ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy
Abstract
1. Introduction
2. Materials and Methods
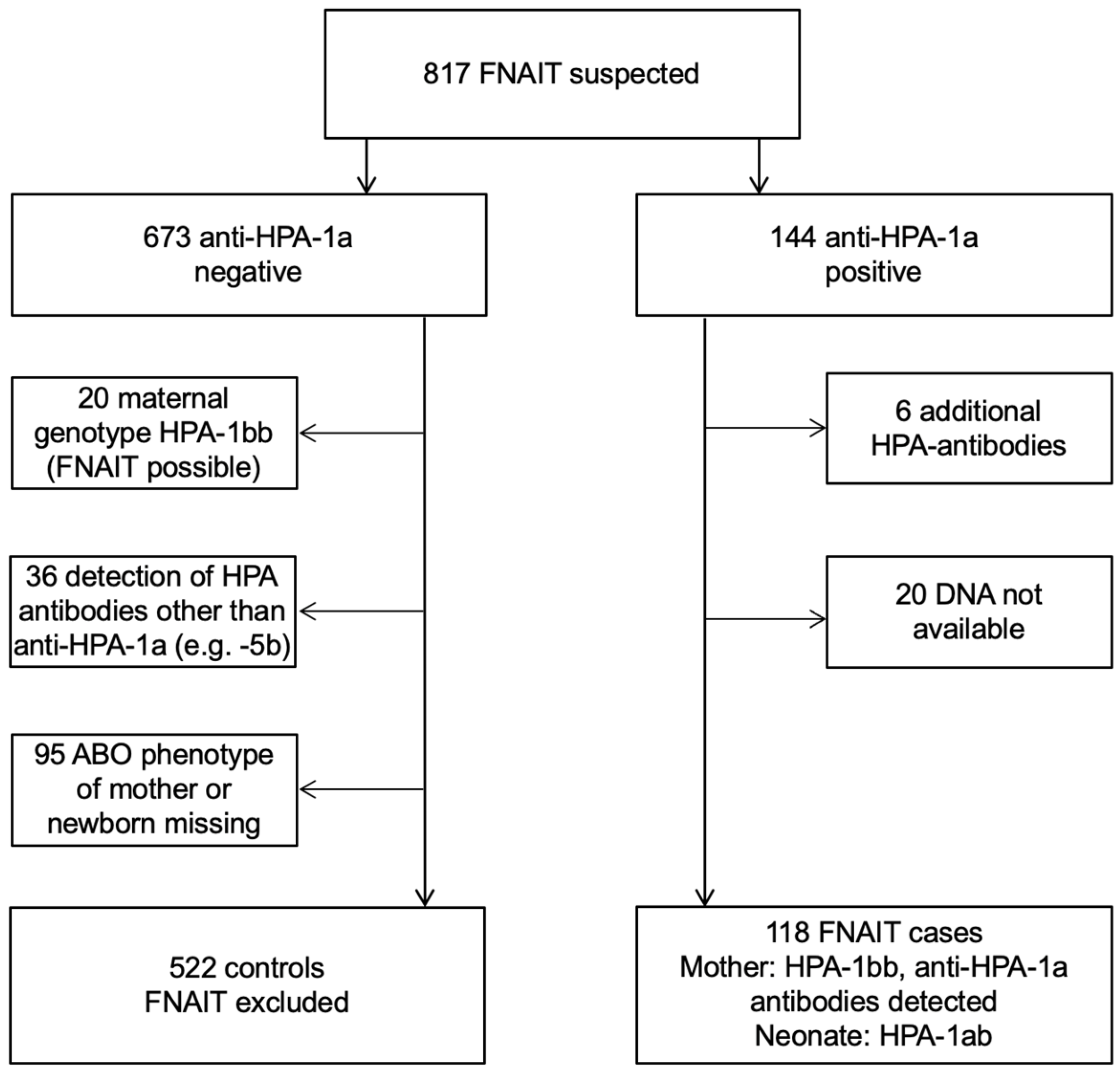
2.1. Patients and Case Definitions
2.2. Work-Up of FNAIT Families
2.3. ABO Genotyping in FNAIT Cases (Mother and Newborn)
2.4. Flow Cytometric Measurement of A Antigens on Adult and Neonatal Platelets
2.5. Antibodies
2.6. Statistical Analysis
3. Results
3.1. ABO Phenotype Frequencies Do Not Differ between FNAIT Cases and Controls
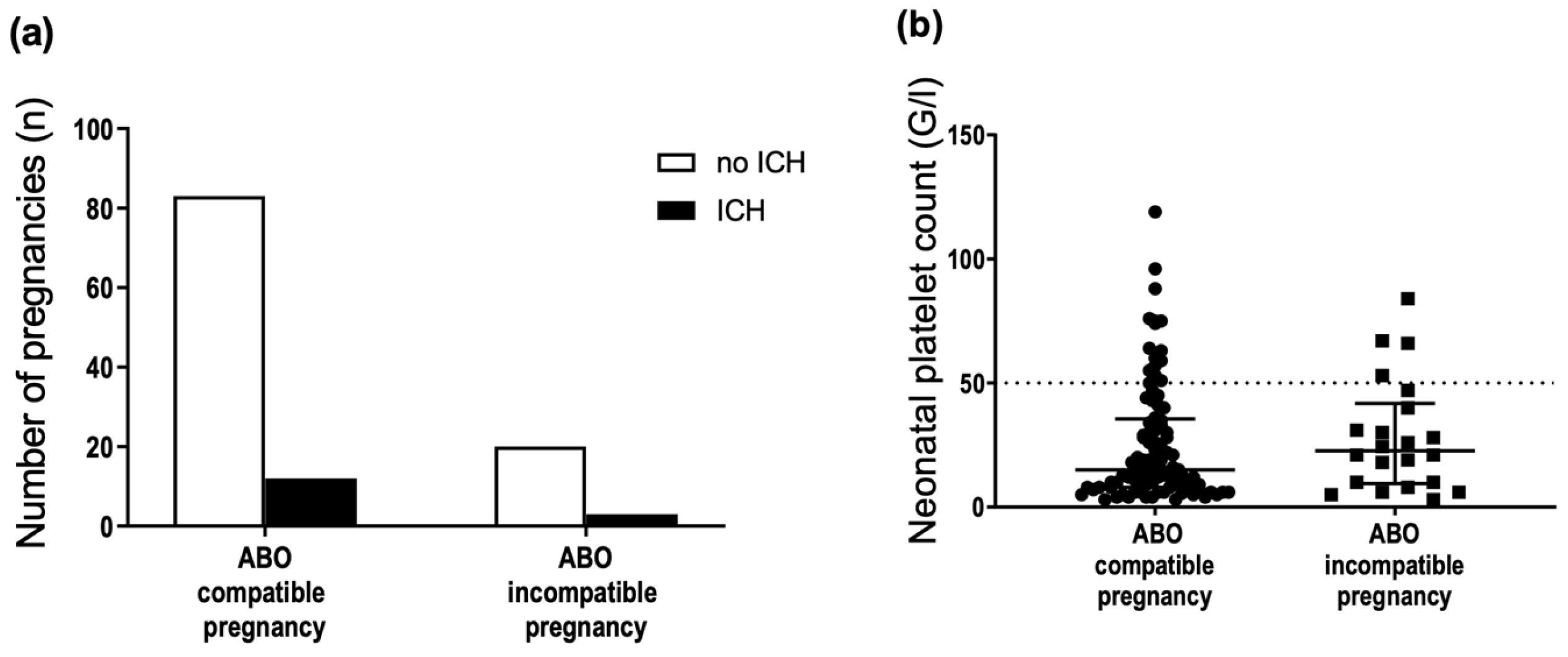
3.2. ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-HPA-1a Immunization by Pregnancy
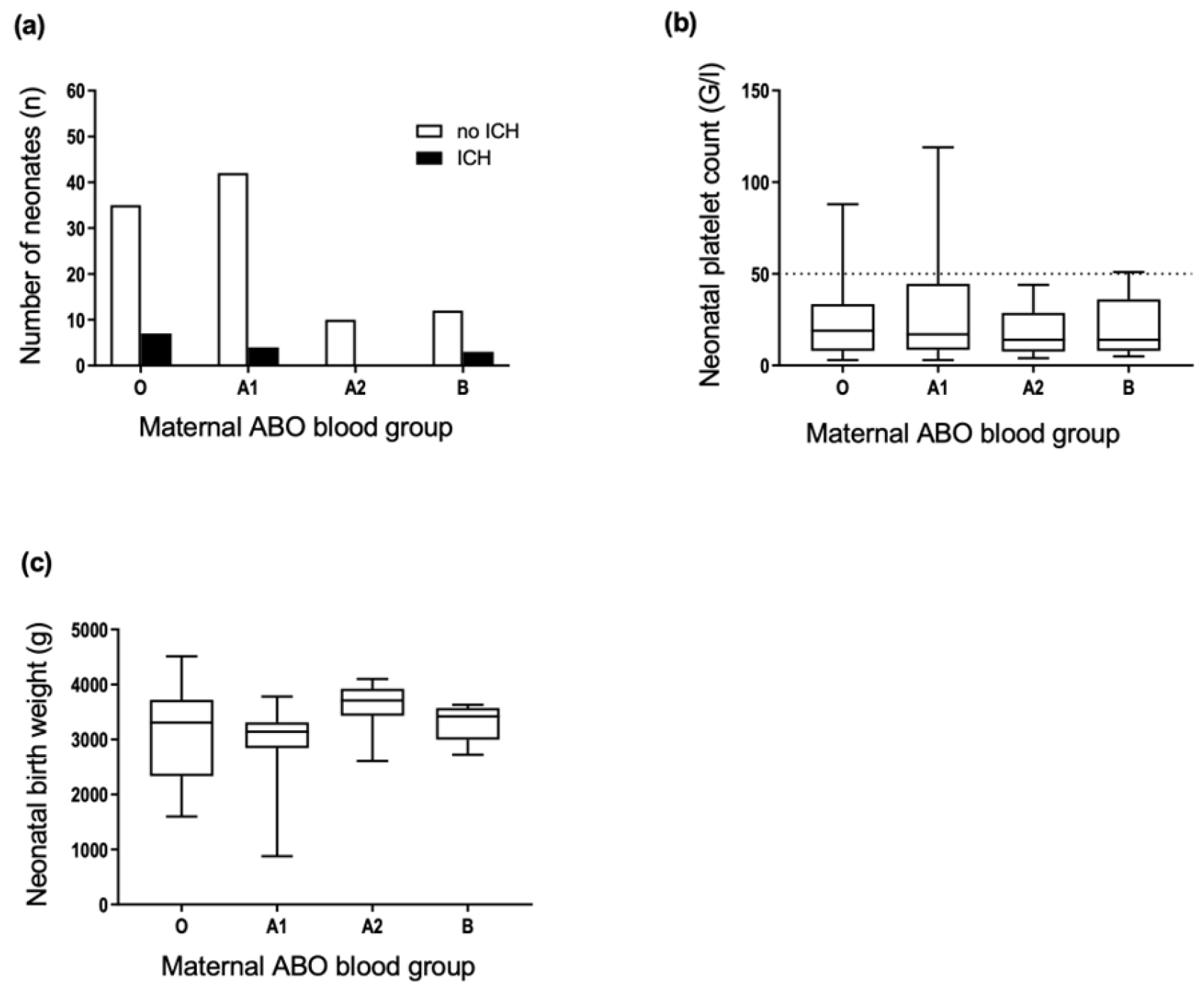
3.3. Maternal ABO Phenotypes Are Not Associated with FNAIT Severity
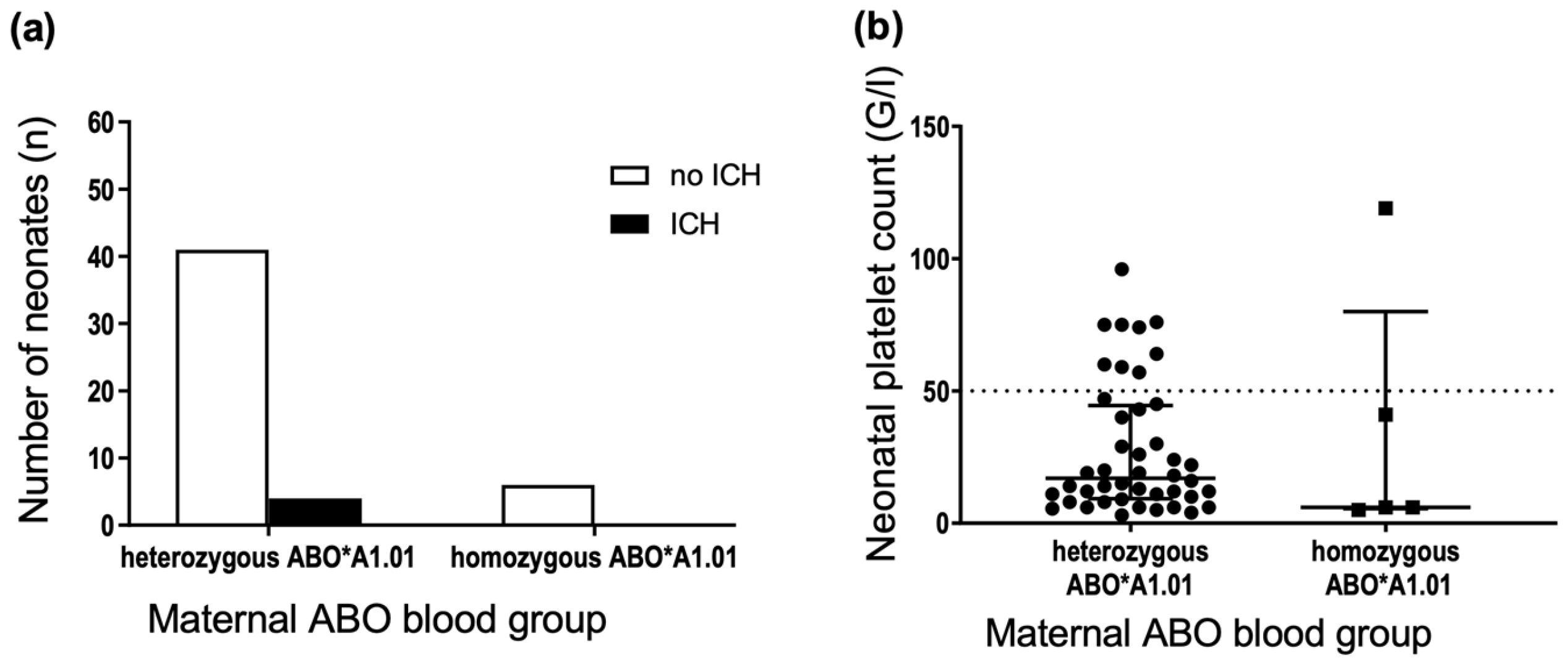
3.4. Maternal Gene Dose of the ABO*A1.01 Allele Is Not Associated with FNAIT Severity
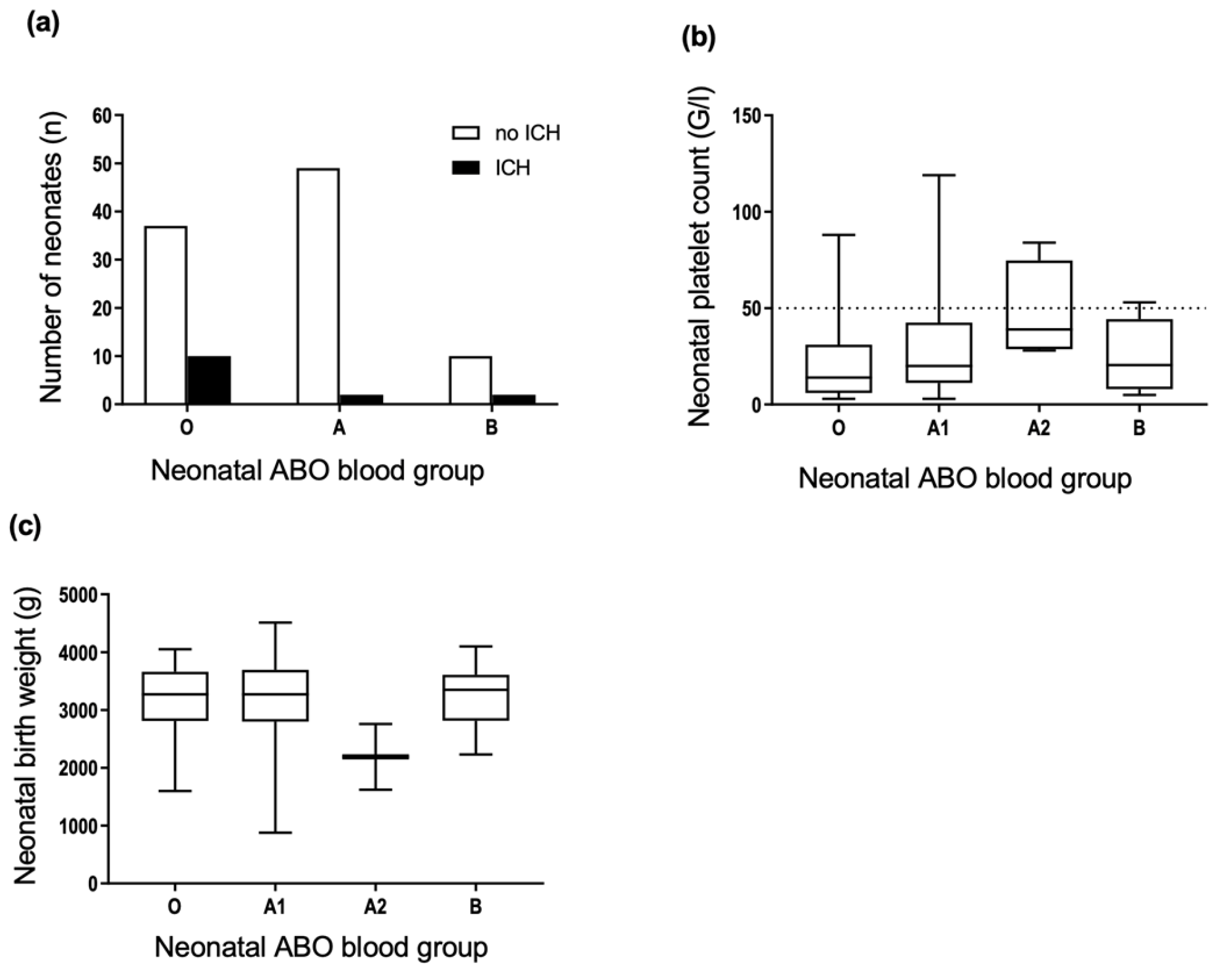
3.5. Neonatal ABO Phenotypes Are Not Associated with FNAIT Severity
3.6. ABO Incompatibility between the Mother and Fetus Is Not Associated with FNAIT Severity
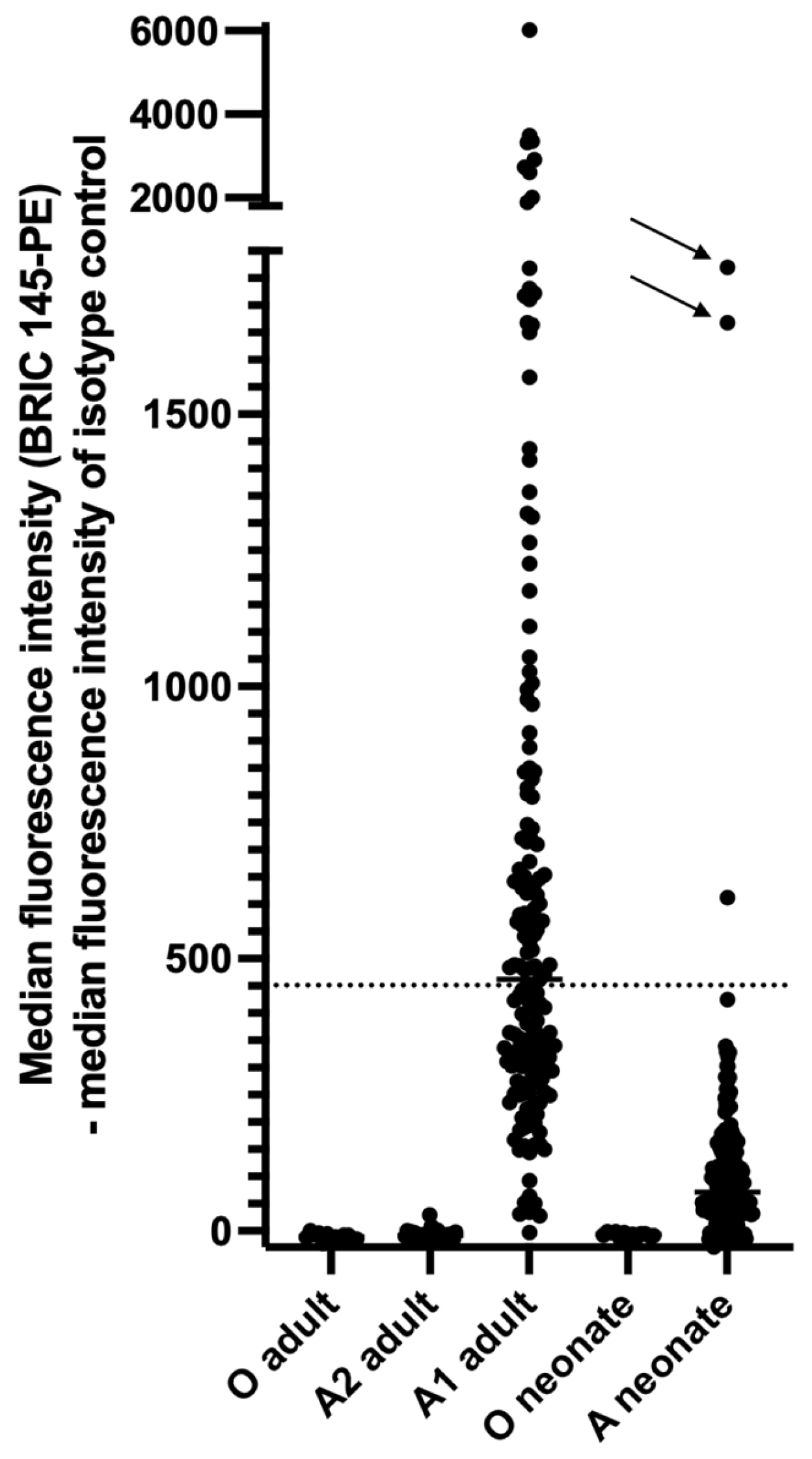
3.7. Blood Group A Antigens Are Weakly Expressed on Newborn Platelets but Strongly Expressed on Platelets of Some Newborns
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vos, T.W.; Winkelhorst, D.; de Haas, M.; Lopriore, E.; Oepkes, D. Epidemiology and management of fetal and neonatal alloimmune thrombocytopenia. Transfus. Apher. Sci. 2020, 59, 102704. [Google Scholar] [CrossRef] [PubMed]
- Alm, J.; Duong, Y.; Wienzek-Lischka, S.; Cooper, N.; Santoso, S.; Sachs, U.J.; Kiefel, V.; Bein, G. Anti-human platelet antigen-5b antibodies and fetal and neonatal alloimmune thrombocytopenia; incidental association or cause and effect? Br. J. Haematol. 2022, 198, 14–23. [Google Scholar] [CrossRef]
