Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population, and Data Assessment
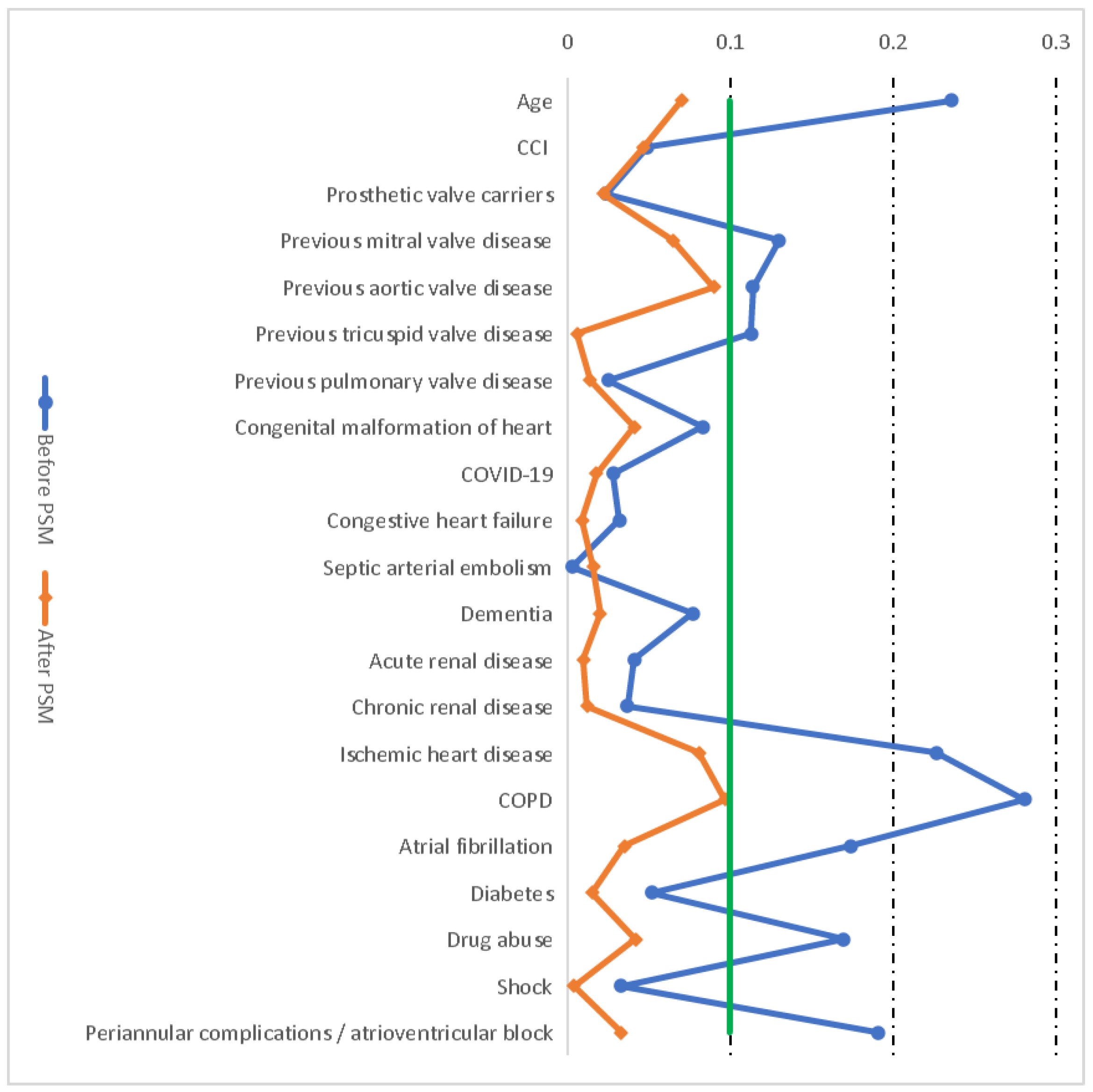
2.2. Propensity Score Matching
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Incidence of Patients Admitted to Hospitals with IE and Hospital Department of Admission According to Sex
3.2. Clinical Characteristics and Hospital Outcomes for Women and Men Admitted to the Hospital for IE
3.3. Variables Associated with IHM for Women and Men Admitted to the Hospital with a Diagnosis of IE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahtela, E.; Oksi, J.; Porela, P.; Ekström, T.; Rautava, P.; Kytö, V. Trends in occurrence and 30-day mortality of infective endocarditis in adults: Population-based registry study in Finland. BMJ Open 2019, 9, e026811. [Google Scholar] [CrossRef] [PubMed]
- Sunder, S.; Grammatico-Guillon, L.; Lemaignen, A.; Lacasse, M.; Gaborit, C.; Boutoille, D.; Tattevin, P.; Denes, E.; Guimard, T.; Dupont, M.; et al. Incidence, characteristics, and mortality of infective endocarditis in France in 2011. PLoS ONE 2019, 14, e0223857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polishchuk, I.; Stavi, V.; Awesat, J.; Ben Baruch Golan, Y.; Bartal, C.; Sagy, I.; Jotkowitz, A.; Barski, L. Sex differences in infective endocarditis. Am. J. Med. Sci. 2021, 361, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Bansal, A.; Cremer, P.C.; Jaber, W.A.; Rampersad, P.; Menon, V. Sex differences in the utilization and outcomes of cardiac valve replacement surgery for infective endocarditis: Insights from the National Inpatient Sample. J. Am. Heart Assoc. 2021, 10, e020095. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Fernández-Pérez, C.; Bernal, J.L.; Ferrera, C.; García-Arribas, D.; Pérez-García, C.N.; San Román, J.A.; Maroto, L.; Macaya, C.; et al. The evolving nature of infective endocarditis in Spain: A population-based study (2003 to 2014). J. Am. Coll. Cardiol. 2017, 70, 2795–2804. [Google Scholar] [CrossRef]
