Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease
Abstract
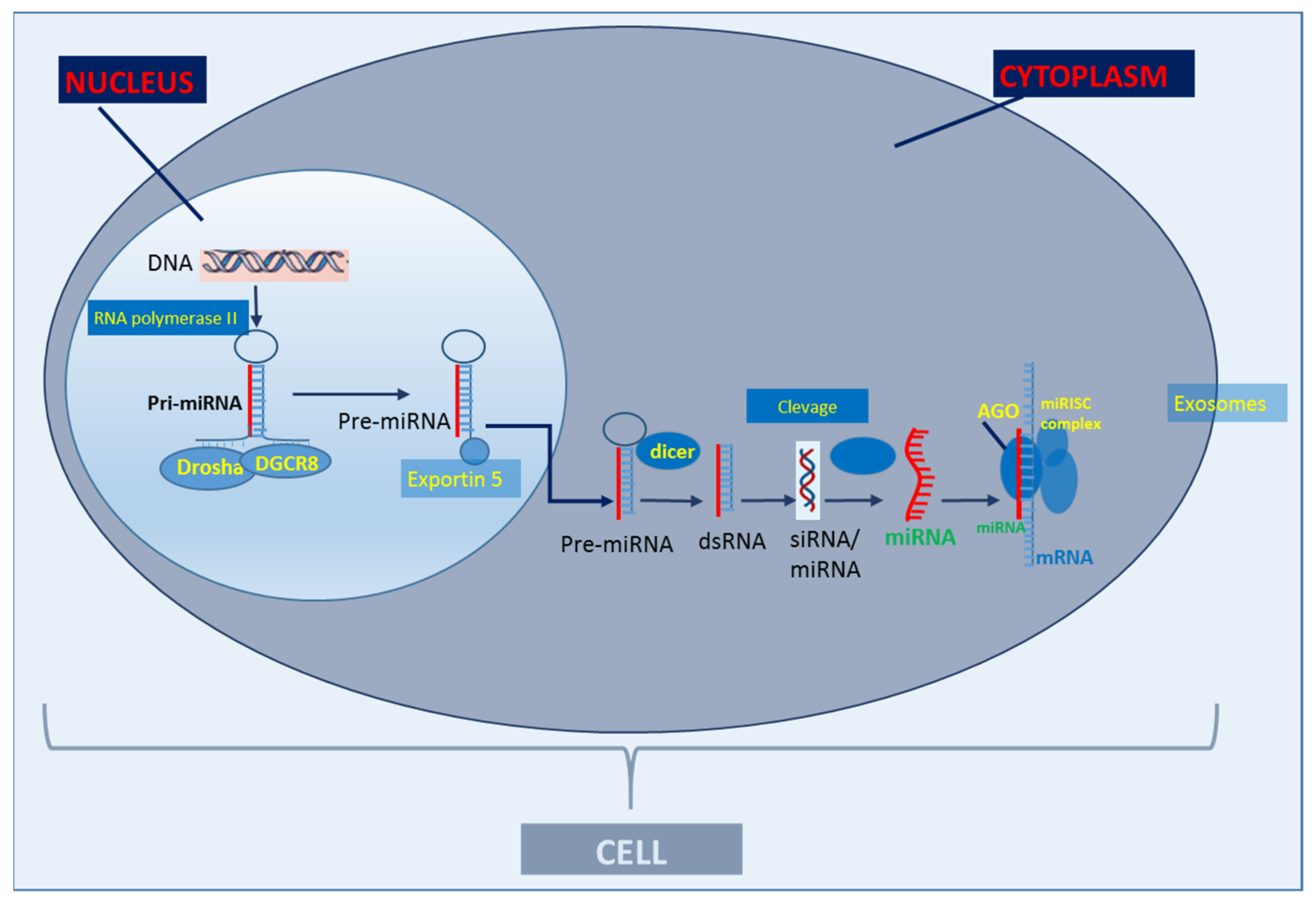
1. Introduction
2. Atherosclerotic Stable CAD
2.1. miRs Diagnostic for CAD
2.1.1. Platelets Activity
2.1.2. Vascular Smooth Muscle Cells
2.1.3. Arterial Endothelial Cells
2.1.4. Macrophages
2.1.5. Cardiomyocytes
| Postulated Role | microRNA | Down- vs. Up- Regulated | Diagnostic/Therapeutic | Reference |
|---|---|---|---|---|
| Expressed in many cells | ||||
| Highly expressed in VSMCs, ECs, cardiac fibroblasts, cardiomyocytes, and platelet apoptosis and eNOS activity | miR-21-5p | Up | D, up-regulated in CAD patients compared to controls (AUC: 0.767, p < 0.001) | Abdallah H.Y., 2022 [88] |
| Platelets | ||||
| Humans: collagen-induced platelet aggregation Mice: expression of the P2Y12 receptor | miR-126-3p | Up | D, monitors P2Y12 inhibition | Kaudewitz D., 2016 [26] |
| Responsive to antiplatelet therapy | miR-126-3p | Up | T, an antagomir against miR-126-3p reduces platelets aggregation | Kaudewitz D., 2016 [26] |
| Marker of platelet activation, that targets the COX-1 receptor through the regulation of TXS | miR-34b-3p | Up | D, miR-34b-3p may facilitate the antiplatelet efficiency of aspirin through inhibiting TXS | Liu W.W., 2009 [28] |
| Marker of response to clopidogrel, that targets the P2Y12 receptor | miR-223-3p | Down | D, high on-clopidogrel platelet reactivity | Shi R., 2016 [29] |
| miRs released by platelets, that are responsive to antiplatelet therapy | miR-126 miR-150 miR-191 miR-223 | Up Up Up Up | D, antiplatelet therapy significantly reduces their levels | Czajka P., 2021 [31] |
| VSMCs | ||||
| High expression is needed to maintain a contractile phenotype of VSMCs | miR-22 | Down | T, a stent with an miR-22 coating showed significant capability to inhibit in-stent restenosis (an animal study) | Yang F., 2018 [40] |
| Mitigates atherosclerosis, VSMCs contractility, increases fibrous cap area, and reduces the necrotic core area | miR-145 | Down Down | T, delivery of miR-145 may limit atherosclerotic plaque growth, and restore contractile levels in VSMCs | Patel N., 2022 [42] |
| Down-regulation of miR-145 plays a critical role in the pathogenesis of atherosclerotic plaques, and neointimal hyperplasia | miR-145 | Down | D, reduced plasma miR-145 levels correlate with an increase in CAD severity (SYNTAX score) | Gao H., 2015 [44] |
| Induces VSMC senescence, promotes the expression of age-associated pro-inflammatory secretory factors, and increases the binding capacity of ox-LDL to macrophages | miR-34a | Up | D, increased expression in CAD, compared to healthy controls (AUC: 0.899, p < 0.001), associated with Gensini score (p < 0.001) | Li H., 2022 [50] |
| Arterial endothelial cells | ||||
| Plays a crucial anti-atherogenic role by regulating the function of ECs and enhancing endothelial repair | miR-126-3p | Down | D, reduced expression is associated with more severe and complex CAD | Li H., 2016 [57] |
| Decreases size of atherosclerotic lesions, alleviates ox-LDL-induced EC injury | miR-126-3p | Down Up | D, decreased expression in CAD patients, compared to healthy controls, but up-regulated in ACS | Wang X., 2017, [58] |
| Induces EC apoptosis, development of atherosclerosis | miR-142-3p | Up | T, down-regulation of miR-142-3p suppresses ECs apoptosis | Qin, B., 2018 [60] |
| Induces apoptosis and oxidative stress, and is pro-atherosclerotic | miR-92a-3p miR-486 | Up Up | D, discriminate between stable and vulnerable CAD | Niculescu L.S., 2015 [66] |
| Lipid metabolism | miR-122 | Up | D, increased in CAD patients, and with CAD severity (Gensini score) | Gao W., 2012 [67] |
| Recovery of ischemic tissue | miR-17 | Down | D, reduced expression is associated with more severe and complex CAD | Chen J., 2015 [68] |
| Rate of apoptosis in ECs | miR-17-5p | Up | T, inhibition of miR-17 suppresses apoptosis, hence, decreases infarct size area, and improves microcirculation of heart tissue, decreasing heart failure symptoms | Yang S., 2018 [69] |
| Macrophages | ||||
| Regulator of cholesterol and fatty acid homeostasis, reverse cholesterol transport, increases HDL-cholesterol level | miR-33a miR-33b | Up Up | T, inhibition of miR-33a facilitates atherosclerotic regression | Price N.L., 2017 [72] |
| Inhibits oxidized LDL-induced lipid accumulation and inflammatory response | miR-146a | Up | D, patients with stable CAD had 3.62-fold higher expression level, compared to controls (AUC:0.767) | Abdallah H.Y., 2022 [88] |
| Reduced in diabetics, has a role in lipid metabolism | miR-155 | Down | D, patients with stable CAD had 1.89-fold lower expression level, compared to controls (AUC:0.767) | Abdallah H.Y., 2022 [88] |
| Cardiomyocytes | ||||
| Indicates myocardial damage | miR-223-5p | Up | D, increased expression, compared to healthy control group, with a AUC of 0.933 for predicting CAD severity | Guo J.F., 2018 [86] |
| Suppresses EC proliferation rate, viability, and migration activity involved in heart development, and indicates myocardial damage | miR-133a | Up | D, increased expression in CAD, compared to healthy controls, but with a low AUC of 0.597 correlates with Gensini score of CAD severity (r = 0.303, p = 0.007) | Zhu L., 2017 [87] |
Indicates myocardial damage Indicates myocardial damage Fibrous cap increase Indicates myocardial damage Fibrous cap thinning Plaque neovascularization Indicates myocardial damage | miR-133a miR-182 miR-145 miR-205 miR-208a miR-21 miR-126 miR-223 | Down Up Up Up Down Up Up Up | CAD vs. controls: AUC: 0.863, p < 0.001 AUC: 0.959, p < 0.001 AUC: 0.836, p < 0.001 AUC: 0.959, p < 0.001 AUC: 0.616, p = 0.015 AUC: 0.767, p < 0.001 AUC: 0.767, p < 0.001 AUC: 0.616, p = 0.015 | Abdallah H.Y., 2022 [88] |
| Induces angiogenesis and myocardial damage Indicate myocardial damage | miR-1 miR-133a | Up Up | D, increased expression, compared to a healthy control group | Kuwabara Y., 2011 [90] |
| Expressed in myocardial cells | miR-23a | Up | D, up-regulated, positive correlation with CAD severity | Lu H.Q., 2013 [91] |
| Cardiac myofibroblast differentiation, smooth muscle cell modulator, increases fibrous cap area, reduces necrotic core | miR-145 | Down | D, reduced in patients with cardiac ischemia | Zhang M., 2017 [92] |
| Indicates myocardial damage, cardiac hypertrophy Protects against H2O2-induced apoptosis | miR-208b miR-499 | Up Up | D, independent predictors of a high SYNTAX score miR-208b: AUC: 0.775, p < 0.001 miR-499: AUC: 0.713, p < 0.001 | Wang W., 2019 [93] |
| Increases foam cell formation | miR-23a | Up | D, correlates with CAD severity (Gensini score) | Wang S., 2016 [94] |
