Recent Advances in Gene Therapy for Familial Hypercholesterolemia: An Update Review
Abstract
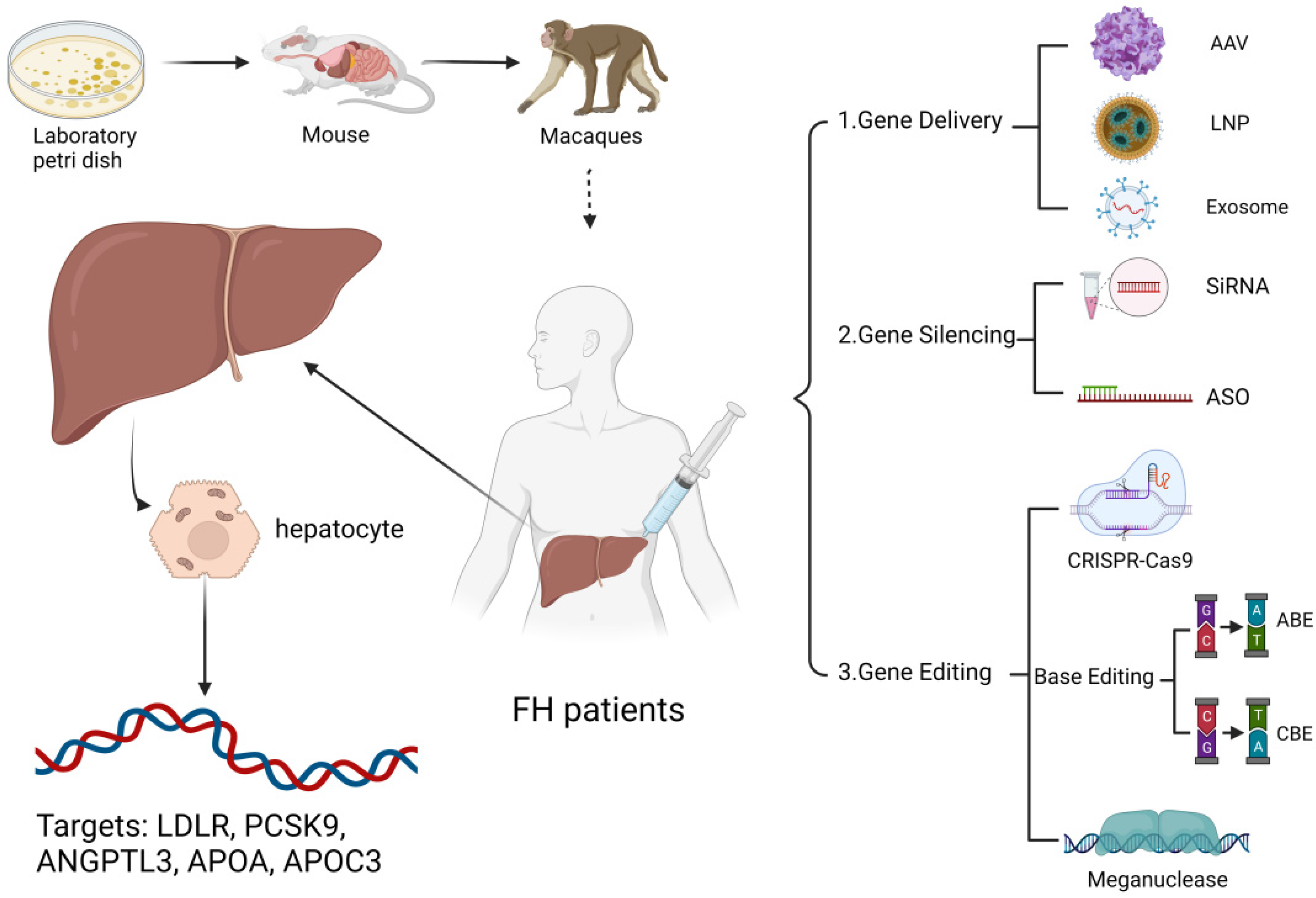
1. Introduction
Search Strategy
2. Replacing a Disease-Causing Gene with a Healthy Copy of the Gene
2.1. Adeno-Associated Virus (AAV) Mediated Therapy
2.2. Exosome-Mediated Therapy
3. Inactivating a Disease-Causing Gene That Is Not Functioning Properly
3.1. Small Interfering RNA (SiRNA)
3.2. Antisense Oligonucleotides (ASO)
4. Introducing a New or Modified Gene into the Body for Therapy
4.1. CRISPR-Cas 9
4.2. Base Editing (BE)
4.3. Meganuclease
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV. | Adeno-associated virus |
| ANGPTL3 | Angiotensin-like protein 3 |
| ASGPR | Glucose-lowering glycoprotein receptor |
| ASO | Antisense oligonucleotides |
| ASCVD | Atherosclerotic cardiovascular disease |
| BE | Base editing |
| CDS | Encoding sequence |
| CHD | Coronary heart disease |
| DKO | Double-knockout |
| FCS | Familial Chylomicronemia Syndrome |
| FH | Familial hypercholesterolemia |
| GalNAc | N-acetylgalactosamine carbohydrate |
| HeFH | Heterozygous familial hypercholesterolemia |
| HFD | High-fat diet |
| HLCs | Hepatocyte-like cells |
| HoFH | Homozygous familial hypercholesterolemia |
| iPSCs | Induced pluripotent stem cells |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| Lp(a) | Lipoprotein(a) |
| LOF | Loss of function |
| NHPs | Non-human primates |
| PCHD | Premature coronary heart disease |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| RNAi | RNA interference |
| siRNA | Small interfering ribonucleic acid |
| TBG | Thyroxin-binding globulin |
| TG | Triglycerides |
| TC | Total cholesterol |
| TRLs | Triglyceride-rich lipoproteins |
| VLDL | Very low density lipoprotein |
| WPRE | Marmot hepatitis virus post-transcriptional regulatory element |
References
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.Y.; Cheng, X.S. Novel Approaches for the Treatment of Familial Hypercholesterolemia: Current Status and Future Challenges. J. Atheroscler. Thromb. 2018, 25, 665–673. [Google Scholar] [CrossRef]
- Raal, F.J.; Santos, R.D.; Blom, D.J.; Marais, A.D.; Charng, M.J.; Cromwell, W.C.; Lachmann, R.H.; Gaudet, D.; Tan, J.L.; Chasan-Taber, S.; et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 998–1006. [Google Scholar] [CrossRef]
- Cuchel, M.; Meagher, E.A.; du Toit Theron, H.; Blom, D.J.; Marais, A.D.; Hegele, R.A.; Averna, M.R.; Sirtori, C.R.; Shah, P.K.; Gaudet, D.; et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: A single-arm, open-label, phase 3 study. Lancet 2013, 381, 40–46. [Google Scholar] [CrossRef]
- Gidding, S.S.; Champagne, M.A.; de Ferranti, S.D.; Defesche, J.; Ito, M.K.; Knowles, J.W.; McCrindle, B.; Raal, F.; Rader, D.; Santos, R.D.; et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement from the American Heart Association. Circulation 2015, 132, 2167–2192. [Google Scholar] [CrossRef]