- Kamphuis, M.M.; Paridaans, N.P.; Porcelijn, L.; Lopriore, E.; Oepkes, D. Incidence and consequences of neonatal alloimmune thrombocytopenia: A systematic review. Pediatrics 2014, 133, 715–721. [Google Scholar] [CrossRef]
- Urbaniak, S.J.; Greiss, M.A. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000, 14, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Liverpool medical institution. Lancet 1960, 275, 526–527. [CrossRef][Green Version]
- Levine, P. The protective action of ABO Incompatibility on Rh isoimmunization and Rh hemolytic disease-theoretical and clinical implications. Am. J. Hum. Genet. 1959, 11, 418. [Google Scholar]
- Zwiers, C.; Koelewijn, J.M.; Vermij, L.; van Sambeeck, J.; Oepkes, D.; de Haas, M.; van der Schoot, C.E. ABO incompatibility and RhIG immunoprophylaxis protect against non-D alloimmunization by pregnancy. Transfusion 2018, 58, 1611–1617. [Google Scholar] [CrossRef]
- Gratwohl, A.A.; Shulman, N.R. ABO-Kompatibilität und isoimmune neonatale Thrombopenie. Schweiz. Med. Wochenschr. 1977, 107, 1464. [Google Scholar]
- Bertrand, G.; Drame, M.; Martageix, C.; Kaplan, C. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood 2011, 117, 3209–3213. [Google Scholar] [CrossRef]
- Ahlen, M.T.; Husebekk, A.; Killie, M.K.; Kjeldsen-Kragh, J.; Olsson, M.L.; Skogen, B. The development of severe neonatal alloimmune thrombocytopenia due to anti-HPA-1a antibodies is correlated to maternal ABO genotypes. Clin. Dev. Immunol. 2012, 2012, 156867. [Google Scholar] [CrossRef]