3. Acute Coronary Syndrome
3.1. miRs Diagnostic in ACS
Differences between STEMI and NSTEMI
| Study Groups, N of Participants | microRNA | Down vs. Up-Regulated | Rationale for Use of Individual microRNA | AUC, or OR (95% CI), p-Value | Reference |
|---|---|---|---|---|---|
| STEMI/NSTEMI, 93 Healthy Controls, 66 | miR-1 | Up | D, early marker, up-regulated expression, compared to healthy control group | AUC: 0.774, p < 0.001 | Ai J., 2010 [102] |
| STEMI/NSTEMI, 17 Healthy Controls, 25 | miR-1 miR-126-3p | Up Down | D, early markers, changed expressions, compared to healthy control group | AUC: 0.92, p = 0.001 AUC: 0.860, p = 0.01 | Long G., 2012 [103] |
| STEMI/NSTEMI, 33 Healthy Controls, 33 | miR-1 miR-133a miR-208a miR-499 | Up Up Up Up | D, early markers, increased expressions, compared to healthy control group | AUC: 0.850, p = 0.001 AUC: 0.870, p = 0.01 AUC: 0.970, p = 0.001 AUC: 0.820, p = 0.01 | Wang G.K., 2010 [105] |
| STEMI/NSTEMI, 142 Non-ACS chest pain, 100 Healthy Controls, 85 | miR-499 | Up | D, early marker of ACS, 1 h after onset of chest pain, correlated with CK-MB level and cTn, but not superior to cTn (AUC: 0.90) | AUC: 0.860, p < 0.001 | Zhang L., 2015 [107] |
| STEMI/NSTEMI, 27 Healthy Controls, 30 | miR-126-3p | Down | D, early marker, diagnostic effect superior to cTn (AUC 0.787) and CK-MB (AUC 0.863) | AUC: 0.992, p < 0.001 | He Y., 2017 [108] |
| STEMI, 25 Healthy Controls, 11 | miR-1 miR-133a miR-208b miR-499-5p | Up Up Up Up | D, with a peak within 12 h from onset of chest pain, expression levels of miR-208b correlated with peak cTn and the LV ejection fraction | AUC: 0.980, p < 0.001 AUC: 0.859, p = 0.007 AUC: 1.000, p < 0.001 AUC: 0.989, p < 0.001 | Gidlöf O.; 2011 [109] |
| STEMI, 106 NSTEMI, 68 Non-ACS chest pain, 163 | miR-1 | Up | D, early marker of ACS within 3 h since onset of chest pain, similar AUC to cTn (AUC: 0.862, p < 0.001) | AUC: 0.863, p < 0.001 | Su T., 2020 [110] |
| STEMI, 15 NSTEMI, 14 Healthy Controls, 21 | miR-17-5p miR-126-5p miR-145-3p | Up Up Up | D, within 4 h after the onset of chest pain | AUC: 0.857, p < 0.001 AUC: 0.802, p < 0.001 AUC: 0.720, p = 0.01 | Xue S., 2019 [111] |
| STEMI, 20 CAD, 20 Healthy Controls, 20 | miR-151-3p | Up | D, proceeded release of necrotic markers, increased expression, compared to healthy controls and stable CAD | STEMI vs. controls: AUC: 0.758, p = 0.005 STEMI vs. CAD AUC: 0.754, p = 0.006 | Horvath M., 2020 [112] |
| STEMI, 20 CAD, 20 Healthy Controls, 20 | miR-331 | Up | D, proceeded release of necrotic markers, increased expression, compared to healthy and stable CAD | STEMI vs. controls: AUC: 0.790, p = 0.002 STEMI vs. CAD AUC: 0.773, p = 0.003 | Horvath M., 2020 [112] |
| STEMI/NSTEMI, 78 Unstable angina, 201 Healthy Controls, 65 | miR-143 miR-145 | Down Down | D, down-regulated compared to controls, good predictive value for the onset of ACS | 0.087 (0.026–0.384), p = 0.019 0.179 (0.08–0.399), p < 0.001 | Meng L., 2022 [113] |
| ACS, 500 Healthy Controls, 500 | miR-143 | Down | D, down-regulated compared to controls, good predictive value for the onset of ACS | 0.56 (0.38–0.82), p = 0.003 | Dégano I.R., 2020 [114] |
| STEMI, 62 Healthy Controls, 26 | miR-23a-3p miR-30d-5p miR-146a-5p | Down Down Down | D, for STEMI vs. healthy controls; p, correlated with GRACE and APACHE scores of in-hospital mortality, and 1-month survival D, for STEMI vs. healthy controls D, for STEMI vs. healthy controls | AUC: 0.806, p < 0.05 p = 0.045 (log-rank tests) AUC 0.745, p <0.05 AUC 0.800, p < 0.05 | Bukauskas T., 2019 [115] |
| NSTEMI, 137 Chest pain *, 905 | miR-126 miR-133 miR-134 | Up Up Up | D, diagnostic for NSTEMI, but not superior to cTn (AUC: 0.937) | AUC: 0.578, p = 0.003 AUC: 0.656, p < 0.001 AUC: 0.506, p = 0.032 | Biener M., 2021 [119] |
| NSTEMI, 145 Healthy Controls, 30 | miR-1 miR-133 miR-208 miR-499 | Up Up Up Up | D, for NSTEMI vs. healthy controls, miR-133, miR-208 and miR-499 superior to cTn (AUC: 0.778) | AUC: 0.772, p < 0.05 AUC: 0.928, p < 0.05 AUC: 0.994, p < 0.05 AUC: 0.994, p < 0.05 | Liu G., 2018 [120] |
| STEMI, 16 NSTEMI, 27 | miR-134 miR-134 miR-124 miR-133b | Up Up Up Up | D, for STEMI, but not superior to cTn D, for occluded IRA D, for occluded IRA D, for occluded IRA | AUC: 0.725, p = 0.002 AUC: 0.686, p = 0.016 AUC: 0.787, p < 0.001 AUC: 0.704, p = 0.006 | Gacoń J., 2016 [122] |
| NSTEMI | miR-223-3p | Down | Marker of response to clopidogrel, targets P2Y12 receptor D, lower response to clopidogrel in NSTEMI | 0.111, (0.018–0.692), p = 0.019 | Zhang Y.Y., 2014 [123] |
3.2. MiRs Worth of Examination as They Might Be Prognostic for Outcomes
3.2.1. miRs Predictive of Plaque Instability and Myocardial Infarction in Patients with Stable CAD or after Index ACS
| Study Groups | microRNA | Down vs. Up-Regulated | Prognostic/Therapeutic | Statistical Analysis AUC, or HR/OR (95% CI), p-Value | Reference |
|---|---|---|---|---|---|
| Cardiovascular events | |||||
| 2812 general population subjects | miR-423-3p | Up | p, for ACS during a median follow-up of 6 y. | Better model including miR-423 (AUC: 0.806) vs. traditional risk factors (AUC: 0.782) | Wang X., 2020 [125] |
| 62 STEMI patients 26 healthy controls | miR-23a-3p | Down | p, correlates with GRACE and APACHE scores of in-hospital mortality, and 1-month survival | AUC 0.806, p < 0.05 p = 0.045 (log-rank tests) | Bukauskas T., 2019 [115] |
| 598 ACS patients randomized to ticagrelor vs. prasugrel treatment | miR-223-3p | Up | p, CVD/re-MI/IS at 30d. CVD/re-MI/IS at 1 y. | 15.74 (2.07–119.9), p = 0.008 3.18 (1.40–7.19), p = 0.006 | Hromadka M., 2021 [127] |
| 598 ACS patients randomized to ticagrelor vs. prasugrel treatment | miR-126 to miR-223 ratio | Low | p, CVD/re-MI/IS at 30d. CVD/re-MI/IS at 1 y. | 0.14 (0.03–0.61), p = 0.009 0.37 (0.17–0.82), p = 0.014 | Hromadka M., 2021 [127] |
| 50 STEMI patients 10 healthy controls | miR-223-3p miR-142-3p miR-146a-5p | Up Up Up | p, for CVD/readmission for HF/new cardiovascular intervention | AUC 0.832, p =0.002 AUC 0.732, p = 0.031 AUC 0.848, p = 0.001 | Scărlătescu A.I., 2022 [128] |
| 340 ACS patients 533 patients with stable CAD | miR-197 miR-223 miR-126 miR-197 miR-223 | Up Up Up Up Up | p, for CVD after MI at 4 y. p, for CVD after adjustment to age at 4 y. | 2.24 (1.25; 4.01), p = 0.006 4.94 (1.42; 17.2), p = 0.012 3.47 (1.39; 8.66), p = 0.008 3.37 (1.35; 8.39), p = 0.009 3.54 (1.41; 8.92), p = 0.007 | Schulte C., 2015 [129] |
| 430 ACS patients 682 patients with stable CAD | miR-19b miR-132 miR-140-3p miR-150 miR-186 miR-210 | Up Up Up Up Up Up | p, for CVD at 4 years | 3.59 (1.27–10.15), 0.025 2.85 (1.33–6.08), 0.022 2.88 (1.36–6.09), 0.022 2.14 (1.21–3.79), 0.022 2.08 (1.18–3.66), 0.022 3.10 (1.12–8.55), 0.039 | Karakas M., 2017 [130] |