- Moyle, M.; Tate, B. Homozygous familial hypercholesterolaemia presenting with cutaneous xanthomas: Response to liver transplantation. Australas. J. Dermatol. 2004, 45, 226–228. [Google Scholar] [CrossRef]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef]
- Maestro, S.; Weber, N.D.; Zabaleta, N.; Aldabe, R.; Gonzalez-Aseguinolaza, G. Novel vectors and approaches for gene therapy in liver diseases. JHEP Rep. 2021, 3, 100300. [Google Scholar] [CrossRef]
- Pasi, K.J.; Rangarajan, S.; Mitchell, N.; Lester, W.; Symington, E.; Madan, B.; Laffan, M.; Russell, C.B.; Li, M.; Pierce, G.F.; et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N. Engl. J. Med. 2020, 382, 29–40. [Google Scholar] [CrossRef]
- Nelson, C.E.; Wu, Y.; Gemberling, M.P.; Oliver, M.L.; Waller, M.A.; Bohning, J.D.; Robinson-Hamm, J.N.; Bulaklak, K.; Castellanos Rivera, R.M.; Collier, J.H.; et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; He, L.; Pu, W.; Yu, W.; Li, Y.; Wu, Y.T.; Xu, C.; Wei, Y.; Ding, Q.; et al. In Vivo AAV-CRISPR/Cas9-Mediated Gene Editing Ameliorates Atherosclerosis in Familial Hypercholesterolemia. Circulation 2020, 141, 67–79. [Google Scholar] [CrossRef]
- Cuchel, M.; Bajaj, A.; Carr, R.; Sikora, T.; Duell, P.B.; Tardif, J.-C.; Roeters van Lennep, J.E.; Linton, M.F.; Averna, M.; Cho, Y.; et al. Use of prophylactic steroids to mitigate potential T-cell response in AAV8-mediated hLDLR gene transfer in subjects with homozygous familial hypercholesterolemia. Mol. Ther. 2020, 28, 271. [Google Scholar]
- Wang, L.; Muthuramu, I.; Somanathan, S.; Zhang, H.; Bell, P.; He, Z.; Yu, H.; Zhu, Y.; Tretiakova, A.P.; Wilson, J.M. Developing a second-generation clinical candidate AAV vector for gene therapy of familial hypercholesterolemia. Mol. Ther. Methods Clin. Dev. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Masana, L. Review of the scientific evolution of gene therapy for the treatment of homozygous familial hypercholesterolaemia: Past, present and future perspectives. J. Med. Genet. 2019, 56, 711–717. [Google Scholar] [CrossRef]
- Taheri, F.; Taghizadeh, E.; Baniamerian, F.; Rostami, D.; Rozeian, A.; Mohammad Gheibi Hayat, S.; Jamialahmadi, T.; Reiner, Ž.; Sahebkar, A. Cellular and Molecular Aspects of Managing Familial Hypercholesterolemia: Recent and Emerging Therapeutic Approaches. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1018–1028. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.C.; Choi, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl. Med. 2019, 8, 880–886. [Google Scholar] [CrossRef]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef]