- Chew, D.S.; Rennert-May, E.; Lu, S.; Parkins, M.; Miller, R.J.H.; Somavaji, R. Sex differences in health resource utilization, costs and mortality during hospitalization for infective endocarditis in the United States. Am. Heart J. Plus. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Sevilla, T.; Revilla, A.; López, J.; Vilacosta, I.; Sarriá, C.; Gómez, I.; García, H.; San Román, J.A. Influence of sex on left-sided infective endocarditis. Rev. Esp. Cardiol. 2010, 63, 1497–1500. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Varela Barca, L.; Vidal-Bonnet, L.; Fariñas, M.C.; Muñoz, P.; Valerio Minero, M.; de Alarcón, A.; Gutiérrez Carretero, E.; Gutiérrez Cuadra, M.; Moreno Camacho, A.; Kortajarena Urkola, X.; et al. Analysis of sex differences in the clinical presentation, management and prognosis of infective endocarditis in Spain. Heart 2021, 107, 1717–1724. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Real Decreto 69/2015, de 6 de Febrero, por el que se Regula el Registro de Actividad de Atención Sanitaria Especializada. (Spanish National Hospital Discharge Database) BOE 2015; 35: 10789-809. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/BOE_RD_69_2015_RAE_CMBD.pdf (accessed on 31 May 2022).
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística Population Estimates. Available online: https://www.ine.es./dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981 (accessed on 31 May 2022).
- Austin, P.C. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat. Med. 2011, 30, 1292–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de Sanidad, Consumo y Bienestar Social. Solicitud de Extracción de Datos—Extraction Request (Spanish National Hospital Discharge Database). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SolicitudCMBDdocs/2018_Formulario_Peticion_Datos_RAE_CMBD.pdf (accessed on 31 May 2022).