- Sachs, U.J.; Wienzek-Lischka, S.; Duong, Y.; Qiu, D.; Hinrichs, W.; Cooper, N.; Santoso, S.; Bayat, B.; Bein, G. Maternal antibodies against paternal class I human leukocyte antigens are not associated with foetal and neonatal alloimmune thrombocytopenia. Br. J. Haematol. 2020, 189, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R.; Edwards, J.T.; Hessner, M.J.; Klein, J.P.; Aster, R.H. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood 2000, 96, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, K.; Ueki, J.; Takenaka, M.; Furihata, K. Study on the expression of ABH antigens on platelets. Blood 1993, 82, 993–999. [Google Scholar] [CrossRef]
- O’Donghaile, D.; Jenkins, P.V.; McGrath, R.T.; Preston, L.; Field, S.P.; Ward, S.E.; O’Sullivan, J.M.; O’Donnell, J.S. Expresser phenotype determines ABO(H) blood group antigen loading on platelets and von Willebrand factor. Sci. Rep. 2020, 10, 18366. [Google Scholar] [CrossRef] [PubMed]
- Wienzek-Lischka, S.; König, I.R.; Papenkort, E.-M.; Hackstein, H.; Santoso, S.; Sachs, U.J.; Bein, G. HLA-DRB3*01:01 is a predictor of immunization against human platelet antigen-1a but not of the severity of fetal and neonatal alloimmune thrombocytopenia. Transfusion 2017, 57, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Moureau, P.; Andre, A. Blood groups of human blood platelets. Nature 1954, 174, 88. [Google Scholar] [CrossRef]
- Dunstan, R.A.; Simpson, M.B.; Knowles, R.W.; Rosse, W.F. The origin of ABH antigens on human platelets. Blood 1985, 65, 615–619. [Google Scholar] [CrossRef]
- Mollicone, R.; Caillard, T.; Le Pendu, J.; François, A.; Sansonetti, N.; Villarroya, H.; Oriol, R. Expression of ABH and X (Lex) antigens on platelets and lymphocytes. Blood 1988, 71, 1113–1119. [Google Scholar] [CrossRef]