- Tang, M.; Chen, Y.; Li, B.; Sugimoto, H.; Yang, S.; Yang, C.; LeBleu, V.S.; McAndrews, K.M.; Kalluri, R. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21557. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. BioMed Res. Int. 2020, 2020, 5424281. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Zhang, Y.; Wang, J.; Wang, C.; Liu, Y.; Yang, G.; Yuan, L. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics 2021, 11, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med. 2019, 380, 549–558. [Google Scholar] [CrossRef]
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226. [Google Scholar] [CrossRef]
- German, C.A.; Shapiro, M.D. Small Interfering RNA Therapeutic Inclisiran: A New Approach to Targeting PCSK9. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Prat, A. The multifaceted biology of PCSK9. Endocr. Rev. 2021, 43, 558–582. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef]
- Lim, G.B. ANGPTL3 inhibition for hypercholesterolaemia. Nat. Rev. Cardiol. 2021, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Redon, V.; Yu, H.; Querbes, W.; Pirruccello, J.; Liebow, A.; Deik, A.; Trindade, K.; Wang, X.; Musunuru, K.; et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis 2018, 268, 196–206. [Google Scholar] [CrossRef]
- Watts, G.F.; Schwabe, C.; Scott, R.S.; Gladding, P.A.; Sullivan, D.R.; Baker, J.R.; Clifton, P.M.; Hamilton, J.; Given, B.D.; Melquist, S.; et al. RNA Interference Targeting Hepatic Angiopoietin-Like Protein 3 Results in Prolonged Reductions in Plasma Triglycerides and LDL-C in Human Subjects. Circulation 2019, 140, E987–E988. [Google Scholar]
- Tromp, T.R.; Stroes, E.S.G.; Hovingh, G.K. Gene-based therapy in lipid management: The winding road from promise to practice. Expert Opin. Investig. Drugs 2020, 29, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.; Peloso, G.M.; Auer, P.L.; Crosslin, D.R.; Stitziel, N.O.; Lange, L.A.; Lu, Y.; Tang, Z.Z.; Zhang, H.; Hindy, G.; et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar] [CrossRef]
- Ballantyne, C.M. RNA interference targeting apolipoprotein C-III results in deep and prolonged reductions in plasma triglycerides. In Proceedings of the American Heart Association Scientific Sessions, Philadelphia, PA, USA, 16–18 November 2020. [Google Scholar]
- Duarte Lau, F.; Giugliano, R.P. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Lamina, C.; Kronenberg, F. Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol. 2019, 4, 575–579. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Balog, C.; Swerdlow, D.I.; Scrimgeour, A.C.; Rambaran, C.; Wilson, R.J.; Boyce, M.; Ray, K.K.; Cho, L.; et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals with Elevated Plasma Lipoprotein(a) Levels. JAMA 2022, 327, 1679–1687. [Google Scholar] [CrossRef]