| 7 STEMI patients 7 healthy controls | miR-146a | Up | p, MACE at 3 years | 1.329 (1.06–1.664), p = 0.01 | Xiao S., 2021 [132] |
| 21 ACS patients 8 healthy controls | miR-208b | Up | p, elevated miR-208b expression was associated with reduced 6-month survival | 5.08 (1.13–22.82), p = 0.03 | Alavi-Moghaddam M., 2018 [133] |
| 142 patients with ACS or ischemic carotid event | miR-1-3p | Up | p, expression levels during incident ischemia are risk factors of CVD at 6 y. | 2.73 (1.22–6.12), p = 0.014 | Badacz R., 2021 [134] |
| Left ventricular remodeling | |||||
| 80 patients with STEMI | miR-1 | Up | p, for LVEDV increase >10% at 6 months, better value in combination with CK-MB, Nt-pro-BNP and CMR | miR-1: AUC: 0.680 miR-1 + CMR + NT-pro-BNP+ CK-MB: AUC: 0.890 | Ma Q., 2020 [136] |
| 44 ACS patients | miR-1 miR-21 miR-29a | Up Up Up | p, for absolute change for LVEDV at 6 months | miR-1 and miR-29b correlated with lower infarct zone, miR-29b correlated with absolute change in LVEDV | Grabmaier, U., 2017 [137] |
| 14 patients with STEMI | miR-30a-5p | Up | p, for LVEF < 50%, and NT-proBNP > 150pg/mL at 6 months | AUC: 0.750 (0.58–0.92) | Maciejak A., 2018 [138] |
| 198 ACS patients | miR-21 miR-146a | Up Up | miR-21 and miR-146a are early biomarkers of LVR p, for LVEDV increase >20% at 1 year | miR-146a: AUC: 0.818 miR-21: AUC: 0.719 in combination they have a higher predictive power | Liu, X., 2015 [139] |
| 359 patients with MI | miR-34a miR-208a | Up Up | p, for CVD and LVEDV increase >10% at 6 months | miR-34a: OR 17.91 (2.07–98.81), p = 0.003 miR-208b: OR 4.18 (1.36–12.83), p = 0.012 combination of the two miRs: OR 18.73 (1.96–101.23), p < 0.001) | Lv P., 2014 [140] |
| 113 MI patients 59 healthy controls | miR-150 | Down | p, for HF and LVEF at 1-year, better value in combination with BNP | HR: 1.233 (1.125–1.352) miR-150 alone: AUC: 0.764 miR-150 plus BNP: AUC: 0.807 | Lin X., 2019 [141] |
| 12 ACS patients 12 healthy controls | miR-29a | Up | The greater increase in miR-29a, the greater increase in LVEDV at 90 days post MI | early miR-29a increase correlates with a negative outcome of post-MI LVR | Zile M.R., 2011 [142] |
| Ischemia/reperfusion injury | |||||
| 44 patients deceased (19 for ACS, 25 as SCD) 18 trauma victims | miR-1 miR-499 miR-208 | Up Up Up | p, miR-1 and miR-499 are sensitive markers to diagnose SCD compared ACS, and miR-208 for ACS vs. controls | SCD vs. ACS (AUC: 0.917) SCD vs. ACS (AUC: 0.898) ACS vs. controls (AUC: 0.855) | Pinchi E., 2019 [143] |
| 24 patients deceased for ACS 8 patients deceased in accidents | miR-1 | Up | p, might play role in cardiac remodeling | 3.8-fold increase in miR-1 in remote myocardium | Boštjancic E., 2010 [144] |
| 47 patients deceased for ACS, including 23 from VF 8 trauma victims | miR-133a/b | Down | p, may contribute to VF | For VF, 2.9-fold decrease in miR-133a/b level in remote myocardium | Boštjancic E., 2018 [145] |
3.2.2. miRs Associated with Myocardial I/R Injury and LVR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tanase, D.M.; Gosav, E.M.; Ouatu, A.; Badescu, M.C.; Dima, N.; Ganceanu-Rusu, A.R.; Popescu, D.; Floria, M.; Rezus, E.; Rezus, C. Current Knowledge of MicroRNAs (miRNAs) in Acute Coronary Syndrome (ACS): ST-Elevation Myocardial Infarction (STEMI). Life 2021, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, J.; Xu, N.; Han, G.; Geng, Q.; Song, J.; Li, S.; Zhao, J.; Chen, H. Signature of circulating microRNAs as po-tential biomarkers in vulnerable coronary artery disease. PLoS ONE 2013, 8, e80738. [Google Scholar] [CrossRef]
- Badacz, R.; Przewlocki, T.; Gacoń, J.; Stępień, E.; Enguita, F.J.; Karch, I.; Żmudka, K.; Kabłak-Ziembicka, A. Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Adv. Interv. Cardiol. 2018, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kabłak-Ziembicka, A.; Przewłocki, T. Clinical Significance of Carotid Intima-Media Complex and Carotid Plaque As-sessment by Ultrasound for the Prediction of Adverse Cardiovascular Events in Primary and Secondary Care Patients. J. Clin. Med. 2021, 10, 4628. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiol-ogy (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Przewłocki, T.; Kablak-Ziembicka, A.; Tracz, W.; Kozanecki, A.; Kopeć, G.; Rubiś, P.; Kostkiewicz, M.; Rosławiecka, A.; Rzeźnik, D.; Stompór, T. Renal artery stenosis in patients with coronary artery disease. Kardiol. Pol. 2008, 66, 856–862. [Google Scholar]
- Yates, L.A.; Norbury, C.J.; Gilbert, R.J. The Long and Short of MicroRNA. Cell 2013, 153, 516–519. [Google Scholar] [CrossRef]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. Role of MicroRNAs in the Pathogenesis of Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 632392. [Google Scholar] [CrossRef]
- Cipollone, F.; Felicioni, L.; Sarzani, R.; Ucchino, S.; Spigonardo, F.; Mandolini, C.; Malatesta, S.; Bucci, M.; Mammarella, C.; Santovito, D.; et al. A unique Microrna signature associated with plaque instability in humans. Stroke 2011, 42, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P.; Šabovič, M.; Mijovski, M.B.; Nikolajević, J.; Antignani, P.L.; Paraskevas, K.I.; Mikhailidis, D.P.; Blinc, A. Inflammatory and Prothrombotic Biomarkers, DNA Polymorphisms, MicroRNAs and Personalized Medicine for Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2022, 23, 12054. [Google Scholar] [CrossRef]
- Puz, P.; Lasek-Bal, A.; Warsz-Wianecka, A.; Kaźmierski, M. Prevalence of atherosclerotic stenosis of carotid and cerebral arteries in patients with stable or unstable coronary artery disease. Pol. Arch. Intern. Med. 2020, 130, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gacoń, J.; Przewłocki, T.; Podolec, J.; Badacz, R.; Pieniążek, P.; Mleczko, S.; Ryniewicz, W.; Żmudka, K.; Kabłak-Ziembicka, A. Prospective study on the prognostic value of repeated carotid intima-media thickness assessment in patients with coronary and extra coronary steno-occlusive arterial disease. Pol. Arch. Intern. Med. 2019, 129, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Vedanthan, R.; Seligman, B.; Fuster, V. Global perspective on acute coronary syndrome: A burden on the young and poor. Circ Res. 2014, 114, 1959–1975. [Google Scholar] [CrossRef]
- Stefan, J.; Bueno, H. Epidemiology of acute coronary syndromes. In The ESC Textbook of Cardiovascular Medicine, 3rd ed.; Camm, A.J., Ed.; The European Society of Cardiology Series; ESC Publications: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Sayed, D.; Abdellatif, M. MicroRNAs in Development and Disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef]