- Santoso, S.; Kiefel, V.; Mueller-Eckhardt, C. Blood group A and B determinants are expressed on platelet glycoproteins IIa, IIIa, and Ib. Thromb. Haemost. 1991, 65, 196–201. [Google Scholar] [CrossRef]
- Hou, M.; Stockelberg, D.; Rydberg, L.; Kutti, J.; Wadenvik, H. Blood group A antigen expression in platelets is prominently associated with glycoprotein Ib and IIb. Evidence for an A1/A2 difference. Transfus. Med. 1996, 6, 51–59. [Google Scholar] [CrossRef]
- Stockelberg, D.; Hou, M.; Rydberg, L.; Kutti, J.; Wadenvik, H. Evidence for an expression of blood group A antigen on platelet glycoproteins IV and V. Transfus. Med. 1996, 6, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.G.; Smith, J.W.; Horsewood, P.; Warner, M.N.; Warkentin, T.E.; Finberg, R.W.; Hayward, C.P. ABH antigens on human platelets: Expression on the glycosyl phosphatidylinositol-anchored protein CD109. J. Lab. Clin. Med. 1998, 132, 142–148. [Google Scholar] [CrossRef]
- Holgersson, J.; Breimer, M.E.; Jacobsson, A.; Svensson, L.; Ulfvin, A.; Samuelsson, B.E. Glycolipid- and glycoprotein-based blood group A antigen expression in human thrombocytes. A1/A2 difference. Glycoconj. J. 1990, 7, 601–608. [Google Scholar] [CrossRef]
- Cooling, L.L.; Zhang, D.; Koerner, T.A. Human platelets express gangliosides with LKE activity and ABH blood group activity. Transfusion 2001, 41, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Skogen, B.; Rossebø Hansen, B.; Husebekk, A.; Havnes, T.; Hannestad, K. Minimal expression of blood group A antigen on thrombocytes from A2 individuals. Transfusion 1988, 28, 456–459. [Google Scholar] [CrossRef]