- Macchi, C.; Sirtori, C.R.; Corsini, A.; Santos, R.D.; Watts, G.F.; Ruscica, M. A new dawn for managing dyslipidemias: The era of rna-based therapies. Pharmacol. Res. 2019, 150, 104413. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, F.; Liu, F.; Chen, J.; Zhao, X.; Xu, Q. Efficient Delivery of Antisense Oligonucleotides Using Bioreducible Lipid Nanoparticles In Vitro and In Vivo. Mol. Ther. Nucleic Acids 2020, 19, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Nicholls, S.J.; Langsted, A.; Ray, K.K.; Tybjærg-Hansen, A. Advances in lipid-lowering therapy through gene-silencing technologies. Nat. Rev. Cardiol. 2018, 15, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Gennemark, P.; Walter, K.; Clemmensen, N.; Rekić, D.; Nilsson, C.A.M.; Knöchel, J.; Hölttä, M.; Wernevik, L.; Rosengren, B.; Kakol-Palm, D.; et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci. Transl. Med. 2021, 13, eabe9117. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.; Helmlinger, G.; Nilsson, C.; Zhudenkov, K.; Skrtic, S.; Hamrén, B.; Peskov, K.; Hurt-Camejo, E.; Jansson-Löfmark, R. Comparative quantitative systems pharmacology modeling of anti-PCSK9 therapeutic modalities in hypercholesterolemia. J. Lipid Res. 2019, 60, 1610–1621. [Google Scholar] [CrossRef]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Alexander, V.J.; Xia, S.; Hurh, E.; Hughes, S.G.; O’Dea, L.; Geary, R.S.; Witztum, J.L.; Tsimikas, S. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur. Heart J. 2019, 40, 2785–2796. [Google Scholar] [CrossRef]
- Miname, M.H.; Rocha, V.Z.; Santos, R.D. The Role of RNA-Targeted Therapeutics to Reduce ASCVD Risk: What Have We Learned Recently? Curr. Atheroscler. Rep. 2021, 23, 40. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Packard, C.J.; Chapman, M.J.; Katzmann, I.; Laufs, U. Targeting RNA With Antisense Oligonucleotides and Small Interfering RNA: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Vermersch, E.; Jouve, C.; Hulot, J.S. CRISPR/Cas9 gene-editing strategies in cardiovascular cells. Cardiovasc. Res. 2020, 116, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Glass, Z.; Chen, J.; Haas, M.; Jin, X.; Zhao, X.; Rui, X.; Ye, Z.; Li, Y.; Zhang, F.; et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A.C.; Evitt, N.H.; Lv, W.; Musunuru, K. Reduced Blood Lipid Levels With In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation 2018, 137, 975–977. [Google Scholar] [CrossRef]
- Ruscica, M.; Zimetti, F.; Adorni, M.P.; Sirtori, C.R.; Lupo, M.G.; Ferri, N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol. Res. 2020, 153, 104653. [Google Scholar] [CrossRef]
- Okada, H.; Nakanishi, C.; Yoshida, S.; Shimojima, M.; Yokawa, J.; Mori, M.; Tada, H.; Yoshimuta, T.; Hayashi, K.; Yamano, T.; et al. Function and Immunogenicity of Gene-corrected iPSC-derived Hepatocyte-Like Cells in Restoring Low Density Lipoprotein Uptake in Homozygous Familial Hypercholesterolemia. Sci. Rep. 2019, 9, 4695. [Google Scholar] [CrossRef]
