A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Study Design
2.2. Endoscopic Evaluation Prior to Removal
2.3. Removal Procedure, Classification, and Complications
2.4. Data Analysis
3. Results
3.1. Group 1 (n = 2)
3.2. Group 2 (n = 5)
3.3. Group 3 (n = 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boogaard, R.; Huijsmans, S.H.; Pijnenburg, M.W.H.; Tiddens, H.A.W.M.; de Jongste, J.C.; Merkus, P.J.F.M. Tracheomalacia and Bronchomalacia in Children. Chest 2005, 128, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Vijayasekaran, D.; Balasubramanian, S.; Sivabalan, S.; Vindhiya, K. Clinical Characteristics and Associated Congenital Lesions with Tracheomalacia in Infants. Indian Pediatr. 2018, 55, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Weigle, C.G.M. Treatment of an infant with tracheobronchomalacia at home with a lightweight, high-humidity, continuous positive airway pressure system. Crit. Care Med. 1990, 18, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Pizer, B.L.; Freeland, A.P.; Wilkinson, A.R. Prolonged positive airway pressure for severe neonatal tracheobronchomalacia. Arch. Dis. Child. 1986, 61, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.; Cohen, R.; Filler, R. Treatment of tracheomalacia: Eight years’ experience. J. Pediatr. Surg. 1986, 21, 781–785. [Google Scholar] [CrossRef]
- Serrano, C.; Lostalé, F.; Rodríguez-Panadero, F.; de Blas, I.; Laborda, A.; de Gregorio, M.A. Stents traqueales metálicos autoexpandibles. Estudio comparativo de 3 tipos diferentes de stents en un modelo animal. Arch. Bronconeumol. 2016, 52, 123–130. [Google Scholar] [CrossRef]
- Tappin, S.W. Canine tracheal collapse. J. Small Anim. Pract. 2016, 57, 9–17. [Google Scholar] [CrossRef]
- Della Maggiore, A. Tracheal and Airway Collapse in Dogs. Vet.-Clin. North Am. Small Anim. Pract. 2014, 44, 117–127. [Google Scholar] [CrossRef]