- Sousa, C.; Nogueira, P.J.; Pinto, F.J. Gender based analysis of a population series of patients hospitalized with infective endocarditis in Portugal. How do women and men compare? Int. J. Cardiovasc. Sci. 2021, 34, 347–355. [Google Scholar] [CrossRef]
- Talha, K.M.; Baddour, L.M.; Thornhill, M.H.; Arshad, V.; Tariq, W.; Tleyjeh, I.M.; Scott, C.G.; Hyun, M.C.; Bailey, K.R.; Anavekar, N.S.; et al. Escalating incidence of infective endocarditis in Europe in the 21st century. Open Heart 2021, 8, e001846. [Google Scholar] [CrossRef]
- Talha, K.M.; Dayer, M.J.; Thornhill, M.H.; Tariq, W.; Arshad, V.; Tleyjeh, I.M.; Bailey, K.R.; Palraj, R.; Anavekar, N.S.; Rizwan Sohail, M.; et al. Temporal trends of infective endocarditis in North America from 2000 to 2017-A Systematic Review. Open Forum. Infect. Dis. 2021, 8, ofab479. [Google Scholar] [CrossRef] [PubMed]
- Veloso, T.R.; Chaouch, A.; Roger, T.; Giddey, M.; Vouillamoz, J.; Majcherczyk, P.; Que, Y.A.; Rousson, V.; Moreillon, P.; Entenza, J.M. Use of a human-like low-grade bacteremia model of experimental endocarditis to study the role of Staphylococcus aureus adhesins and platelet aggregation in early endocarditis. Infect. Immun. 2013, 81, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, W.G.; Mügge, A.; Martin, R.P.; Lindert, O.; Hausmann, D.; Nonnast-Daniel, B.; Laas, J.; Lichtlen, P.R. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N. Engl. J. Med. 1991, 324, 795–800. [Google Scholar] [CrossRef]
- Shah, A.S.V.; McAllister, D.A.; Gallacher, P.; Astengo, F.; Rodríguez Pérez, J.A.; Hall, J.; Lee, K.K.; Bing, R.; Anand, A.; Nathwani, D.; et al. Incidence, Microbiology, and Outcomes in Patients Hospitalized With Infective Endocarditis. Circulation 2020, 141, 2067–2077. [Google Scholar] [CrossRef]
- Jensen, A.D.; Bundgaard, H.; Butt, J.H.; Bruun, N.E.; Voldstedlund, M.; Torp-Pedersen, C.; Gislason, G.; Iversen, K.; Chamat, S.; Dahl, A.; et al. Temporal changes in the incidence of infective endocarditis in Denmark 1997–2017: A nationwide study. Int. J. Cardiol. 2021, 326, 145–152. [Google Scholar] [CrossRef]
- Li, H.L.; Tromp, J.; Teramoto, K.; Tse, Y.K.; Yu, S.Y.; Lam, L.Y.; Li, K.Y.; Wu, M.Z.; Ren, Q.W.; Wong, P.F.; et al. Temporal trends and patterns of infective endocarditis in a Chinese population: A territory-wide study in Hong Kong (2002–2019). Lancet Reg. Health West Pac. 2022, 22, 100417. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017, 317, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Loubon, C.; Muñoz-Moreno, M.F.; Andrés-García, I.; Álvarez, F.J.; Gómez-Sánchez, E.; Bustamante-Munguira, J.; Lorenzo-López, M.; Tamayo-Velasco, Á.; Jorge-Monjas, P.; Resino, S.; et al. Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century. J. Clin. Med. 2019, 8, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [Green Version]
- Habib, G. Infective endocarditis in Portugal: Changing epidemiology but still a deadly disease. Rev. Port. Cardiol. 2021, 40, 219–220. [Google Scholar] [CrossRef]