- Weber, M.; Baker, M.B.; Moore, J.P.; Searles, C.D. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun. 2010, 393, 643–648. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- Nikas, J.B.; Low, W.C. ROC-supervised principal component analysis in connection with the diagnosis of diseases. Am. J. Transl. Res. 2011, 3, 180–196. [Google Scholar]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating Micrornas in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef]
- Zhang, C. MicroRNA-145 in vascular smooth muscle cell biology: A new therapeutic target for vascular disease. Cell Cycle 2009, 8, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Gnatenko, D.V.; Dunn, J.J.; Schwedes, J.; Bahou, W.F. Transcript profiling of human platelets using microarray and serial analysis of gene expression (SAGE). Methods Mol. Biol. 2009, 496, 245–272. [Google Scholar] [PubMed]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef]
- Pordzik, J.; Pisarz, K.; De Rosa, S.; Jones, A.D.; Eyileten, C.; Indolfi, C.; Małek, L.; Postula, M. The Potential Role of Platelet-Related microRNAs in the Development of Cardiovascular Events in High-Risk Populations, Including Diabetic Patients: A Review. Front. Endocrinol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaudewitz, D.; Skroblin, P.; Bender, L.H.; Barwari, T.; Willeit, P.; Pechlaner, R.; Sunderland, N.P.; Willeit, K.; Morton, A.C.; Arm-strong, P.C.; et al. Association of MicroRNAs and YRNAs With Platelet Function. Circ Res. 2016, 118, 420–432. [Google Scholar] [CrossRef]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating micrornas as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Wang, H.; Chen, X.H.; Fu, S.W.; Liu, M.L. miR-34b-3p may promote antiplatelet efficiency of aspirin by inhibiting thromboxane synthase expression. Thromb. Haemost. 2019, 119, 1451–1460. [Google Scholar] [CrossRef]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.-J.; Lu, R.-Y.; Zhang, Y.-Y.; Zeng, S.; Liu, X.; Zhao, J.-H.; Zhang, W.-C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb. Res. 2013, 131, 508–513. [Google Scholar] [CrossRef]
- Krammer, T.L.; Mayr, M.; Hackl, M. microRNAs as promising biomarkers of platelet activity in antiplatelet therapy monitoring. Int. J. Mol. Sci. 2020, 21, 3477. [Google Scholar] [CrossRef]
- Czajka, P.; Fitas, A.; Jakubik, D.; Eyileten, C.; Gasecka, A.; Wicik, Z.; Siller-Matula, J.M.; Filipiak, K.J.; Postula, M. MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review. Front. Physiol. 2021, 12, 652579. [Google Scholar] [CrossRef]
- Wang, D.; Atanasov, A.G. The microRNAs Regulating Vascular Smooth Muscle Cell Proliferation: A Minireview. Int. J. Mol. Sci. 2019, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Burba, I.; Ruggeri, C.; Zeni, F.; Bertolotti, M.; Scopece, A.; Pompilio, G.; Raucci, A. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Warren, D.T. Vascular smooth muscle cell contractile function and mechanotransduction. Vessel Plus 2018, 2, 36. [Google Scholar] [CrossRef]
- Nguyen, D.N.D.; Chilian, W.M.; Zain, S.M.; Daud, M.F.; Pung, Y.-F. MicroRNA regulation of vascular smooth muscle cells and its significance in cardiovascular diseases. Can. J. Physiol. Pharmacol. 2021, 99, 827–838. [Google Scholar] [CrossRef]
- Baran, J.; Kleczyński, P.; Niewiara, Ł.; Podolec, J.; Badacz, R.; Gackowski, A.; Pieniążek, P.; Legutko, J.; Żmudka, K.; Przewłocki, T.; et al. Importance of Increased Arterial Resistance in Risk Prediction in Patients with Cardiovascular Risk Factors and Degenerative Aortic Stenosis. J. Clin. Med. 2021, 10, 2109. [Google Scholar] [CrossRef]
- Arnăutu, S.F.; Morariu, V.I.; Arnăutu, D.A.; Tomescu, M.C. The Predictive Value of Carotid Artery Strain and Strain-Rate in Assessing the 3-Year Risk for Stroke and Acute Coronary Syndrome in Patients with Metabolic Syndrome. Rev. Cardiovasc. Med. 2022, 23, 146. [Google Scholar] [CrossRef]
- Zhang, J.; Starkuviene, V.; Erfle, H.; Wang, Z.; Gunkel, M.; Zeng, Z.; Sticht, C.; Kan, K.; Rahbari, N.; Keese, M. High-content analysis of microRNAs involved in the phenotype regulation of vascular smooth muscle cells. Sci. Rep. 2022, 12, 3498. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 2018, 137, 1824–1841. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Xie, B.-D.; Sun, L.; Chen, W.; Jiang, S.-L.; Liu, W.; Bian, F.; Tian, H.; Li, R.-K. Phenotypic switching of vascular smooth muscle cells in the ‘normal region’ of aorta from atherosclerosis patients is regulated by miR-145. J. Cell. Mol. Med. 2016, 20, 1049–1061. [Google Scholar] [CrossRef]
- Patel, N.; Chin, D.D.; Magee, G.A.; Chung, E.J. Therapeutic Response of miR-145 Micelles on Patient-Derived Vascular Smooth Muscle Cells. Front. Digit. Health 2022, 4, 836579. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M.; et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012, 126, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guddeti, R.R.; Matsuzawa, Y.; Liu, L.-P.; Su, L.-X.; Guo, D.; Nie, S.-P.; Du, J.; Zhang, M. Plasma Levels of microRNA-145 Are Associated with Severity of Coronary Artery Disease. PLoS ONE 2015, 10, e0123477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Qian, H.-L.; Chen, S.-Y.; Huang, W.-P.; Huang, D.-N.; Hao, H.-Y.; Ren, K.-F.; Wang, Y.-B.; Fu, G.-S.; Ji, J. miR-22 eluting cardiovascular stent based on a self-healable spongy coating inhibits in-stent restenosis. Bioact. Mater. 2021, 6, 4686–4696. [Google Scholar] [CrossRef]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.H.; et al. Local MicroRNA modulation using a novel anti-miR-21-eluting stent effectively prevents experimental in-stent restenosis. Arter. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef]
- Dai, H.; Wang, J.; Shi, Z.; Ji, X.; Huang, Y.; Zhou, R. Predictive value of miRNA-21 on coronary restenosis after percuta-neous coronary intervention in patients with coronary heart disease. A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24966. [Google Scholar] [CrossRef]
- Tekieli, Ł.; Musiałek, P.; Kablak-Ziembicka, A.; Trystuła, M.; Przewłocki, T.; Legutko, J.; Dzierwa, K.; Maciejewski, D.; Michalski, M.; Pieniążek, P. Severe, recurrent in-stent carotid restenosis: Endovascular approach, risk factors. Results from a prospective academic registry of 2637 consecutive carotid artery stenting procedures (TARGET-CAS). Adv. Interv. Cardiol. 2019, 15, 465–471. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; McCormick, R.; Molloy, A.P.; McArdle, A.; Goljanek-Whysall, K. Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell 2016, 15, 361–369. [Google Scholar] [CrossRef]