- Julmy, F.; Achermann, F.; Schulzki, T.; Carrel, T.; Nydegger, U. PLTs of blood group A1 donors express increased surface A antigen owing to apheresis and prolonged storage. Transfusion 2003, 43, 1378–1385. [Google Scholar] [CrossRef]
- Cooling, L.L.W.; Kelly, K.; Barton, J.; Hwang, D.; Koerner, T.A.W.; Olson, J.D. Determinants of ABH expression on human blood platelets. Blood 2005, 105, 3356–3364. [Google Scholar] [CrossRef]
- Sant’Anna Gomes, B.M.; Estalote, A.C.; Palatnik, M.; Pimenta, G.; Pereira, B.d.B.; Do Nascimento, E.M. Prevalence, serologic and genetic studies of high expressers of the blood group A antigen on platelets. Transfus. Med. 2010, 20, 303–314. [Google Scholar] [CrossRef][Green Version]
- DeLelys, M.E.; Ochoa, G.; Cserti-Gazdewich, C.M.; Vietz, C.; Preffer, F.I.; Dzik, W. Relationship between ABO genotype and A antigen expression on platelets. Transfusion 2013, 53, 1763–1771. [Google Scholar] [CrossRef]
- Xu, X.; Xu, F.; Ying, Y.; Hong, X.; Liu, Y.; Chen, S.; He, J.; Zhu, F.; Hu, W. ABO antigen levels on platelets of normal and variant ABO blood group individuals. Platelets 2019, 30, 854–860. [Google Scholar] [CrossRef]
- Curtis, B.R.; Fick, A.; Lochowicz, A.J.; McFarland, J.G.; Ball, R.H.; Peterson, J.; Aster, R.H. Neonatal alloimmune thrombocytopenia associated with maternal-fetal incompatibility for blood group B. Transfusion 2008, 48, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Zipursky, A.; Pollock, J.; Neelands, P.; Chown, B.; Israels, L. The transplacental passage of foetal red blood cells and the pathogenesis of Rh immunisation during pregnancy. Lancet 1963, 282, 489–493. [Google Scholar] [CrossRef]
- Chown, B. Anaemia from bleeding of the fetus into the mother’s circulation. Lancet 1954, 266, 1213–1215. [Google Scholar] [CrossRef]