- Bottero, E.; Bellino, C.; De Lorenzi, D.; Ruggiero, P.; Tarducci, A.; D’Angelo, A.; Gianella, P. Clinical Evaluation and Endoscopic Classification of Bronchomalacia in Dogs. J. Vet.- Intern. Med. 2013, 27, 840–846. [Google Scholar] [CrossRef]
- Done, S.H.; Drew, R.A. Observations on the pathology of tracheal collapse in dogs. J. Small Anim. Pract. 1976, 17, 783–791. [Google Scholar] [CrossRef]
- Weisse, C.; Berent, A.; Violette, N.; McDougall, R.; Lamb, K. Short-, intermediate-, and long-term results for endoluminal stent placement in dogs with tracheal collapse. J. Am. Vet.- Med. Assoc. 2019, 254, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Antón-Pacheco, J.L.; Cabezalí, D.; Tejedor, R.; López, M.; Luna, C.; Comas, J.V.; De Miguel, E. The role of airway stenting in pediatric tracheobronchial obstruction. Eur. J. Cardio-Thorac. Surg. 2008, 33, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Cofer, S.A.; Badaoui, J.N.; Boesch, R.P.; Balakrishnan, K. Endotracheal metallic stent removal: A novel ABC (airway balloon collapse) technique. Int. J. Pediatr. Otorhinolaryngol. 2020, 140, 110490. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, B.; Xu, X. Follow-Up Investigation of 41 Children After Metallic Airway Stent Implantation: An 8-Year Experience. Front. Pediatr. 2020, 8, 579209. [Google Scholar] [CrossRef]
- Mondal, A.; Ha, J.; Jo, V.Y.; Wu, F.-Y.; Kaza, A.K.; Dupont, P.E. Preclinical evaluation of a pediatric airway stent for tracheobronchomalacia. J. Thorac. Cardiovasc. Surg. 2020, 161, e51–e60. [Google Scholar] [CrossRef]
- Lopez-Minguez, S.; Serrano-Casorran, C.; Guirola, J.A.; Rodriguez-Zapater, S.; Bonastre, C.; De Gregorio, M.A. New tracheal stainless steel stent pilot study: Twelve month follow-up in a rabbit model. PeerJ 2019, 7, e7797. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Serrano-Casorran, C.; Dvm, S.L.; Dvm, S.R.; Bonastre, C.; Guirola, J.A.; De Gregorio, M.A.; Lopez-Minguez, S.; Zapater, S.R.; De Gregorio, M. A new airway spiral stent designed to maintain airway architecture with an atraumatic removal after full epithelization—Research of feasibility and viability in canine patients with tracheomalacia. Pediatr. Pulmonol. 2020, 55, 1757–1764. [Google Scholar] [CrossRef]
- Baxter, J.D.; Dunbar, J.S. LXXVI Tracheomalacia. Ann. Otol. Rhinol. Laryngol. 1963, 72, 1013–1023. [Google Scholar] [CrossRef]
- Vasko, J.S.; Ahn, C. Surgical Management of Secondary Tracheomalacia. Ann. Thorac. Surg. 1968, 6, 269–272. [Google Scholar] [CrossRef]
- Picot, C.; Monnet, P.; Bethenod, M.; Beraud, C.; Jaubert de Beaujeu, M.; Salle, B. Tracheomalacia in infants. Arch. Fr. Pediatr. 1969, 26, 493–506. [Google Scholar] [PubMed]
- Cox, W.L.; Shaw, R.R. Congenital chondromalacia of the trachea. J. Thorac. Cardiovasc. Surg. 1965, 49, 1033–1039. [Google Scholar] [CrossRef]
- Bairdain, S.; Smithers, C.J.; Hamilton, T.E.; Zurakowski, D.; Rhein, L.; Foker, J.E.; Baird, C.; Jennings, R.W. Direct tracheobronchopexy to correct airway collapse due to severe tracheobronchomalacia: Short-term outcomes in a series of 20 patients. J. Pediatr. Surg. 2015, 50, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.; Keyes, C. Airway stents. Ann. Cardiothorac. Surg. 2018, 7, 273–283. [Google Scholar] [CrossRef]
- Cooper, J.; Pearson, F.; Patterson, G.; Todd, T.; Ginsberg, R.; Goldberg, M.; Waters, P. Use of silicone stents in the management of airway problems. Ann. Thorac. Surg. 1989, 47, 371–378. [Google Scholar] [CrossRef]
- Dumon, J.-F. A Dedicated Tracheobronchial Stent. Chest 1990, 97, 328–332. [Google Scholar] [CrossRef]
- Chastre, J.; Fagon, J.-Y. Ventilator-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Xiong, X.F.; Xu, L.; Fan, L.L.; Cheng, D.Y.; Zheng, B.X. Long-term follow-up of self-expandable metallic stents in benign tracheobronchial stenosis: A retrospective study. BMC Pulm. Med. 2019, 19, 33. [Google Scholar] [CrossRef]