- Li, H.; Chen, M.; Feng, Q.; Zhu, L.; Bai, Z.; Wang, B.; Guo, Z.; Hou, A.; Li, H. MicroRNA-34a in coronary heart disease: Correlation with disease risk, blood lipid, stenosis degree, inflammatory cytokines, and cell adhesion molecules. J. Clin. Lab. Anal. 2021, 36, e24138. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Schober, A.; Maleki, S.S.; Nazari-Jahantigh, M. Regulatory Non-coding RNAs in Atherosclerosis. In Textbook: Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; Von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of MicroRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yang, T.-L. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 495, 1482–1489. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, X.; Liu, Y.-Z.; Meng, Z.; Wang, D.; Yang, F.; Shi, Q.-W. Plasma MicroRNA-126-5p is Associated with the Complexity and Severity of Coronary Artery Disease in Patients with Stable Angina Pectoris. Cell. Physiol. Biochem. 2016, 39, 837–846. [Google Scholar] [CrossRef]
- Wang, X.; Lian, Y.; Wen, X.; Guo, J.; Wang, Z.; Jiang, S.; Hu, Y. Expression of miR-126 and its potential function in coronary ar-tery disease. Afr. Health Sci. 2017, 17, 474–480. [Google Scholar] [CrossRef][Green Version]
- Huang, R.; Hu, Z.; Cao, Y.; Li, H.; Zhang, H.; Su, W.; Xu, Y.; Liang, L.; Melgiri, N.; Jiang, L. MiR-652-3p inhibition enhances endothelial repair and reduces atherosclerosis by promoting Cyclin D2 expression. eBioMedicine 2019, 40, 685–694. [Google Scholar] [CrossRef]
- Qin, B.; Shu, Y.; Long, L.; Li, H.; Men, X.; Feng, L.; Yang, H.; Lu, Z. MicroRNA-142-3p Induces Atherosclero-sis-Associated Endothelial Cell Apoptosis by Directly Targeting Rictor. Cell. Physiol. Biochem. 2018, 47, 1589–1603. [Google Scholar] [CrossRef]
- Hartmann, P.; Zhou, Z.; Natarelli, L.; Wei, Y.; Nazari-Jahantigh, M.; Zhu, M.; Grommes, G.; Steffens, S.; Weber, C.; Schober, A. Endothelial dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nat. Commun. 2016, 7, 10521. [Google Scholar] [CrossRef]
- Jiang, L.; Qiao, Y.; Wang, Z.; Ma, X.; Wang, H.; Li, J. Inhibition of microRNA-103 attenuates inflammation and endoplasmic re-ticulum stress in atherosclerosis through disrupting the PTEN-mediated MAPK signaling. J. Cell Physiol. 2020, 235, 380–393. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, L.; Martin, M.; Hsu, C.-Y.; Fang, L.; Lin, F.-M.; Lin, T.-Y.; Geary, M.J.; Geary, G.G.; Zhao, Y.; et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of MicroRNA-92a. Circulation 2015, 131, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miao, C.; Cui, J.; Bian, X. miR-92a-3p promotes ox-LDL induced-apoptosis in HUVECs via targeting SIRT6 and activating MAPK signaling pathway. Braz. J. Med. Biol. Res. 2021, 54, e9386. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Ito, H. Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy. J. Clin. Med. 2021, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, L.S.; Simionescu, N.; Sanda, G.M.; Carnuta, M.G.; Stancu, C.S.; Popescu, A.C.; Popescu, M.; Vlad, A.; Dimulescu, D.R.; Simionescu, M.; et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE 2015, 10, e0140958. [Google Scholar] [CrossRef]
- Gao, W.; He, H.-W.; Wang, Z.-M.; Zhao, H.; Lian, X.-Q.; Wang, Y.-S.; Zhu, J.; Yan, J.-J.; Zhang, D.-G.; Yang, Z.-J.; et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012, 11, 55. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Hu, Q.; Yang, S.; Zhang, B.; Jiang, H. MiR-17-5p as circulating biomarkers for the severity of coronary atherosclerosis in coronary artery disease. Int. J. Cardiol. 2015, 197, 123–124. [Google Scholar] [CrossRef]
- Yang, S.; Fan, T.; Hu, Q.; Xu, W.; Yang, J.; Xu, C.; Zhang, B.; Chen, J.; Jiang, H. Downregulation of microRNA-17-5p improves cardiac function after myocardial infarction via attenuation of apoptosis in endothelial cells. Mol. Genet. Genomics 2018, 293, 883–894. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Rotllan, N.; Zhang, X.; Fernandez-Fuertes, M.; Ramirez-Hidalgo, C.; Araldi, E. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol. Med. 2017, 9, 1244–1262. [Google Scholar] [CrossRef]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef]
- Price, N.L.; Rotllan, N.; Canfrán-Duque, A.; Zhang, X.; Pati, P.; Arias, N.; Moen, J.; Mayr, M.; Ford, D.A.; Baldán, Á.; et al. Genetic Dissection of the Impact of miR-33a and miR-33b during the Progression of Atherosclerosis. Cell Rep. 2017, 21, 1317–1330. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Corbalán-Campos, J.; Heyll, K.; Weber, C.; Schober, A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of MicroRNA-155 on atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Androulidaki, A.; Iliopoulos, D.; Arranz, A.; Doxaki, C.; Schworer, S.; Zacharioudaki, V.; Margioris, A.N.; Tsichlis, P.N.; Tsatsanis, C. The kinase Akt1 controls macro-phage response to lipopolysaccharide by regulating microRNAs. Immunity 2009, 31, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Bjerre Knudsen, L. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.; Gambardella, J.; Sardu, C.; Lombardi, A.; Santulli, G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Non-Coding RNA 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Pieniazek, P.; Tracz, W.; Tekieli, L.; Przewlocki, T.; Kablak-Ziembicka, A.; Motyl, R.; Moczulski, Z.; Stepniewski, J.; Trystula, M.; et al. Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions. Med. Sci. Monit. 2012, 18, MT7–MT18. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Su, Y.L.; Wang, X.; Mann, M.; Adamus, T.P.; Wang, D.; Moreira, D.F.; Zhang, Z.; Ouyang, C.; He, X.; Zhang, B.; et al. Myeloid cell-targeted miR-146a mimic inhibits NF-kappaB-driven inflammation and leukemia progression in vivo. Blood 2020, 135, 167–180. [Google Scholar] [CrossRef]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lip-oprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef]