- Doudna, J.A. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Wang, X.; Musunuru, K. In Vivo Base Editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a Therapeutic Alternative to Genome Editing. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1741–1747. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Grizot, S.; Epinat, J.C.; Thomas, S.; Duclert, A.; Rolland, S.; Pâques, F.; Duchateau, P. Generation of redesigned homing endonucleases comprising DNA-binding domains derived from two different scaffolds. Nucleic Acids Res. 2010, 38, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Smith, J.; Breton, C.; Clark, P.; Zhang, J.; Ying, L.; Che, Y.; Lape, J.; Bell, P.; Calcedo, R.; et al. Meganuclease targeting of PCSK9 in macaque liver leads to stable reduction in serum cholesterol. Nat. Biotechnol. 2018, 36, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Breton, C.; Warzecha, C.C.; Bell, P.; Yan, H.; He, Z.; White, J.; Zhu, Y.; Li, M.; Buza, E.L.; et al. Long-term stable reduction of low-density lipoprotein in nonhuman primates following in vivo genome editing of PCSK9. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
| Way. | Target | Drug Name | Study Design | Sample Size | Primary Outcome | Lipid-Lowering Effect | Trial Number |
|---|---|---|---|---|---|---|---|
| AAV-mediated therapy | LDLR | AAV8.TBG.hLDLR | Phase 1/2 | 9 participants | Number of participants with product-related adverse events, up to 24 weeks | Not shown | NCT02651675 |
| Exosome-mediated therapy | LDLR | ExoLdlr | Phase 1 | 30 participants | Changes of TC, TG, LDL-C, HDL-C at day 19 | Underway | NCT05043181 |
| SiRNA | PCSK9 | Inclisiran (ALN-PCSsc) | Phase 3 | 1617 participants | Percentage change in LDL-C from the baseline to day 510 | LDL-C reduction 50% | NCT03400800 |
| ANGPTL3 | ARO-ANG3 | Phase 1 | 93 participants | Number of participants with treatment-related adverse events, post-dose 113 (±3 days) | LDL-C reduction 42% | NCT03747224 | |
| Phase 2 | 204 participants | Percentage change in fasting TG from the baseline, at week 24 | Underway | NCT04832971 | |||
| APOC3 | ARO-APOC3 | Phase 1 | 112 participants | Number of participants with possible treatment-related adverse events as of day 113 (±3 days) | Not shown | NCT03783377 | |
| Lp(a) | Olpasiran (AMG 890) | Phase 1 | 79 participants | Changes in the data, such as participant incidence of treatment adverse events and Lp(a) levels, up to 365 days | Lp(a) reduction 76–91% in patients with Lp(a) ≥ 200 nM/L, 71% to 97% in patients with 70 nM/L ≤ Lp(a) ≤ 199 nM/L | NCT03626662 | |
| Phase 2 | 290 participants | Percentage change in Lp(a), relative to the baseline values, at week 36 | Underway | NCT04270760 | |||
| SLN360 | Phase 1 | 88 participants | Safety and tolerability of SLN360 in patients with elevated Lp(a) | Lp(a) reduction 70% (300 mg) or 80% (600 mg) | NCT04606602 | ||