- Curlier, E.; Hoen, B.; Alla, F.; Selton-Suty, C.; Schubel, L.; Doco-Lecompte, T.; Minary, L.; Erpelding, M.L.; Duval, X.; Chirouze, C.; et al. Relationships between sex, early valve surgery and mortality in patients with left-sided infective endocarditis analysed in a population-based cohort study. Heart 2014, 100, 1173–1178. [Google Scholar] [CrossRef]
- Lipsky, M.S.; Su, S.; Crespo, C.J.; Hung, M. Men and oral health: A review of sex and gender differences. Am. J. Mens. Health 2021, 15, 15579883211016361. [Google Scholar] [CrossRef]
- Mabilangan, C.; Cole, H.; Hiebert, B.; Keynan, Y.; Arora, R.C.; Shah, P. Short- and long-term outcomes of medically treated isolated left-sided endocarditis: A retrospective study with 5-year longitudinal follow-up. Can. J. Cardiol. 2020, 36, 1534–1540. [Google Scholar] [CrossRef]
- Aksoy, O.; Meyer, L.T.; Cabell, C.H.; Kourany, W.M.; Pappas, P.A.; Sexton, D.J. Gender differences in infective endocarditis: Pre- and co-morbid conditions lead to different management and outcomes in female patients. Scand. J. Infect. Dis. 2007, 39, 101–107. [Google Scholar] [CrossRef]
- Weber, C.; Gassa, A.; Rokohl, A.; Sabashnikov, A.; Deppe, A.C.; Eghbalzadeh, K.; Merkle, J.; Hamacher, S.; Liakopoulos, O.J.; Wahlers, T. Severity of presentation, not sex, increases risk of surgery for infective endocarditis. Ann. Thorac. Surg. 2019, 107, 1111–1117. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Differences in frailty in older men and women. Med. J. Aust. 2020, 212, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Pal, L.M.; Manning, L. Palliative care for frail older people. Clin. Med. 2014, 14, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Sambola, A.; Fernández-Hidalgo, N.; Almirante, B.; Roca, I.; González-Alujas, T.; Serra, B.; Pahissa, A.; García-Dorado, D.; Tornos, P. Sex differences in native-valve infective endocarditis in a single tertiary-care hospital. Am. J. Cardiol. 2010, 106, 92–98. [Google Scholar] [CrossRef] [PubMed]
- van Melle, J.P.; Roos-Hesselink, J.W.; Bansal, M.; Kamp, O.; Meshaal, M.; Pudich, J.; Luksic, V.R.; Rodriguez-Alvarez, R.; Sadeghpour, A.; Hanzevacki, J.S.; et al. Infective endocarditis in adult patients with congenital heart disease. Int. J. Cardiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ahtela, E.; Oksi, J.; Vahlberg, T.; Sipilä, J.; Rautava, P.; Kytö, V. Short- and long-term outcomes of infective endocarditis admission in adults: A population-based registry study in Finland. PLoS ONE 2021, 16, e0254553. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Haruna, T.; Haruna, Y.; Nakane, E.; Yamaji, Y.; Hayashi, H.; Hanyu, M.; Inoko, M. Thirty-Day Readmission After Infective Endocarditis: Analysis from a Nationwide Readmission Database. J. Am. Heart Assoc. 2019, 8, e011598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Hansen, M.; Cohen, G.; Boyle, K.; Daneman, N.; Adhikari, N.K. Accuracy of administrative data for identification of patients with infective endocarditis. Int. J. Cardiol. 2016, 224, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.Y.; Liang, L.Y.; Lin, C.C.; Chen, Y.J.; Wu, M.Y.; Chen, S.H.; Wu, P.H.; Kuo, C.C.; Chi, C.Y. Electronic medical record-based deep data cleaning and phenotyping improve the diagnostic validity and mortality assessment of infective endocarditis: Medical big data initiative of CMUH. Biomedicine 2021, 11, 59–67. [Google Scholar] [CrossRef]