- Arroyo, A.B.; Águila, S.; Fernández-Pérez, M.P.; Reyes-García, A.M.L.; Reguilón-Gallego, L.; Zapata-Martínez, L.; Vicente, V.; Mar-tínez, C.; González-Conejero, R. miR-146a in Cardiovascular Diseases and Sepsis: An Additional Burden in the Inflamma-tory Balance? Thromb. Haemost. 2021, 121, 1138–1150. [Google Scholar] [CrossRef]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction−induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2018, 120, 4433–4443. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Y.; Gao, L.; Mao, C.; Zeng, H.; Wang, X.; Sun, Y.; Gu, J.; Wang, Y.; Chen, K.; et al. MicroRNA-146a protects against myocardial ischaemia reperfusion injury by targeting Med1. Cell Mol. Biol. Lett. 2019, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Elfaki, I.; Khullar, N.; Waza, A.; Jha, C.; Mir, M.; Nisa, S.; Mohammad, B.; Mir, T.; Maqbool, M.; et al. Role of Selected miRNAs as Diagnostic and Prognostic Biomarkers in Cardiovascular Diseases, Including Coronary Artery Disease, Myocardial Infarction and Atherosclerosis. J. Cardiovasc. Dev. Dis. 2021, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; E Creemers, E.; Pinto-Sietsma, S.-J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-F.; Zhang, Y.; Zheng, Q.-X.; Zhang, Y.; Zhou, H.-H.; Cui, L.-M. Association between elevated plasma microRNA-223 content and severity of coronary heart disease. Scand. J. Clin. Lab. Investig. 2018, 78, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. The correlations of circulating microRNA-133a with the risk and severity of coronary heart disease. Int. J. Clin. Exp. Med. 2017, 10, 972–978. [Google Scholar]
- Abdallah, H.Y.; Hassan, R.; Fareed, A.; Abdelgawad, M.; Mostafa, S.A.; Mohammed, E.A.-M. Identification of a circulating microRNAs biomarker panel for non-invasive diagnosis of coronary artery disease: Case–control study. BMC Cardiovasc. Disord. 2022, 22, 286. [Google Scholar] [CrossRef]
- De Rosa, S.; Fichtlscherer, S.; Lehmann, R.; Assmus, B.; Dimmeler, S.; Zeiher, A.M. Transcoronary Concentration Gradients of Circulating MicroRNAs. Circulation 2011, 124, 1936–1944. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef]
- Lu, H.-Q.; Liang, C.; He, Z.-Q.; Fan, M.; Wu, Z.-G. Circulating miR-214 is associated with the severity of coronary artery disease. J. Geriatr. Cardiol. 2013, 10, 34–38. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Y.-J.; Sara, J.D.; Liu, L.-J.; Zhao, X.; Gao, H. Circulating MicroRNA-145 is Associated with Acute Myocardial Infarction and Heart Failure. Chin. Med. J. 2017, 130, 51–56. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Gao, L.; Li, Y.; Sun, Y.; Yao, H.-C. Plasma miR-208b and miR-499: Potential Biomarkers for Severity of Coronary Artery Disease. Dis. Markers 2019, 2019, 9842427. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, W.; Wang, C. MiR-23a Regulates the Vasculogenesis of Coronary Artery Disease by Targeting Epidermal Growth Factor Receptor. Cardiovasc. Ther. 2016, 34, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Da-Silva, T.; Napoleão, P.; Costa, M.; Gabriel, A.; Selas, M.; Silva, F.; Enguita, F.; Ferreira, R.; Carmo, M. Circulating miRNAs Are Associated with the Systemic Extent of Atherosclerosis: Novel Observations for miR-27b and miR-146. Diagnostics 2021, 11, 318. [Google Scholar] [CrossRef]
- Przewłocki, T.; Kablak-Ziembicka, A.; Kozanecki, A.; Rzeźnik, D.; Pieniazek, P.; Musiałek, P.; Piskorz, A.; Sokołowski, A.; Rosławiecka, A.; Tracz, W. Polyvascular extracoronary atherosclerotic disease in patients with coronary artery disease. Kardiol. Pol. 2009, 67, 978–984. [Google Scholar]
- Vrsalovic, M.; Presecki, A.V.; Aboyans, V. Cardiac troponins predict mortality and cardiovascular outcomes in patients with peripheral artery disease: A systematic review and meta-analysis of adjusted observational studies. Clin. Cardiol. 2022, 45, 198–204. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Rosławiecka, A.; Badacz, R.; Sokołowski, A.; Rzeźnik, D.; Trystuła, M.; Musiałek, P.; Przewłocki, T. Simple clinical scores to predict blood pressure and renal function response to renal artery stenting for atherosclerotic renal artery stenosis. Pol. Arch. Intern. Med. 2020, 130, 953–959. [Google Scholar] [CrossRef]
- Faccini, J.; Ruidavets, J.-B.; Cordelier, P.; Martins, F.; Maoret, J.-J.; Bongard, V.; Ferrieres, J.; Roncalli, J.; Elbaz, M.; Vindis, C. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Sci. Rep. 2017, 7, srep42916. [Google Scholar] [CrossRef] [PubMed]
- Gacoń, J.; Badacz, R.; Stępień, E.; Karch, I.; Enguita, F.J.; Żmudka, K.; Przewłocki, T.; Kabłak-Ziembicka, A. Diagnostic and prognostic micro-RNAs in ischaemic stroke due to carotid artery stenosis and in acute coronary syndrome: A four-year prospective study. Kardiol. Pol. 2018, 76, 362–369. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar] [CrossRef]
- Long, G.; Wang, F.; Duan, Q.; Chen, F.; Yang, S.; Gong, W.; Wang, Y.; Chen, C.; Wang, D.W. Human Circulating MicroRNA-1 and MicroRNA-126 as Potential Novel Indicators for Acute Myocardial Infarction. Int. J. Biol. Sci. 2012, 8, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczyk, E.; Eljaszewicz, A.; Kazimierczyk, R.; Tynecka, M.; Zembko, P.; Tarasiuk, E.; Kaminski, K.; Sobkowicz, B.; Moniuszko, M.; Tycinska, A. Altered microRNA dynamics in acute coronary syndrome. Adv. Interv. Cardiol. 2020, 16, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Widera, C.; Gupta, S.K.; Lorenzen, J.M.; Bang, C.; Bauersachs, J.; Bethmann, K.; Kempf, T.; Wollert, K.C.; Thum, T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell Cardiol. 2011, 51, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis. 2015, 7, 303–308. [Google Scholar] [CrossRef]
- He, Y.; Zhong, J.; Huang, S.; Shui, X.; Kong, D.; Chen, C.; Lei, W. Elevated circulating miR-126-3p expression in patients with acute myocardial infarction: Its diagnostic value. Int. J. Clin. Exp. Pathol. 2017, 10, 11051–11056. [Google Scholar]
- Gidlöf, O.; Andersson, P.; van der Pals, J.; Götberg, M.; Erlinge, D. Cardiospecific microRNA Plasma Levels Correlate with Troponin and Cardiac Function in Patients with ST Elevation Myocardial Infarction, Are Selectively Dependent on Renal Elimination, and Can Be Detected in Urine Samples. Cardiology 2011, 118, 217–226. [Google Scholar] [CrossRef]
- Su, T.; Shao, X.; Zhang, X.; Yang, C.; Shao, X. Value of circulating miRNA-1 detected within 3 h after the onset of acute chest pain in the diagnosis and prognosis of acute myocardial infarction. Int. J. Cardiol. 2019, 307, 146–151. [Google Scholar] [CrossRef]
- Xue, S.; Liu, D.; Zhu, W.; Su, Z.; Zhang, L.; Zhou, C.; Li, P. Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p Are Novel Biomarkers for Diagnosis of Acute Myocardial Infarction. Front. Physiol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Horváth, M.; Horváthová, V.; Hájek, P.; Štěchovský, C.; Honěk, J.; Šenolt, L.; Veselka, J. MicroRNA-331 and microRNA-151-3p as biomarkers in patients with ST-segment elevation myocardial infarction. Sci. Rep. 2020, 10, 5845. [Google Scholar] [CrossRef]
- Meng, L.; Yu, X.; Han, H.; Jia, X.; Hu, B.; Zhang, L.; Wang, Z.; Zhang, W.; Zhong, M.; Zhu, H. Circulating miR-143 and miR-145 as promising biomarkers for evaluating severity of coronary artery stenosis in patients with acute coronary syndrome. Clin. Biochem. 2022, Online ahead of print. [Google Scholar] [CrossRef]