- Bi, Y.; Wu, G.; Yu, Z.; Han, X.; Ren, J. Fluoroscopic removal of self-expandable metallic airway stent in patients with airway stenosis. Medicine 2020, 99, e18627. [Google Scholar] [CrossRef]
- Martins, J.; Lach, A.; Morris, H.L.; Carr, A.J.; Mouthuy, P.-A. Polydioxanone implants: A systematic review on safety and performance in patients. J. Biomater. Appl. 2019, 34, 902–916. [Google Scholar] [CrossRef]
- Rodriguez-Zapater, S.; Serrano-Casorran, C.; Guirola, J.A.; Lopez-Minguez, S.; Bonastre, C.; de Gregorio, M.A. Reactivity Study of a Biodegradable Polydioxanone Tracheal Stent in a Rabbit Model. Arch. Bronconeumol. 2020, 56, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sztanó, B.; Kiss, G.; Márai, K.; Rácz, G.; Szegesdi, I.; Rácz, K.; Katona, G.; Rovó, L. Biodegradable airway stents in infants—Potential life-threatening pitfalls. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Violette, N.P.; Weisse, C.; Berent, A.C.; Lamb, K.E. Correlations among tracheal dimensions, tracheal stent dimensions, and major complications after endoluminal stenting of tracheal collapse syndrome in dogs. J. Vet. Intern. Med. 2019, 33, 2209–2216. [Google Scholar] [CrossRef]
- Sura, P.A.; Krahwinkel, D.J. Self-expanding nitinol stents for the treatment of tracheal collapse in dogs: 12 cases (2001–2004). J. Am. Vet. Med. Assoc. 2008, 232, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Beranek, J.; Jaresova, H.; Rytz, U. Use of nitinol self-expandable stents in 26 dogs with tracheal collapse. Schweiz. Arch. Tierheilkd 2014, 156, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rosenheck, S.; Davis, G.; Sammarco, C.D.; Bastian, R. Effect of variations in stent placement on outcome of endoluminal stenting for canine tracheal collapse. J. Am. Anim. Hosp. Assoc. 2017, 53, 150–158. [Google Scholar] [CrossRef]