- Fedeli, U.; Schievano, E.; Buonfrate, D.; Pellizzer, G.; Spolaore, P. Increasing incidence and mortality of infective endocarditis: A population-based study through a record-linkage system. BMC Infect. Dis. 2011, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, S.; Robicsek, A.; Scranton, R.; Zuckerman, D.; Solomon, D.H. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J. Clin. Epidemiol. 2007, 60, 397–409. [Google Scholar] [CrossRef]
| 2016 | 2017 | 2018 | 2019 | 2020 | p-Value * | ||
|---|---|---|---|---|---|---|---|
| N, (incidence per 100,000 people per year) | Both sexes | 1975 (4.25) | 2090 (4.49) | 2242 (4.8) | 2222 (4.72) | 1930 (4.08) | 0.656 |
| N, (incidence per 100,000 people per year) | Women | 646 (2.73) | 704 (2.97) | 772 (3.24) | 715 (2.98) | 642 (2.66) | 0.672 |
| Men | 1329 (5.83) | 1386 (6.07) | 1470 (6.42) | 1507 (6.53) | 1288 (5.55) | 0.826 | |
| Age, mean (SD) | Women | 70.21 (18.21) | 69.00 (19.34) | 69.88 (18.27) | 70.53 (16.74) | 71.78 (14.77) | 0.064 |
| Men | 65.53 (17.31) | 64.62 (17.18) | 67.23 (15.82) | 66.36 (15.90) | 67.45 (15.24) | <0.001 | |
| CCI index, mean (SD) | Women | 1.27 (1.13) | 1.35 (1.12) | 1.30 (1.11) | 1.46 (1.18) | 1.50 (1.17) | <0.001 |
| Men | 1.30 (1.19) | 1.43 (1.21) | 1.49 (1.25) | 1.43 (1.25) | 1.53 (1.27) | <0.001 | |
| Prosthetic valve carriers, n (%) | Women | 69 (10.68) | 68 (9.66) | 65 (8.42) | 55 (7.69) | 65 (10.12) | 0.287 |
| Men | 121 (9.10) | 117 (8.44) | 136 (9.25) | 121 (8.03) | 103 (8.00) | 0.647 | |
| Previous mitral valve disease, n (%) | Women | 195 (30.19) | 189 (26.85) | 235 (30.44) | 244 (34.13) | 217 (33.80) | 0.021 |
| Men | 311 (23.40) | 349 (25.18) | 370 (25.17) | 364 (24.15) | 367 (28.49) | 0.032 | |
| Previous aortic valve disease, n (%) | Women | 128 (19.81) | 163 (23.15) | 174 (22.54) | 206 (28.81) | 177 (27.57) | <0.001 |
| Men | 357 (26.86) | 410 (29.58) | 420 (28.57) | 444 (29.46) | 422 (32.76) | 0.020 | |
| Previous tricuspid valve disease, n (%) | Women | 43 (6.66) | 54 (7.67) | 90 (11.66) | 82 (11.47) | 74 (11.53) | 0.001 |
| Men | 66 (4.97) | 80 (5.77) | 101 (6.87) | 111 (7.37) | 112 (8.70) | 0.002 | |
| Previous pulmonary valve disease, n (%) | Women | 3 (0.46) | 7 (0.99) | 2 (0.26) | 2 (0.28) | 3 (0.47) | 0.268 |
| Men | 3 (0.23) | 7 (0.51) | 4 (0.27) | 2 (0.13) | 7 (0.54) | 0.244 | |
| Congenital malformation of heart, n (%) | Women | 13 (2.01) | 25 (3.55) | 28 (3.63) | 20 (2.8) | 19 (2.96) | 0.403 |
| Men | 44 (3.31) | 51 (3.68) | 57 (3.88) | 55 (3.65) | 40 (3.11) | 0.820 | |
| Drug abuse, n (%) | Women | 10 (1.55) | 13 (1.85) | 8 (1.04) | 5 (0.70) | 5 (0.78) | 0.208 |