- Bowman, J.M.; Pollock, J.M.; Penston, L.E. Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sang. 1986, 51, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, J.C.; Donohoe, W.T. Rh-immunization by pregnancy: Results of a survey and their relevance to prophylactic therapy. Br. Med. J. 1968, 4, 139–144. [Google Scholar] [CrossRef]
- Prevention of Rh-haemolytic disease: Results of the clinical trial. A combined study from centres in England and Baltimore. Br. Med. J. 1966, 2, 907–914. [CrossRef][Green Version]
- Mueller-Eckhardt, C.; Kiefel, V.; Grubert, A.; Kroll, H.; Weisheit, M.; Schmidt, S.; Mueller-Eckhardt, G.; Santoso, S. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet 1989, 333, 363–366. [Google Scholar] [CrossRef]
- Jin, J.C.; Lakkaraja, M.M.; Ferd, P.; Manotas, K.; Gabor, J.; Wissert, M.; Berkowitz, R.L.; McFarland, J.G.; Bussel, J.B. Maternal sensitization occurs before delivery in severe cases of fetal alloimmune thrombocytopenia. Am. J. Hematol. 2019, 94, E213–E215. [Google Scholar] [CrossRef]
- Desai, R.G.; McCutcheon, E.; Little, B.; Driscoll, S.G. Fetomaternal passage of leukocytes and platelets in erythroblastosis fetalis. Blood 1966, 27, 858–862. [Google Scholar] [CrossRef]
- Habibi, B.; Bretagne, M.; Bretagne, Y.; Forestier, F.; Daffos, F. Blood group antigens on fetal red cells obtained by umbilical vein puncture under ultrasound guidance: A rapid hemagglutination test to check for contamination with maternal blood. Pediatr. Res. 1986, 20, 1082–1084. [Google Scholar] [CrossRef]
- Fischer, K. Morbus Haemolyticus Neonatorum im AB0-System; Georg Thieme Verlag: Stuttgart, Germany, 1961. [Google Scholar]