- Weisse, C. Insights in tracheobronchial stenting. J. Small Anim. Pract. 2014, 55, 181–184. [Google Scholar] [CrossRef]
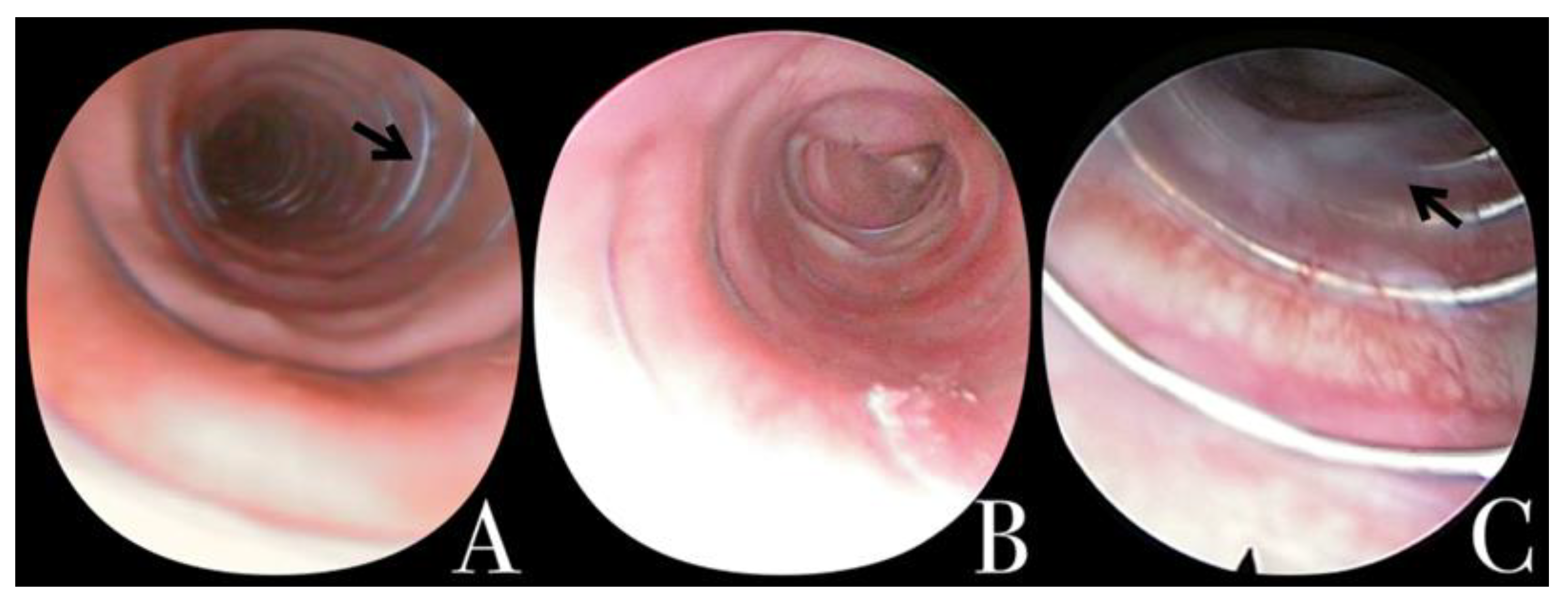
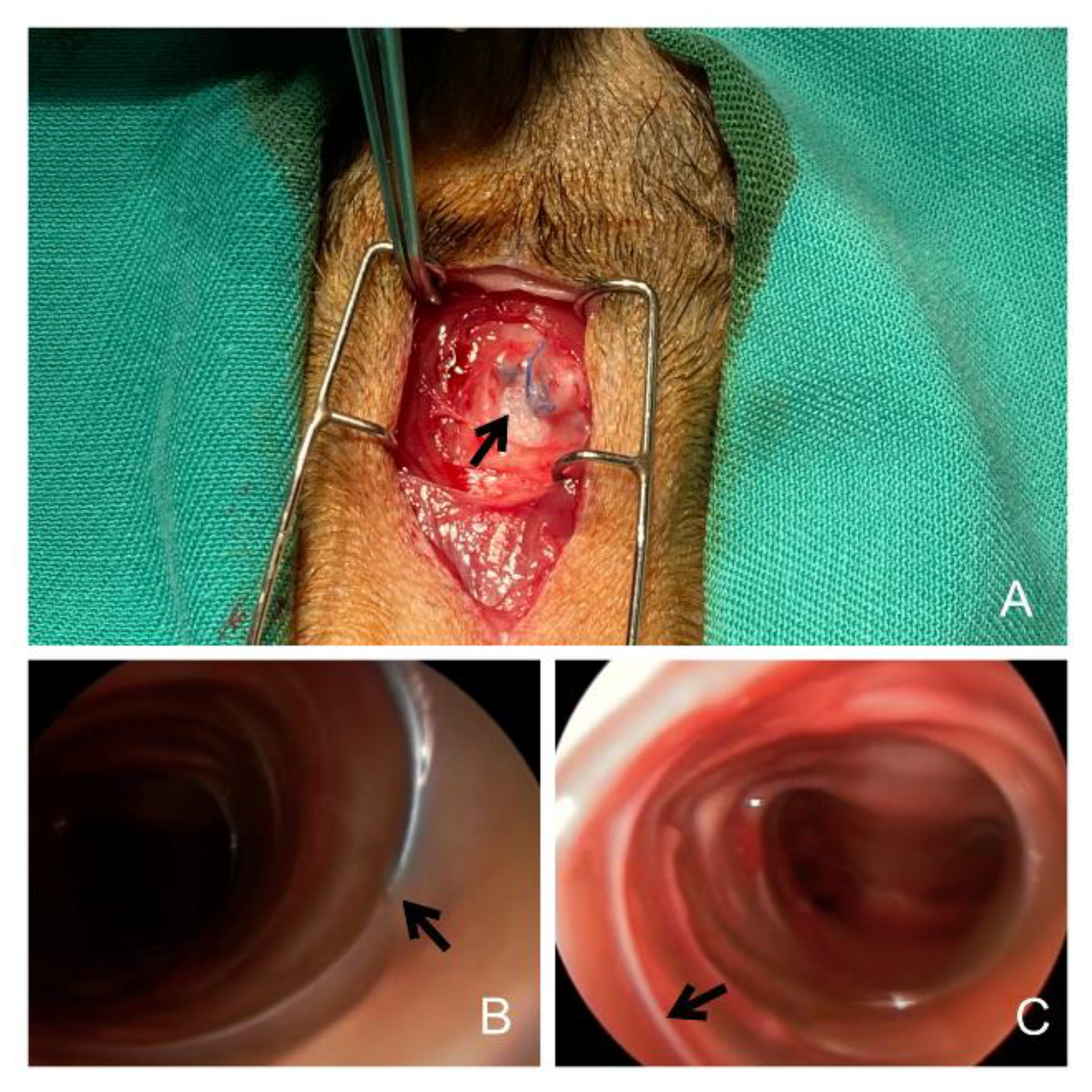
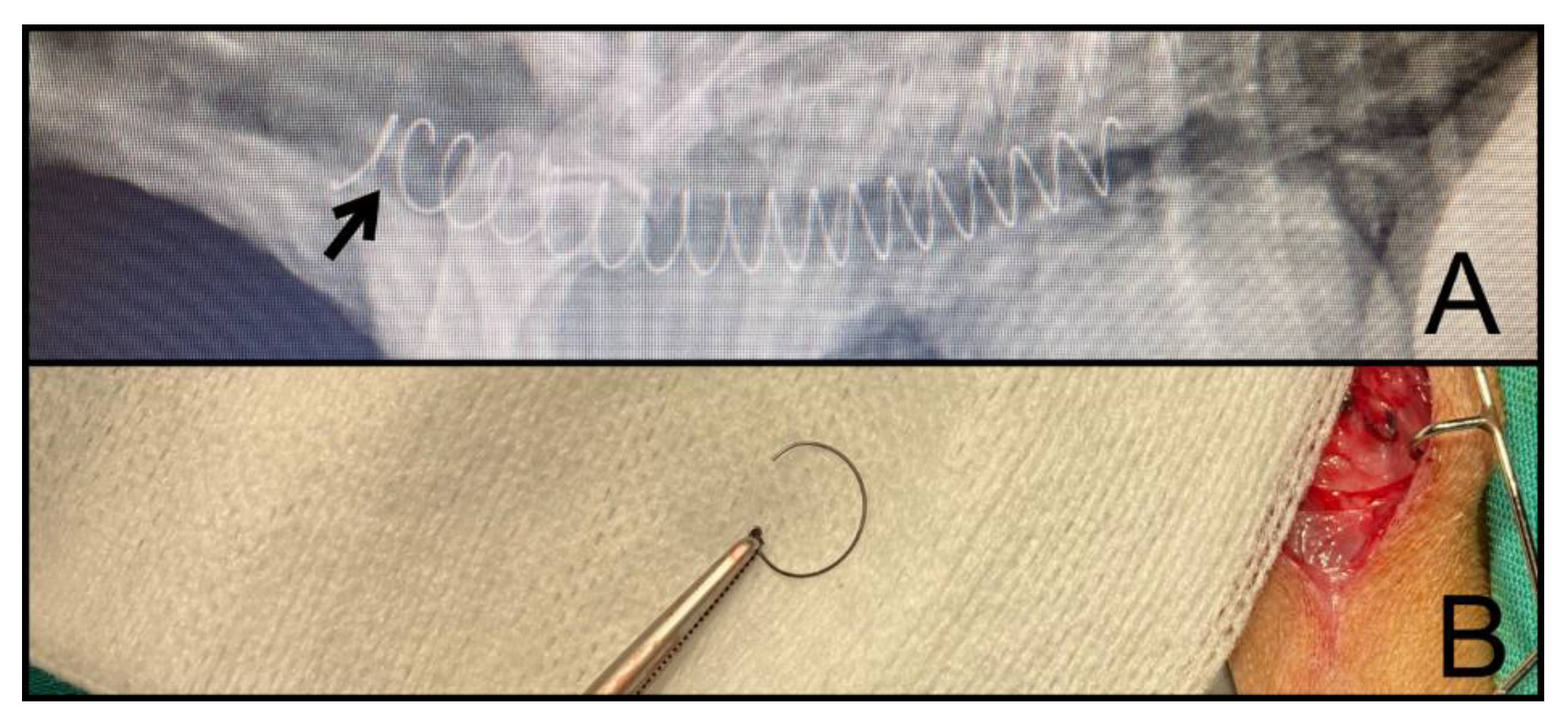
| <30 Days | 90–180 Days | >365 Days | Total | p-Value | ||
|---|---|---|---|---|---|---|
| n | 2 | 5 | 3 | 10 | ||
| Body weight (kg) | 6.23 (1.95) | 4.37 ± 1.59 | 3.25 | 4.80 ± 1.67 | 0.213 | |
| BCS (I-V) | III | - | - | 1/3 (33.3%) | 1/10 (10%) | 0.159 |
| IV | 2/2 (100%) | 3/5 (60%) | - | 5/10 (50%) | ||
| V | - | 2/5 (40%) | 2/3 (66.6%) | 4/10 (40%) | ||
| TC grade | II | - | - | 1/3 (33.3%) | 1/10 (10%) | |
| III | 1/2 (50%) | 2/3 (33.3%) | 3/10 (30%) | |||
| IV | 1/2 (50%) | 3/5 (66.7%) | 2/3 | 6/10 (60%) | ||
| Bronchus collapse | No | 2/2 (100%) | 1/5 (20%) | 1/3 (33.3%) | 4/10 (40%) | 0.053 |
| L | - | 2/5 (40%) | 1/3 (33.3%) | 3/10 (30%) | ||
| L + R | - | 2/5 (40%) | 1/3 (33.3%) | 3/10 (30%) | ||
| Tracheal diameter (mm) | C | 11.70 (2.00) | 10.83 ± 0.42 | 8.5 | 10.73 ± 1.36 | 0.252 |
| I | 10.25 (1.1) | 8.13 ± 1.22 | 7.5 | 8.73 ± 1.47 | 0.172 | |
| IT | 10.60 (0.8) | 8.87 ± 1.53 | 8.3 | 9.35 ± 1.41 | 0.172 | |
| Tracheal length (mm) | 110.00 (20) | 85.67 ± 5.13 | 75 | 92.00 ± 16.19 | 0.117 |
| <30 Days | 90–180 Days | >365 Days | Total | p-Value | ||
|---|---|---|---|---|---|---|
| Removal decision | FR | - | 1/5 (20%) | 1/3 (33.3%) | 2/10 (20%) | 0.081 |
| M | 2/2 (100%) | - | - | 2/10 (20%) | ||
| SL | - | 4/5 (80%) | 2/3 (66.6%) | 6/10 (60%) | ||
| Removal date | day | 17.5 ± 14.84 | 160.00 ± 62.45 | 661.66 ± 289.23 | 279.72 ± 338.35 | 0.117 |
| Removal Duration | min | 3.50 ± 1.15 | 8.00 ± 4.00 | 10 ± 2.00 | 7.16 ± 3.32 | 0.213 |
| Epithelialization | 0 (naked) | 2/2 (100.0%) | - | - | 2/10 (20%) | 0.081 |