| ASO | PCSK9 | AZD8233 (ION-863633) | Phase 1 | 73 participants | Safety, tolerability, and pharmacokinetics of AZD8233, from the randomization to 4 months follow-up | PCSK9 reduction 95%, LDL-C reduction 68% | NCT03593785 |
| ANGPTL3 | Vupanorsen(IONIS-ANGPTL3-Lrx) | Phase 2 | 105 participants | Percentage change from the baseline in the fasting TG levels, week 25 for cohorts B and C, week 27 for cohort A | TC was reduced by 47%, 36% and 53%, respectively, with weekly injections of 20 mg, 40 mg and 80 mg vupanorsen for four weeks. | NCT03371355 | |
| ApoC3 | volanesorsen | Phase 3 | 67 participants | Fasting TG relative to the baseline percentage change, at month 3 | ApoC3 reduction 84%; TG reduction 77% | NCT02211209 | |
| ApoC3 | Olezarsen (AKCEA-APOCIII-LRx) | Phase 1/2a | 56 participants | Safety, tolerability, pharmacokinetics, and pharmacodynamics of IONIS APOC-III-LRx, up to 183 days | ApoC3 reduction 92%; TG reduction 77% | NCT02900027 | |
| Phase 2 | 114 participants | Safety, tolerability and effectiveness of ISIS 678354 in reducing the TG levels at different doses, up to 6 months | Underway | NCT03385239 | |||
| Lp(a) | ISIS 681257 (IONIS-APO(a)Rx-lrx) | Phase 2 | 286 participants | Percentage change from the baseline in Lp(a), week 25 for groups A, B, and C, week 27 for groups D and E | Lp(a) reduction 80% (20 mg per week), 58% (20 mg per 2 weeks), 35%, 56%, 72% (20, 40, 60 mg per 4 weeks) | NCT03070782 | |
| TQJ230 | Phase 3 | 8324 participants | Time to first adverse cardiovascular event in the group of patients with elevated Lp(a) ≥ 70 or 90 mg/dL, time frame: approximately 4 years | Underway | NCT04023552 |
| Approach | Advantage | Challenge |
|---|---|---|
| AAV-mediated therapy |
|
|
| Exosome-mediated therapy |
|
|
| RNA-targeted therapeutics (SiRNA, ASO) |
|
|
| CRISPR-Cas 9 |
|
|
| Base editing |
|
|
| Meganuclease |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Hu, L.; Shen, T.; Yang, R.; Jiang, L. Recent Advances in Gene Therapy for Familial Hypercholesterolemia: An Update Review. J. Clin. Med. 2022, 11, 6773. https://doi.org/10.3390/jcm11226773
Fu Q, Hu L, Shen T, Yang R, Jiang L. Recent Advances in Gene Therapy for Familial Hypercholesterolemia: An Update Review. Journal of Clinical Medicine. 2022; 11(22):6773. https://doi.org/10.3390/jcm11226773
Chicago/Turabian StyleFu, Qingan, Lijuan Hu, Tianzhou Shen, Renqiang Yang, and Long Jiang. 2022. "Recent Advances in Gene Therapy for Familial Hypercholesterolemia: An Update Review" Journal of Clinical Medicine 11, no. 22: 6773. https://doi.org/10.3390/jcm11226773
APA StyleFu, Q., Hu, L., Shen, T., Yang, R., & Jiang, L. (2022). Recent Advances in Gene Therapy for Familial Hypercholesterolemia: An Update Review. Journal of Clinical Medicine, 11(22), 6773. https://doi.org/10.3390/jcm11226773