- Cohen, F.; Zuelzer, W.W. Mechanisms of isoimmunization. II. Transplacental passage and postnatal survival of fetal erythrocytes in heterospecific pregnancies. Blood 1967, 30, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Brinc, D.; Lazarus, A.H. Mechanisms of anti-D action in the prevention of hemolytic disease of the fetus and newborn. Hematology Am. Soc. Hematol. Educ. Program 2009, 2009, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Crow, A.R.; Freedman, J.; Hannach, B.; Lazarus, A.H. Monoclonal antibody-mediated inhibition of the human HLA alloimmune response to platelet transfusion is antigen specific and independent of Fcgamma receptor-mediated immune suppression. Br. J. Haematol. 2000, 110, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, G.; Fantasia, D.; Palka, C.; Morizio, E.; Alfonsi, M.; Calabrese, G. Isolation and enrichment of circulating fetal cells for NIPD: An overview. Diagnostics 2021, 11, 2239. [Google Scholar] [CrossRef]
- Kumpel, B.M.; Sibley, K.; Jackson, D.J.; White, G.; Soothill, P.W. Ultrastructural localization of glycoprotein IIIa (GPIIIa, beta 3 integrin) on placental syncytiotrophoblast microvilli: Implications for platelet alloimmunization during pregnancy. Transfusion 2008, 48, 2077–2086. [Google Scholar] [CrossRef]
- Thiede, H.A.; Choate, J.W.; Gardner, H.H.; Santhay, H. Immunofluorescent examination of the human chorionic villus for blood group A and B substance. J. Exp. Med. 1965, 121, 1039–1050. [Google Scholar] [CrossRef]
- Goto, S.; Hoshino, M.; Tomoda, Y.; Ishizuka, N. Innumoelectron microscopy of the human chorionic villus in search of blood group A and B antigens. Lab. Investig. 1976, 35, 530–536. [Google Scholar]