| Men | 44 (3.31) | 56 (4.04) | 68 (4.63) | 58 (3.85) | 41 (3.18) | 0.274 | |
| LOHS, median (IQR) | Women | 16.5 (27) | 17 (28) | 18 (25) | 19 (24) | 18 (24) | 0.681 |
| Men | 20 (25) | 19 (26) | 19 (26) | 19 (25) | 19 (23) | 0.897 | |
| IHM, n (%) | Women | 125 (19.35) | 128 (18.18) | 142 (18.39) | 144 (20.14) | 140 (21.81) | 0.441 |
| Men | 191 (14.37) | 183 (13.20) | 232 (15.78) | 233 (15.46) | 200 (15.53) | 0.275 |
| BEFORE PSM | AFTER PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | ASD | p-Value | Women | Men | ASD | p-Value | |
| N | 3479 | 6980 | NA | NA | 3479 | 3479 | NA | NA |
| Age, mean (SD) | 70.25 (17.59) | 66.24 (16.33) | 0.236 | <0.001 | 70.25 (17.59) | 70.11 (11.87) | 0.07 | 0.697 |
| CCI index, mean (SD) | 1.38 (1.15) | 1.43 (1.24) | 0.049 | 0.019 | 1.38 (1.15) | 1.42 (1.11) | 0.047 | 0.140 |
| Prosthetic valve carriers, n (%) | 322 (9.26) | 598 (8.57) | 0.024 | 0.242 | 322 (9.26) | 344 (9.89) | 0.022 | 0.370 |
| Previous mitral valve disease, n (%) | 1080 (31.04) | 1761 (25.23) | 0.13 | <0.001 | 1080 (31.04) | 1069 (30.73) | 0.065 | 0.781 |
| Previous aortic valve disease, n (%) | 848 (24.37) | 2053 (29.41) | 0.114 | <0.001 | 848 (24.37) | 789 (22.69) | 0.09 | 0.097 |
| Previous tricuspid valve disease, n (%) | 343 (9.86) | 470 (6.73) | 0.113 | <0.001 | 343 (9.86) | 337 (9.69) | 0.006 | 0.809 |
| Previous pulmonary valve disease, n (%) | 17 (0.49) | 23 (0.33) | 0.025 | 0.214 | 17 (0.49) | 20 (0.57) | 0.014 | 0.621 |
| Congenital malformation of heart, n (%) | 105 (3.02) | 247 (3.54) | 0.083 | 0.164 | 105 (3.02) | 87 (2.52) | 0.041 | 0.705 |
| COVID-19, n (%) | 20 (0.57) | 31 (0.44) | 0.028 | 0.366 | 20 (0.57) | 27 (0.78) | 0.018 | 0.306 |
| Congestive heart failure, n (%) | 392 (11.27) | 717 (10.27) | 0.032 | 0.119 | 392 (11.27) | 382 (10.98) | 0.009 | 0.703 |
| Septic arterial embolism, n (%) | 150 (4.31) | 305 (4.37) | 0.003 | 0.891 | 150 (4.31) | 161 (4.63) | 0.016 | 0.523 |
| Dementia, n (%) | 92 (2.64) | 108 (1.55) | 0.077 | <0.001 | 92 (2.64) | 82 (2.36) | 0.02 | 0.443 |
| Acute renal disease, n (%) | 690 (19.83) | 1500 (21.49) | 0.041 | 0.050 | 690 (19.83) | 676 (19.43) | 0.01 | 0.673 |
| Chronic renal disease, n (%) | 640 (18.4) | 1186 (16.99) | 0.037 | 0.075 | 640 (18.40) | 656 (18.86) | 0.012 | 0.622 |
| Ischemic heart disease, n (%) | 343 (9.86) | 1229 (17.61) | 0.227 | <0.001 | 343 (9.86) | 379 (10.90) | 0.081 | 0.156 |
| COPD, n (%) | 104 (2.99) | 683 (9.79) | 0.281 | <0.001 | 104 (2.99) | 131 (3.77) | 0.097 | 0.072 |
| Atrial fibrillation, n (%) | 1167 (33.54) | 1789 (25.63) | 0.174 | <0.001 | 1167 (33.54) | 1223 (35.15) | 0.035 | 0.157 |
| Diabetes, n (%) | 816 (23.46) | 1792 (25.67) | 0.052 | 0.013 | 816 (23.46) | 793 (22.79) | 0.015 | 0.513 |
| Drug abuse, n (%) | 41 (1.18) | 267 (3.83) | 0.17 | <0.001 | 41 (1.18) | 60 (1.73) | 0.042 | 0.057 |
| Shock, n (%) | 65 (1.87) | 163 (2.34) | 0.033 | 0.123 | 65 (1.87) | 63 (1.81) | 0.004 | 0.858 |
| Periannular complications/atrioventricular block, n (%) | 142 (4.08) | 425 (6.09) | 0.191 | <0.001 | 142 (4.08) | 122 (3.51) | 0.033 | 0.209 |