- Dégano, I.R.; Camps-Vilaró, A.; Subirana, I.; García-Mateo, N.; Cidad, P.; Muñoz-Aguayo, D.; Puigdecanet, E.; Nonell, L.; Vila, J.; Crepaldi, F.M.; et al. Association of Circulating microRNAs with Coronary Artery Disease and Usefulness for Reclassification of Healthy Individuals: The REGICOR Study. J. Clin. Med. 2020, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, T.; Mickus, R.; Cereskevicius, D.; Macas, A. Value of Serum miR-23a, miR-30d, and miR-146a Biomarkers in ST-Elevation Myocardial Infarction. J. Pharmacol. Exp. Ther. 2019, 25, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.J.; Liu, T.; Zhang, H.; Yang, S.J. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 323–329. [Google Scholar] [PubMed]
- Kayvanpour, E.; Gi, W.-T.; Sedaghat-Hamedani, F.; Lehmann, D.H.; Frese, K.S.; Haas, J.; Tappu, R.; Samani, O.S.; Nietsch, R.; Kahraman, M.; et al. microRNA neural networks improve diagnosis of acute coronary syndrome (ACS). J. Mol. Cell Cardiol. 2020, 151, 155–162. [Google Scholar] [CrossRef]
- Esa, J.A.W.N. Circulating Cell and Plasma microRNA Profiles Differ between Non-STSegment and ST-Segment-Elevation Myocardial Infarction. Fam. Med. Med. Sci. Res. 2013, 2, 108. [Google Scholar] [CrossRef]
- Biener, M.; Giannitsis, E.; Thum, T.; Bär, C.; Costa, A.; Andrzejewski, T.; Stoyanov, K.M.; Vafaie, M.; Meder, B.; A Katus, H.; et al. Diagnostic value of circulating microRNAs compared to high-sensitivity troponin T for the detection of non-ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, X.; Meng, X.; Zhang, Z. Sensitive miRNA markers for the detection and management of NSTEMI acute myocardial infarction patients. J. Thorac. Dis. 2018, 10, 3206–3215. [Google Scholar] [CrossRef]
- Zhelankin, A.; Stonogina, D.; Vasiliev, S.; Babalyan, K.; Sharova, E.; Doludin, Y.; Shchekochikhin, D.; Generozov, E.; Akselrod, A. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules 2021, 11, 962. [Google Scholar] [CrossRef]
- Gacoń, J.; Kabłak-Ziembicka, A.; Stępień, E.; Enguita, F.J.; Karch, I.; Derlaga, B.; Żmudka, K.; Przewłocki, T. Decision-making microRNAs (miR-124, -133a/b, -34a and -134) in patients with occluded target vessel in acute coronary syndrome. Kardiol. Pol. 2016, 74, 280–288. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhou, X.; Ji, W.-J.; Shi, R.; Lu, R.-Y.; Li, J.-L.; Yang, G.-H.; Luo, T.; Zhang, J.-Q.; Zhao, J.-H.; et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J. Thromb. Thrombolysis 2013, 38, 65–72. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Jiang, Z.; Wu, F.; Ping, J.; Ming, L. Identification of microRNAs as diagnostic biomarkers for acute myocardial infarction in Asian populations. Medicine 2017, 96, e7173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Y.; Fang, T.; Wang, X.; Chen, L.; Zheng, C.; Kang, Y.; Jiang, L.; You, X.; Gai, S.; et al. Circulating MicroRNA-423-3p Improves the Prediction of Coronary Artery Disease in a General Population―Six-Year Follow-up Results From the China-Cardiovascular Disease Study. Circ. J. 2020, 84, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/β-catenin signaling pathway via targeting MYBL2. J. Cell Physiol. 2019, 234, 22034–22043. [Google Scholar] [CrossRef] [PubMed]
- Hromadka, M.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Tousek, F.; Jarkovsky, J.; Beranova, M.; Jansky, P.; Svoboda, M.; Krepelkova, I.; et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021, 11, 508. [Google Scholar] [CrossRef]
- Scărlătescu, A.I.; Barbălată, T.; Sima, A.V.; Stancu, C.; Niculescu, L.; Micheu, M.M. miR-146a-5p, miR-223-3p and miR-142-3p as Potential Predictors of Major Adverse Cardiac Events in Young Patients with Acute ST Elevation Myocardial Infarction—Added Value over Left Ventricular Myocardial Work Indices. Diagnostics 2022, 12, 1946. [Google Scholar] [CrossRef]
- Schulte, C.; Molz, S.; Appelbaum, S.; Karakas, M.; Ojeda, F.; Lau, D.M.; Hartmann, T.; Lackner, K.J.; Westermann, D.; Schnabel, R.B.; et al. miRNA-197 and miRNA-223 Predict Cardiovascular Death in a Cohort of Pa-tients with Symptomatic Coronary Artery Disease. PLoS ONE 2015, 10, e0145930. [Google Scholar] [CrossRef]
- Karakas, M.; Schulte, C.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Münzel, T.; Schnabel, R.B.; Blankenberg, S.; Zeller, T. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease—Results from the large AtheroGene study. Eur. Heart J. 2016, 38, 516–523. [Google Scholar] [CrossRef]
- Ziaee, S.; Hosseindokht, M.; Cheraghi, S.; Pourgholi, L.; Ahmadi, A.; Sadeghian, S.; Abbasi, S.H.; Davarpasand, T.; Boroumand, M. Predictive Inflammation-related microRNAs for Cardiovascular Events Following Early-Onset Coronary Artery Disease. Arch. Med. Res. 2020, 52, 69–75. [Google Scholar] [CrossRef]
- Xiao, S.; Xue, T.; Pan, Q.; Hu, Y.; Wu, Q.; Liu, Q.; Wang, X.; Liu, A.; Liu, J.; Zhu, H.; et al. MicroRNA-146a Serves as a Biomarker for Adverse Prognosis of ST-Segment Elevation Myocardial Infarction. Cardiovasc. Ther. 2021, 2021, 2923441. [Google Scholar] [CrossRef]
- Alavi-Moghaddam, M.; Chehrazi, M.; Alipoor, S.D.; Mohammadi, M.; Baratloo, A.; Mahjoub, M.P.; Movasaghi, M.; Garssen, J.; Adcock, I.M.; Mortaz, E. A Preliminary Study of microRNA-208b after Acute Myocardial Infarction: Impact on 6-Month Survival. Dis. Markers 2018, 2018, 2410451. [Google Scholar] [CrossRef]
- Badacz, R.; Kleczyński, P.; Legutko, J.; Żmudka, K.; Gacoń, J.; Przewłocki, T.; Kabłak-Ziembicka, A. Expression of miR-1-3p, miR-16-5p and miR-122-5p as Possible Risk Factors of Secondary Cardiovascular Events. Biomedicines 2021, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Badacz, R.; Przewłocki, T.; Pieniążek, P.; Rosławiecka, A.; Kleczyński, P.; Legutko, J.; Żmudka, K.; Kabłak-Ziembicka, A. MicroRNA-134-5p and the Extent of Arterial Occlusive Disease Are Associated with Risk of Future Adverse Cardiac and Cerebral Events in Diabetic Patients Undergoing Carotid Artery Stenting for Symptomatic Carotid Artery Disease. Molecules 2022, 27, 2472. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Jiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocardial infarction using cardiac magnetic reso-nance. Quant Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Grabmaier, U.; Clauss, S.; Gross, L.; Klier, I.; Franz, W.; Steinbeck, G.; Wakili, R.; Theiss, H.; Brenner, C. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—The SITAGRAMI-miR analysis. Int. J. Cardiol. 2017, 244, 30–36. [Google Scholar] [CrossRef]
