTs, n (%) | ||
| Interferon Beta 1-a | 61 (19.7) | 55 (19.2) |
| Glatiramer acetate | 20 (6.5) | 19 (6.7) |
| Teriflunomide | 28 (9.0) | 26 (9.0) |
| Dimethyl fumarate | 47 (15.2) | 45 (15.6) |
| Fingolimod | 35 (11.3) | 35 (12.1) |
| Natalizumab | 47 (15.2) | 43 (14.9) |
| Cladribine | 17 (5.5) | 12 (4.2) |
| Ocrelizumab | 23 (7.4) | 22 (7.7) |
| Interferon Beta 1-b | 7 (2.3) | 7 (2.4) |
| Azathioprine | 1 (0.3) | 1 (0.3) |
| Methotrexate | 3 (0.9) | 3 (1.0) |
| Rituximab | 2 (0.6) | 2 (0.7) |
| Untreated | 19 (6.1) | 18 (6.2) |
| Total | First Vaccine Dose | Second Vaccine Dose | |
|---|---|---|---|
| Study population | 310 | 310 | 288 |
| All AEFIs, n | 913 | 539 | 374 |
| Short-term AEFIs, n (%) | 790 (86.5) | 438 (81.3) | 352 (94.1) |
| Long-term AEFIs, n (%) | 123 (13.5) | 101 (18.7) | 22 (5.9) |
| Variable | Multivariable Analysis | |
|---|---|---|
| Beta Coefficients (SE) | p Value | |
| EDSS | −0.08 (0.08) | 0.30 |
| Disease duration | 0.02 (0.01) | 0.10 |
| Age | −0.02 (0.01) | 0.03 |
| Sex (female vs. male) | 0.361 (0.09) | <0.001 |
| DMTs | ||
| No therapy | Ref | Ref |
| Interferon Beta 1-a | 0.263 (0.185) | 0.156 |
| Interferon Beta 1-b | −0.361 (0.367) | 0.326 |
| Glatiramer Acetate | 0.071 (0.228) | 0.756 |
| Teriflunomide | 0.118 (0.213) | 0.579 |
| Dimethyl fumarate | 0.094 (0.193) | 0.626 |
| Natalizumab | 0.068 (0.197) | 0.730 |
| Fingolimod | 0.099 (0.202) | 0.625 |
| Ocrelizumab | 0.477 (0.208) | 0.022 |
| Cladribine | 0.101 (0.236) | 0.669 |
| Other treatment | −0.171 (0.357) | 0.631 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniscalco, G.T.; Scavone, C.; Mascolo, A.; Manzo, V.; Prestipino, E.; Guglielmi, G.; Aiezza, M.L.; Cozzolino, S.; Bracco, A.; Moreggia, O.; et al. The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study. J. Clin. Med. 2022, 11, 6855. https://doi.org/10.3390/jcm11226855
Maniscalco GT, Scavone C, Mascolo A, Manzo V, Prestipino E, Guglielmi G, Aiezza ML, Cozzolino S, Bracco A, Moreggia O, et al. The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study. Journal of Clinical Medicine. 2022; 11(22):6855. https://doi.org/10.3390/jcm11226855
Chicago/Turabian StyleManiscalco, Giorgia Teresa, Cristina Scavone, Annamaria Mascolo, Valentino Manzo, Elio Prestipino, Gaspare Guglielmi, Maria Luisa Aiezza, Santolo Cozzolino, Adele Bracco, Ornella Moreggia, and et al. 2022. "The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study" Journal of Clinical Medicine 11, no. 22: 6855. https://doi.org/10.3390/jcm11226855
APA StyleManiscalco, G. T., Scavone, C., Mascolo, A., Manzo, V., Prestipino, E., Guglielmi, G., Aiezza, M. L., Cozzolino, S., Bracco, A., Moreggia, O., Di Giulio Cesare, D., Ziello, A. R., Falco, A., Massa, M., Majolo, M., Raiola, E., Soprano, R., Russo, G., Longo, G., ... Capuano, A. (2022). The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study. Journal of Clinical Medicine, 11(22), 6855. https://doi.org/10.3390/jcm11226855