| BEFORE PSM | AFTER PSM | |||||
|---|---|---|---|---|---|---|
| Women | Men | p-Value | Women | Men | p-Value | |
| Staphylococcus bacteremia, n (%) | 992 (28.51) | 2035 (29.15) | 0.496 | 992 (28.51) | 978 (28.11) | 0.709 |
| Streptococcus bacteremia, n (%) | 705 (20.26) | 1715 (24.57) | <0.001 | 705 (20.26) | 848 (24.37) | <0.001 |
| Gram-negative bacilli bacteremia, n (%) | 353 (10.15) | 459 (6.58) | <0.001 | 353 (10.15) | 240 (6.90) | <0.001 |
| Fungemia, n (%) | 15 (0.43) | 34 (0.49) | 0.693 | 15 (0.43) | 14 (0.40) | 0.852 |
| Heart valve surgery n (%) | 567 (16.30) | 1560 (22.35) | <0.001 | 567 (16.30) | 652 (18.74) | 0.007 |
| Dialysis, n (%) | 172 (4.94) | 350 (5.01) | 0.876 | 172 (4.94) | 141 (4.05) | 0.073 |
| Pacemaker implantation, n (%) | 140 (4.02) | 385 (5.52) | 0.001 | 140 (4.02) | 184 (5.29) | 0.012 |
| Mechanical ventilation, n (%) | 359 (10.32) | 788 (11.29) | 0.135 | 359 (10.32) | 309 (8.88) | 0.042 |
| LOHS, median (IQR) | 18 (25) | 19 (25) | 0.085 | 18 (25) | 19 (25) | 0.271 |
| IHM, n (%) | 679 (19.52) | 1039 (14.89) | <0.001 | 679 (19.52) | 556 (15.98) | <0.001 |
| BEFORE PSM | AFTER PSM | |||||
|---|---|---|---|---|---|---|
| Women | Men | p-Value | Women | Men | p-Value | |
| N | 679 | 1039 | NA | 679 | 556 | NA |
| Age, mean (SD) | 75.94 (11.72) | 72.88 (12.03) | <0.001 | 75.94 (11.72) | 75.97 (10.12) | 0.966 |
| <40 years old, n (%) | 4 (1.71) | 10 (2.28) | 0.621 | 4 (1.71) | 0 (0) | NA |
| 40–66 years old, n (%) | 121 (15.37) | 273 (10.62) | <0.001 | 121 (15.37) | 101 (10.58) | 0.003 |
| 67–75 years old, n (%) | 151 (18.48) | 262 (15.35) | 0.047 | 151 (18.48) | 139 (14.32) | 0.018 |
| ≥76 years old, n (%) | 403 (24.56) | 494 (21.82) | 0.045 | 403 (24.56) | 316 (20.80) | 0.012 |
| CCI index, mean (SD) | 1.81 (1.16) | 2.01 (1.25) | 0.001 | 1.81 (1.16) | 1.72 (1.15) | 0.163 |
| Prosthetic valve carriers, n (%) | 61 (18.94) | 79 (13.21) | 0.022 | 61 (18.94) | 48 (13.95) | 0.083 |
| Previous mitral valve disease, n (%) | 227 (21.02) | 260 (14.76) | <0.001 | 227 (21.02) | 173 (14.65) | <0.001 |
| Previous aortic valve disease, n (%) | 175 (20.64) | 342 (16.66) | 0.011 | 175 (20.64) | 142 (20.97) | 0.872 |
| Previous tricuspid valve disease, n (%) | 73 (21.28) | 67 (14.26) | 0.009 | 73 (21.28) | 52 (15.43) | 0.050 |
| Previous pulmonic valve disease, n (%) | 2 (11.76) | 1 (4.35) | 0.397 | 2 (11.76) | 1 (5.00) | 0.465 |
| Congenital malformation of heart, n (%) | 7 (6.67) | 4 (5.67) | 0.718 | 7 (6.67) | 4 (7.55) | 0.832 |
| COVID-19, n (%) | 4 (20.00) | 7 (22.58) | 0.827 | 4 (20.00) | 7 (25.93) | 0.636 |
| Congestive heart failure, n (%) | 103 (26.28) | 168 (23.43) | 0.292 | 103 (26.28) | 100 (26.18) | 0.975 |
| Septic arterial embolism, n (%) | 52 (34.67) | 58 (19.02) | <0.001 | 52 (34.67) | 38 (23.60) | 0.032 |
| Dementia, n (%) | 24 (26.09) | 28 (25.93) | 0.979 | 24 (26.09) | 19 (23.17) | 0.656 |
| Acute renal disease, n (%) | 256 (37.10) | 442 (29.47) | <0.001 | 256 (37.10) | 202 (29.88) | 0.005 |