- Maciejak, A.; Kostarska-Srokosz, E.; Gierlak, W.; Dluzniewski, M.; Kuch, M.; Marchel, M.; Opolski, G.; Kiliszek, M.; Matlak, K.; Dobrzycki, S.; et al. Circulating miR-30a-5p as a prognostic biomarker of left ventricular dysfunction after acute myocardial infarction. Sci. Rep. 2018, 8, 9883. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a Are Associated with Left Ventricular Remodeling after Acute Myocardial Infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Huo, Z. Serum Circulating miR-150 is a Predictor of Post-Acute Myocardial Infarction Heart Failure. Int. Heart J. 2019, 60, 280–286. [Google Scholar] [CrossRef]
- Zile, M.R.; Mehurg, S.M.; Arroyo, J.E.; Stroud, R.E.; DeSantis, S.M.; Spinale, F.G. Relationship between the temporal pro-file of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ. Cardiovasc. Genet. 2011, 4, 614–619. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, P.; Aromatario, M.; Cipolloni, L.; Fabbri, M.; La Russa, R.; Maiese, A.; Neri, M.; Santurro, A.; Scopetti, M.; et al. miR-1, miR-499 and miR-208 are sensitive markers to diagnose sudden death due to early acute myocardial infarction. J. Cell. Mol. Med. 2019, 23, 6005–6016. [Google Scholar] [CrossRef]
- Bostjancic, E.; Zidar, N.; Stajner, D.; Glavac, D. MicroRNA miR-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol. 2010, 56, 27–31. [Google Scholar]
- Boštjančič, E.; Brandner, T.; Zidar, N.; Glavač, D.; Štajer, D. Down-regulation of miR-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomed. Pharmacother. 2018, 99, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, E.; Medzikovic, L.; Ruffenach, G.; Eghbali, M. Role of miRNA-1 and miRNA-21 in Acute Myocardial Is-chemia-Reperfusion Injury and Their Potential as Therapeutic Strategy. Int. J. Mol. Sci. 2022, 23, 1512. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nojima, T.; Fujisaki, N.; Tsukahara, K.; Yamamoto, H.; Yamada, T.; Aokage, T.; Yumoto, T.; Osako, T.; Nakao, A. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020, 7, e501. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, W.; Miszalski-Jamka, T.; Zalewski, J.; Legutko, J.; Żmudka, K.; Paszek, E. Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy. J. Clin. Med. 2022, 11, 5000. [Google Scholar] [CrossRef] [PubMed]
- Bellis, A.; Di Gioia, G.; Mauro, C.; Mancusi, C.; Barbato, E.; Izzo, R.; Trimarco, B.; Morisco, C. Reducing Cardiac Injury during ST-Elevation Myocardial Infarction: A Reasoned Approach to a Multitarget Therapeutic Strategy. J. Clin. Med. 2021, 10, 2968. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. In-flammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef]
- Minicucci, M.F.; Azevedo-Gaiolla, P.S.; Polegato, B.; Paiva, S.; Zornoff, L.A.M. Heart failure after myocardial infarction: Clinical implications and treatment. Clin. Cardiol. 2011, 34, 410–414. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Ren, J.; Li, X.; Gao, X.; Lu, C.; Zhang, Y.; Sun, H.; Wang, Y.; Wang, H.; et al. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE 2012, 7, e50515. [Google Scholar] [CrossRef]
- Yin, C.; Wang, X.; Kukreja, R.C. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett. 2008, 582, 4137–4142. [Google Scholar] [CrossRef]
- Dehaini, H.; Awada, H.; El-Yazbi, A.; Zouein, F.A.; Issa, K.; Eid, A.A.; Ibrahim, M.; Badran, A.; Baydoun, E.; Pintus, G.; et al. MicroRNAs as Potential Pharmaco-Targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-H.; Lee, S.-Y.; Lee, C.Y.; Kim, R.; Kim, P.; Oh, S.; Lee, H.; Lee, M.Y.; Kim, J.; Kim, L.K.; et al. Exogenous miRNA-146a Enhances the Therapeutic Efficacy of Human Mesenchymal Stem Cells by Increasing Vascular Endothelial Growth Factor Secretion in the Ischemia/Reperfusion-Injured Heart. J. Vasc. Res. 2017, 54, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hullinger, T.G.; Montgomery, R.L.; Seto, A.G.; Dickinson, B.A.; Semus, H.M.; Lynch, J.M.; Dalby, C.M.; Robinson, K.; Stack, C.; Latimer, P.A.; et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 2012, 110, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Kalocayova, B.; Devaux, Y.; Bartekova, M. Potential Clinical Implications of miR-1 and miR-21 in Heart Disease and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 700. [Google Scholar] [CrossRef] [PubMed]
- Krzywińska, O.; Bracha, M.; Jeanniere, C.; Recchia, E.; Kędziora-Kornatowska, K.; Kozakiewicz, M. Meta-analysis of the potential role of miRNA-21 in cardiovascylar system function monitoring. BioMed Res. Int. 2020, 2020, 4525410. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.-P.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019, 10, 1802. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, T.; Chen, L.; Wang, N. MicroRNA-93 elevation after myocardial infarction is cardiac protective. Med. Hypotheses 2017, 106, 23–25. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.X.; He, Q.; Zhang, H.; Friedberg, D.; Wang, F.; Redington, A.N. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res. Cardiol. 2018, 113, 36. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. MicroRNAs in the development of left ventricular remodeling and postmyocardial infarction heart failure. Pol. Arch. Intern. Med. 2020, 130, 59–65. [Google Scholar] [CrossRef]
- Maries, L.; Marian, C.; Sosdean, R.; Goanta, F.; Sirbu, I.O.; Anghel, A. MicroRNAs—The Heart of Post-Myocardial Infarction Remodeling. Diagnostics 2021, 11, 1675. [Google Scholar] [CrossRef]
- Bostan, M.-M.; Stătescu, C.; Anghel, L.; Șerban, I.-L.; Cojocaru, E.; Sascău, R. Post-Myocardial Infarction Ventricular Remodeling Biomarkers—The Key Link between Pathophysiology and Clinic. Biomolecules 2020, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A.; Alduraywish, A.; Almaeen, A.; Alruwali, M.; Alruwaili, R.; Alomair, B.M.; Salma, U.; Hedeab, G.M.; Bugti, N.; Abdulhabeeb, I. Therapeutic Value of miRNAs in Coronary Artery Disease. Oxidative Med. Cell Longev. 2021, 2021, 8853748. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; Van Der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabłak-Ziembicka, A.; Badacz, R.; Przewłocki, T. Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. J. Clin. Med. 2022, 11, 6849. https://doi.org/10.3390/jcm11226849
Kabłak-Ziembicka A, Badacz R, Przewłocki T. Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. Journal of Clinical Medicine. 2022; 11(22):6849. https://doi.org/10.3390/jcm11226849
Chicago/Turabian StyleKabłak-Ziembicka, Anna, Rafał Badacz, and Tadeusz Przewłocki. 2022. "Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease" Journal of Clinical Medicine 11, no. 22: 6849. https://doi.org/10.3390/jcm11226849
APA StyleKabłak-Ziembicka, A., Badacz, R., & Przewłocki, T. (2022). Clinical Application of Serum microRNAs in Atherosclerotic Coronary Artery Disease. Journal of Clinical Medicine, 11(22), 6849. https://doi.org/10.3390/jcm11226849