- Kjær, M.; Geisen, C.; Akkök, Ç.A.; Wikman, A.; Sachs, U.; Bussel, J.B.; Nielsen, K.; Walles, K.; Curtis, B.R.; Vidarsson, G.; et al. Strategies to develop a prophylaxis for the prevention of HPA-1a immunization and fetal and neonatal alloimmune thrombocytopenia. Transfus. Apher. Sci. 2020, 59, 102712. [Google Scholar] [CrossRef]
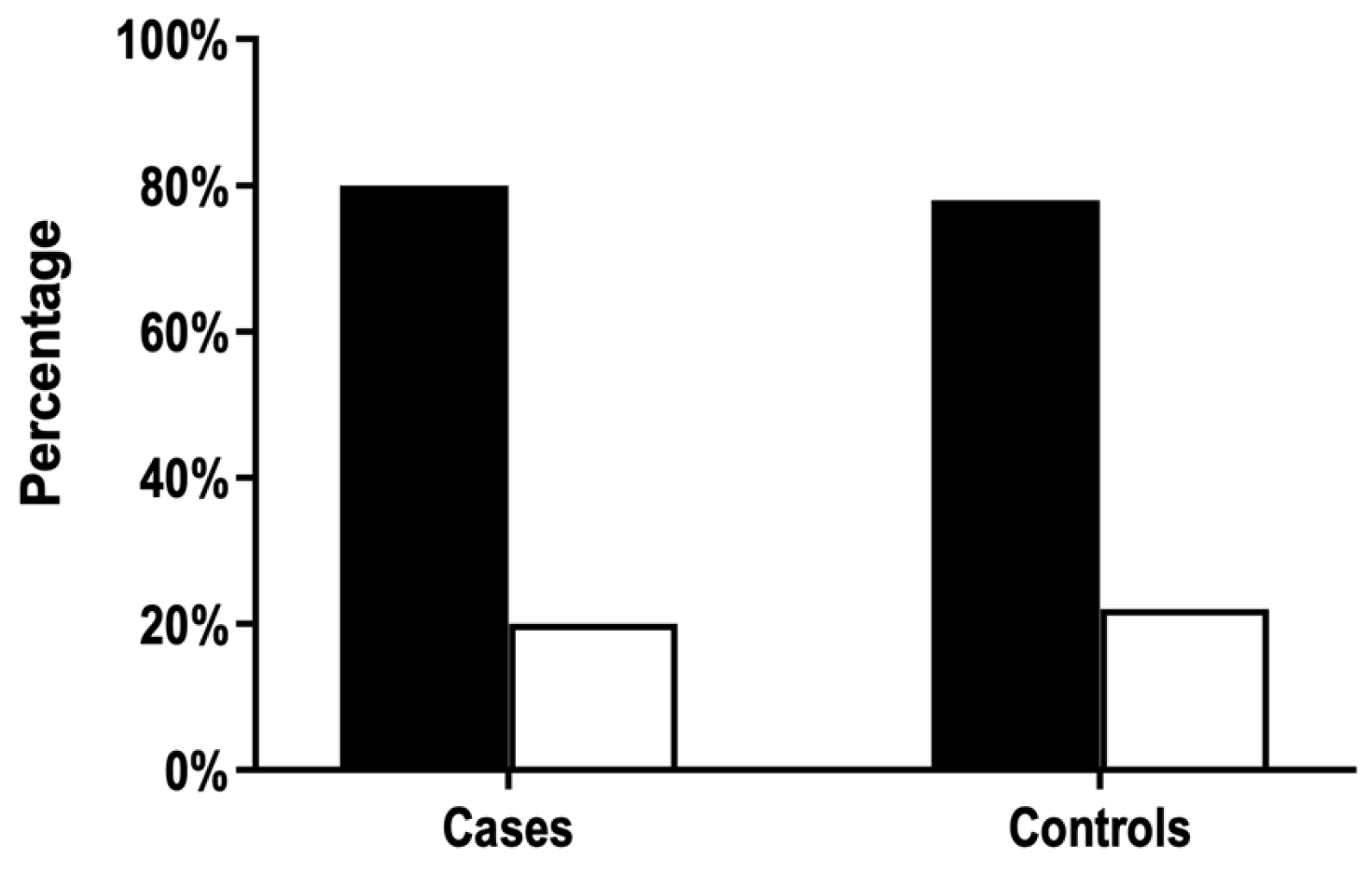
| ABO Phenotype | Cases (Mothers) (n = 118) | Cases (Neonates) (n = 118) | First Time Blood Donors (n = 45,295) |
|---|---|---|---|
| 0 | 35% | 39% | 41% |
| A | 47% | 44% | 42% |
| B | 13% | 10% | 12% |
| AB | 5% | 7% | 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miserre, L.; Wienzek-Lischka, S.; Mann, A.; Cooper, N.; Santoso, S.; Ehrhardt, H.; Sachs, U.J.; Bein, G. ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy. J. Clin. Med. 2022, 11, 6811. https://doi.org/10.3390/jcm11226811
Miserre L, Wienzek-Lischka S, Mann A, Cooper N, Santoso S, Ehrhardt H, Sachs UJ, Bein G. ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy. Journal of Clinical Medicine. 2022; 11(22):6811. https://doi.org/10.3390/jcm11226811
Chicago/Turabian StyleMiserre, Laila, Sandra Wienzek-Lischka, Andreas Mann, Nina Cooper, Sentot Santoso, Harald Ehrhardt, Ulrich J. Sachs, and Gregor Bein. 2022. "ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy" Journal of Clinical Medicine 11, no. 22: 6811. https://doi.org/10.3390/jcm11226811
APA StyleMiserre, L., Wienzek-Lischka, S., Mann, A., Cooper, N., Santoso, S., Ehrhardt, H., Sachs, U. J., & Bein, G. (2022). ABO Incompatibility between the Mother and Fetus Does Not Protect against Anti-Human Platelet Antigen-1a Immunization by Pregnancy. Journal of Clinical Medicine, 11(22), 6811. https://doi.org/10.3390/jcm11226811