| Chronic renal disease, n (%) | 172 (26.88) | 275 (23.19) | 0.081 | 172 (26.88) | 158 (24.09) | 0.249 |
| Ischemic heart disease, n (%) | 89 (25.95) | 230 (18.71) | 0.003 | 89 (25.95) | 25 (17.36) | 0.042 |
| COPD, n (%) | 30 (28.85) | 141 (20.64) | 0.060 | 30 (28.85) | 1 (20.00) | 0.671 |
| Atrial fibrillation, n (%) | 269 (23.05) | 368 (20.57) | 0.109 | 269 (23.05) | 239 (19.54) | 0.036 |
| Diabetes, n (%) | 183 (22.43) | 296 (16.52) | <0.001 | 183 (22.43) | 133 (16.77) | 0.004 |
| Drug Abuse, n (%) | 5 (12.20) | 16 (5.99) | 0.151 | 5 (12.20) | 6 (9.38) | 0.646 |
| Shock, n (%) | 45 (69.23) | 89 (54.60) | 0.044 | 45 (69.23) | 33 (52.38) | 0.052 |
| Periannular complications/atrioventricular block, n (%) | 30 (21.13) | 83 (19.53) | 0.680 | 30 (21.13) | 39 (18.40) | 0.525 |
| Staphylococcus bacteremia, n (%) | 274 (27.62) | 420 (20.64) | <0.001 | 274 (27.62) | 225 (23.01) | 0.019 |
| Streptococcus bacteremia, n (%) | 75 (10.64) | 118 (6.88) | 0.002 | 75 (10.64) | 65 (7.67) | 0.042 |
| Gram-negative bacteremia, n (%) | 72 (20.40) | 102 (22.22) | 0.530 | 72 (20.40) | 52 (21.67) | 0.709 |
| Fungemia, n (%) | 6 (40.00) | 15 (44.12) | 0.788 | 6 (40.00) | 9 (64.29) | 0.196 |
| Heart valve surgery, n (%) | 130 (22.93) | 253 (16.22) | <0.001 | 130 (22.93) | 111 (17.02) | 0.010 |
| Dialysis, n (%) | 81 (47.09) | 131 (37.43) | 0.035 | 81 (47.09) | 54 (38.30) | 0.119 |
| Pacemaker implantation, n (%) | 17 (12.14) | 37 (9.61) | 0.399 | 17 (12.14) | 17 (9.24) | 0.400 |
| Mechanical ventilation, n (%) | 152 (42.34) | 278 (35.28) | 0.022 | 152 (42.34) | 111 (35.92) | 0.091 |
| LOHS, Median (IQR) | 14 (21) | 15 (21) | 0.855 | 14 (21) | 16 (21) | 0.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Miguel-Yanes, J.M.; Jimenez-Garcia, R.; De Miguel-Diez, J.; Hernández-Barrera, V.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Noriega, C.; Lopez-de-Andres, A. Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020. J. Clin. Med. 2022, 11, 6847. https://doi.org/10.3390/jcm11226847
De Miguel-Yanes JM, Jimenez-Garcia R, De Miguel-Diez J, Hernández-Barrera V, Carabantes-Alarcon D, Zamorano-Leon JJ, Noriega C, Lopez-de-Andres A. Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020. Journal of Clinical Medicine. 2022; 11(22):6847. https://doi.org/10.3390/jcm11226847
Chicago/Turabian StyleDe Miguel-Yanes, Jose M., Rodrigo Jimenez-Garcia, Javier De Miguel-Diez, Valentin Hernández-Barrera, David Carabantes-Alarcon, Jose J. Zamorano-Leon, Concepción Noriega, and Ana Lopez-de-Andres. 2022. "Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020" Journal of Clinical Medicine 11, no. 22: 6847. https://doi.org/10.3390/jcm11226847
APA StyleDe Miguel-Yanes, J. M., Jimenez-Garcia, R., De Miguel-Diez, J., Hernández-Barrera, V., Carabantes-Alarcon, D., Zamorano-Leon, J. J., Noriega, C., & Lopez-de-Andres, A. (2022). Differences in Sex and the Incidence and In-Hospital Mortality among People Admitted for Infective Endocarditis in Spain, 2016–2020. Journal of Clinical Medicine, 11(22), 6847. https://doi.org/10.3390/jcm11226847