| 1 (partial) | - | 1/5 (20%) | - | 1/10 (10%) | ||
| 2 (total) | - | 4/5 (80%) | 3/3 (100%) | 7/10 (70%) | ||
| SS imprint | 1/2 (50.0%) | 5/5 (100.0%) | 3/3 (100%) | 9/10 (90%) | 0.268 | |
| Tracheal congestion | 2/2 (100%) | 1/5 (20%) | 1/3 (33.3%) | 4/10 (40%) | 0.105 | |
| Secretion retention | 1 (moderate) | 1/5 (20%) | 1/10 (10%) | |||
| Granuloma | 0 (absence) | 2/2 (100%) | 4/5 (80%) | 3/3 (100%) | 9/10 (90%) | 0.452 |
| 1 (unique) | - | 1/5 (20%) | - | 1/10 (10%) | ||
| No MSS contact | 0 (absence) | 2/2 (100%) | 1/5 (20%) | 3/3 (100%) | 7/10 (70%) | 0.105 |
| 2 (both sides) | - | 4/5 (80%) | - | 3/10 (30%) | ||
| Stenosis | - | 1/3 (33.3%) | - | 1/10 (10%) | 0.452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Minguez, S.; Rodriguez-Zapater, S.; Bonastre, C.; Rodriguez, J.; De Gregorio, M.A.; Guirola, J.A.; Serrano-Casorran, C. A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model. J. Clin. Med. 2022, 11, 6757. https://doi.org/10.3390/jcm11226757
Lopez-Minguez S, Rodriguez-Zapater S, Bonastre C, Rodriguez J, De Gregorio MA, Guirola JA, Serrano-Casorran C. A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model. Journal of Clinical Medicine. 2022; 11(22):6757. https://doi.org/10.3390/jcm11226757
Chicago/Turabian StyleLopez-Minguez, Sandra, Sergio Rodriguez-Zapater, Cristina Bonastre, Jose Rodriguez, Miguel Angel De Gregorio, Jose Andres Guirola, and Carolina Serrano-Casorran. 2022. "A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model" Journal of Clinical Medicine 11, no. 22: 6757. https://doi.org/10.3390/jcm11226757
APA StyleLopez-Minguez, S., Rodriguez-Zapater, S., Bonastre, C., Rodriguez, J., De Gregorio, M. A., Guirola, J. A., & Serrano-Casorran, C. (2022). A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model. Journal of Clinical Medicine, 11(22), 6757. https://doi.org/10.3390/jcm11226757






